We report the case of a 64-year-old woman who presented with localized postradiotherapy lichen planus (LP) who subsequently had new lesions at a distant site months after completion of radiotherapy. To our knowledge, this is a unique case of radiation-induced LP initially localized to the radiation field with subsequent generalization.
Introduction
Isoradiotopic responses were first described in 2004 by Shurman et al1 as the development of unrelated dermatoses within previously irradiated skin. Several case reports describe development of auto-antibody–mediated diseases in the radiation treatment field including bullous pemphigoid2 and pemphigus vulgaris.3, 4 To our knowledge, only 5 cases of postradiotherapy LP have been reported since 2002.1, 5, 6, 7, 8 We present a case of postradiotherapy LP that was localized to the radiation treatment field with subsequent generalization to a distant site in a patient with no history of LP.
Case
A 64-year-old woman with invasive lobular carcinoma received radiation treatment of the right breast and axilla. She received a total dose of 61 Gy in 33 fractions after undergoing lumpectomy and sentinel lymph node biopsy. In 2015, 3 months after completion of radiation treatment, she reported having a pruritic, irritating rash localized to the radiation field. Physical examination found multiple 4- to 8-mm well-demarcated, violaceous papules on her right axilla, lateral breast, and inframammary fold confined to the radiation site (Fig 1). The papules exhibited fine overlying scale without white lines indicative of Wickham striae. There was no evidence of scalp, mucosal, or nail involvement. Three to four months before the onset of rash, she was started on anastrozole and zoledronic acid injections. The patient had no history of liver disease, and her dermatologic history was unremarkable except for a single asymptomatic pink papule in the right axilla in 2011 and a subtle pink plaque on the left upper back in 2013, which a punch biopsy determined to be nodular granulomatous mixed dermatitis. Biopsy of the new rash found changes suggestive of LP including vacuolar alteration of the basal layer, dyskeratosis, and a bandlike infiltrate of lymphocytes and occasional eosinophils (Fig 2). Her symptoms were well controlled with application of 0.1% triamcinolone ointment 1 to 2 times per day, 3 times per week. In 2016, 10 months after her initial presentation, she returned with 2 new pruritic papules on her upper right posterior shoulder resembling the initial eruption (Fig 3).
Fig 1.
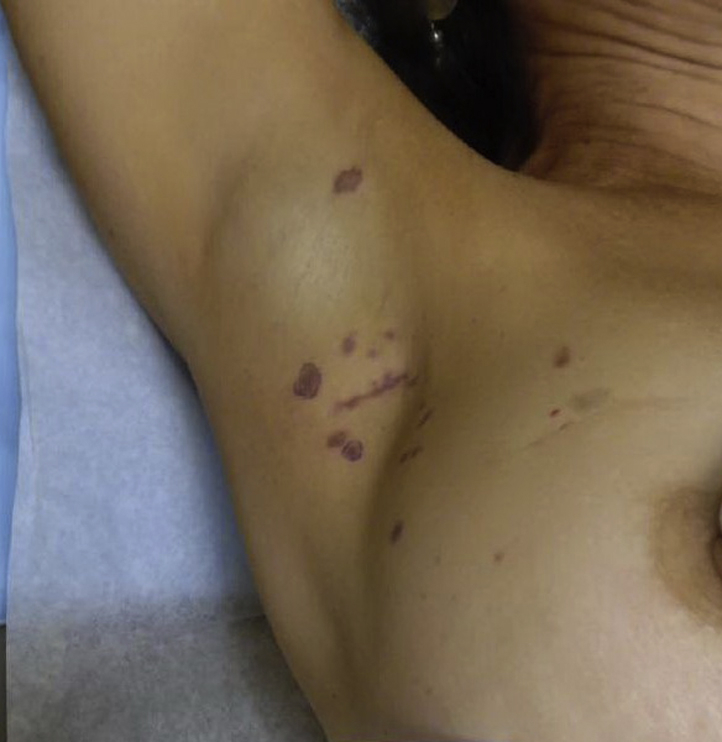
Well-demarcated, polygonal, violaceous papules in radiation field of right axilla, lateral breast, and inframammary fold.
Fig 2.
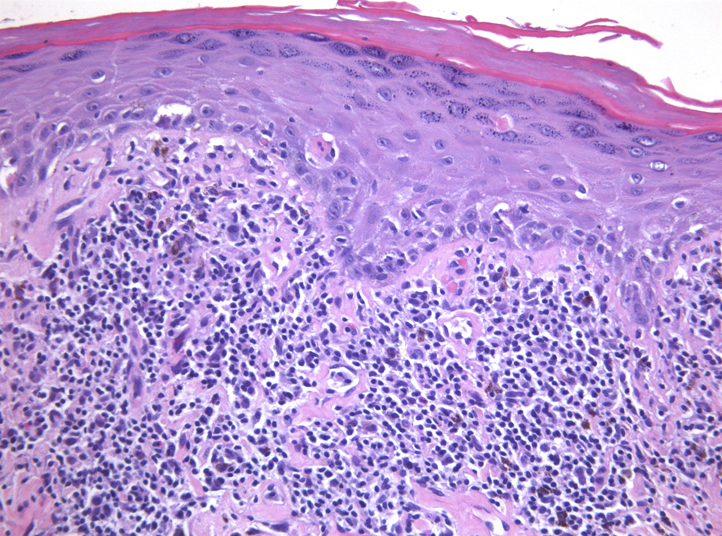
Biopsy result shows vacuolar alteration of the basal layer and dyskeratosis with a subepithelial bandlike infiltrate of lymphocytes and eosinophils below. (Original magnification: ×20).
