Abstract
Immune checkpoint inhibitors have drastically changed in the treatment of many kinds of malignancies, especially malignant melanoma. The focus of the recent experiments has not only been on their efficacy but also immune-related adverse events (irAEs). We report a case of fulminant hepatitis due to nivolumab. In this case, the patient had undergone long-term nivolumab therapy. He did not complain of any symptoms but his liver enzyme levels were extremely elevated (grade 4). We promptly decided to start oral corticosteroids in the patient. His liver function rapidly improved. The dose of corticosteroids was gradually reduced. Our case demonstrates that sudden onset fulminant hepatitis can occur despite the safe use of long-term nivolumab therapy. The irAE can improve rapidly with proper corticosteroid treatment. This report will be useful for the physicians who always use immune checkpoint inhibitors.
Keywords: Malignant melanoma, Nivolumab, Hepatitis, Long-term administration, Successful treatment
Introduction
Immune checkpoint inhibition with anti-CTLA-4 blockade (e.g., ipilimumab) and anti-PD-1 antibodies (e.g., nivolumab) has improved the poor prognosis of unresectable malignant melanoma [1, 2]. These newly approved medications induce many types of immune-related adverse events (irAEs), including pneumonitis, colitis, hepatitis, and endocrinopathies such as hypophysitis and thyroiditis. Although irAEs induced by ipilimumab have occasionally been reviewed in the literature [3], studies on nivolumab are also gradually being described. Anti-PD-1 agents often have toxicity profiles different from ipilimumab. Therefore, we report a case of hepatitis induced by nivolumab.
Case Report
A 52-year-old male with lung and liver metastasis of malignant melanoma was put on nivolumab therapy (3 mg/kg every 3 weeks). After approximately 34 weeks of nivolumab administration (Fig. 1), laboratory tests revealed a sudden elevation of liver enzymes (AST/ALT) to 1,225/824 U/L (grade 4). The patient did not have any symptoms. Computed tomography detected the exacerbation of liver metastasis, identifying a lesion that was slightly larger than the nondiffuse, localized lesion that had been observed 3 months earlier (Fig. 2). Therefore, we suspected hepatitis associated with nivolumab because this drug has a low probability of causing serious liver damage to localized lesions, even when they are large. The patient stopped taking nivolumab and was treated with 70 mg/day (1.0 mg/kg/day) of systemic corticosteroids. His AST/ALT level promptly improved to 112/389 U/L within 3 days. The dose of corticosteroids was then gradually reduced.
Fig. 1.
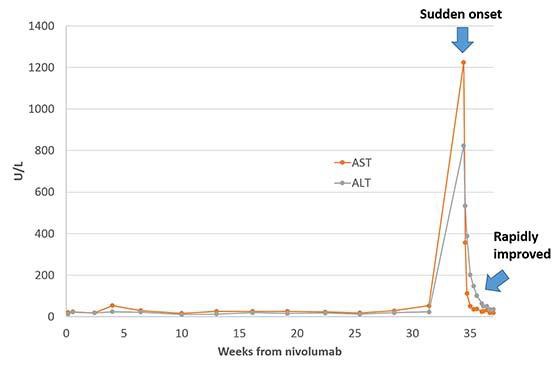
Laboratory data fluctuation. A line graph demonstrates the fluctuations of AST/ALT levels. The horizontal axis shows the number of weeks after nivolumab induction. At week 34, these enzymes were suddenly elevated. After corticosteroid initiation, the levels of these enzymes rapidly improved.
Fig. 2.
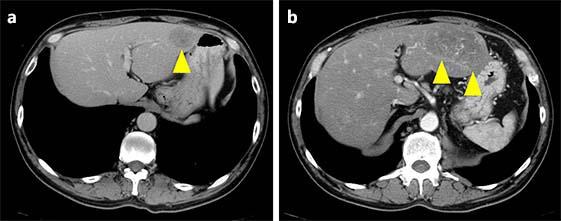
CT of the abdomen. a CT scan before hepatitis: a large metastatic lesion is present in the left lobe (yellow arrowhead). b Three months later, during hepatitis, the metastatic lesion became larger (yellow arrowheads). However, the lesion was localized, not diffuse.
Discussion
It is known that both ipilimumab and nivolumab can cause hepatitis [2]. The median onset of ipilimumab-related hepatitis is approximately 8–12 weeks after initial treatment [3]. Therefore, the interval from nivolumab initiation to the occurrence of hepatitis was much longer than that reported in previous cases. Hepatitis induced by nivolumab was also observed at 7–12 weeks in a pooled analysis of a nivolumab phase III trial. However, the incidence of grade 2–3 nivolumab-induced hepatitis was approximately 1% in a phase III clinical study. In that study, the liver function test improved to grade 1 within 4–15 days of initiation of corticosteroid treatment [4].
The liver dysfunction caused by long-term nivolumab therapy in this case is noteworthy. Although many diseases induce liver dysfunction, in this case, we definitively excluded other causes of hepatitis such as viral infection (e.g., HBV, HCV, HSV, CMV, and EBV), medication other than nivolumab, and other forms of autoimmune hepatitis. To manage hepatitis associated with nivolumab, these possibilities must be excluded as causes of the disease [5]. The recommended initial treatment of hepatitis in the Risk Evaluation and Mitigation Strategy (REMS) is to administer systemic corticosteroids (1–2 mg/kg/day of prednisone). If the symptoms continue after 3–5 days, alternative immunosuppressive therapy, such as 500 mg of oral mycophenolate mofetil every 12 h, must be considered. In this case, because the laboratory test showed an AST/ALT level >20× the upper limit of normal, we considered systemic corticosteroids to be the most appropriate treatment. Fortunately, the AST/ALT level rapidly improved to grade 1–2 in 3 days, and modification was not necessary.
It is very important to note that severe hepatitis can occur despite long-term nivolumab therapy. In this case, severe hepatitis was rapidly managed and successfully controlled with systemic corticosteroids. It was possible to quickly reach a correct diagnosis because we were aware of the possibility of drug-induced hepatitis, even though the period of occurrence of nivolumab-induced hepatitis had passed. Therefore, we believe that this report may be very informative when treating hepatitis as an irAE of nivolumab. In addition, to our knowledge, this patient exhibited the longest time between nivolumab administration and the onset of hepatitis that has been reported to date.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to declare.
References
- 1.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 2.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 4.Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 5.Villadolid J, Amin A. Immune checkpoint inhibitors in clinical practice: update on management of immune-related toxicities. Transl Lung Cancer Res. 2015;4:560–575. doi: 10.3978/j.issn.2218-6751.2015.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]