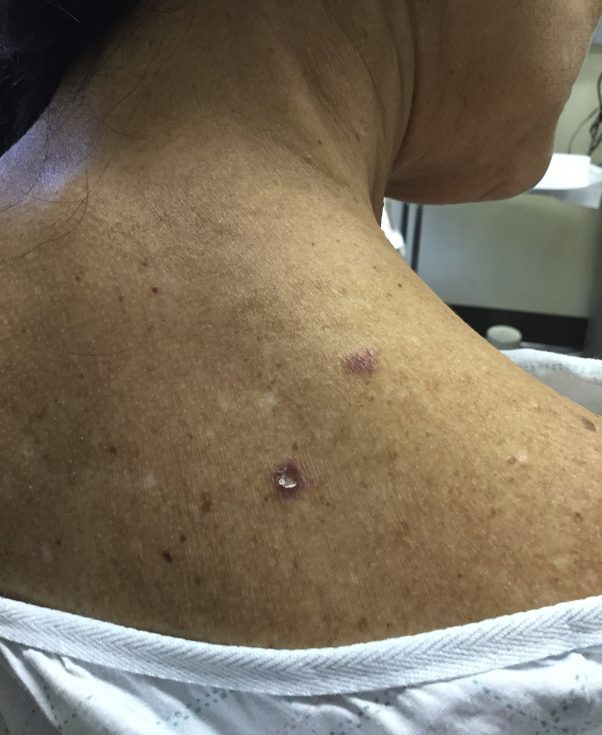
Fig 3.
Two new pruritic violaceous papules on the upper right posterior shoulder 10 months after initial presentation.
Discussion
The pathophysiologic role of radiation in LP remains unclear. Ultraviolet radiation is considered the inciting factor for actinic LP, yet some studies show patients with generalized LP respond favorably to narrow-band ultraviolet B treatments. The most commonly reported postradiotherapy dermatoses include bullous pemphigoid2 and pemphigus vulgaris.3, 4 To our knowledge, only 5 cases of radiation-induced LP have been reported,1, 5, 6, 7, 8 including one case of lichen ruber planus8 (Table I). Primary cancer types included 3 cases of breast cancer,5, 7, 8 1 case of poorly differentiated thyroid cancer,6 and 1 case of penile squamous cell carcinoma.1 Three studies reported total radiation doses ranging from 5940 cGy to 60 Gy, similar to that of our patients' accumulative dose of 61 Gy.6, 7, 8 Only 1 case reported a history of LP 7 months before radiotherapy.7 The latency period from the end of radiation to onset of LP ranged from occurring during radiation therapy (in a case of lichen ruber planus)8 to 4 months, which was comparable to that of our patient who had LP 4 months after stopping radiotherapy.1, 6, 7 All cases were initially localized to the radiation field, and only the case of lichen ruber planus generalized to regions external to the radiation field.1, 5, 6, 7, 8 Most cases were pruritic, exhibited Wickham striae, and did not involve mucosal membranes or nails.1, 5, 6, 8 All cases reported improvement or resolution with topical steroids.1, 5, 6, 7, 8
Table I.
Previous case reports of postradiation LP
| Study | Sex | Age | Cancer | Total RT (Gy) | Progression of disease |
||||
|---|---|---|---|---|---|---|---|---|---|
| History of LP? | Time interval after RT | Localization | Characteristics | Outcome | |||||
| Kim et al6 | M | 58 | Thyroid | 59.4 | No | 1 mo | Localized | Pruritic, Wickham striae: present, MM: no involvement, nails: no involvement | Resolved over 5 mo with topical fluocinonide/clobetasol propionate 0.05% creams. Episodic recurrences within radiation site |
| Shurman et al1 | M | 68 | Penile SCC | Not reported | Not reported | 2 mo | Localized | Pruritic, Wickham striae: present, MM: no involvement, nails: no involvement | Responded well to topical steroids |
| Eichbaum et al8 | F | 56 | DCIS of the breast | 50.4 | Not reported | 0 mo (onset during RT) | Localized, progressed to generalized | Lichen ruber planus: disseminated, pruritic, erythema, Wickham striae: present, MM: present | Resolved after 5 mo of topical betamethasone propionate for the body and topical tretinoin for the oral lesions |
| Pretel and Espana7 | F | 44 | Infiltrating DC of the breast | 60 | Yes | 4 mo | Localized | Not reported | Resolved with 1 mo topical clobetasol propionate 0.05% |
| Vergilis-Kalner et al5 | F | 59 | Metastatic breast cancer | Not reported | Not reported | Not reported | Localized | Pruritic, painful, erythema, MM: no involvement, nails: no involvement | Some improvement with triamcinolone acetonide 0.1% ointment twice daily |
cGy, Centigray; DC, ductal carcinoma; DCIS, ductal carcinoma in situ; MM, mucous membranes; RT, radiotherapy; SCC, squamous cell carcinoma.
Interestingly, although our case showed the bandlike lymphocytic infiltrate characteristic of LP, eosinophils were also present, prompting lichenoid drug reaction to be initially included on the differential diagnosis. Although there are limited reports of lichenoid drug reactions caused by anastrozole and the bisphosphonate class, we favor the diagnosis of postradiation LP because of the asymmetric distribution of the rash confined to the radiation field, timing occurring greater than 3 to 4 months after initial drug administration, and lack of changes made to the medication schedule.
Shurman et al1 proposed the term isoradiotopic response to describe the phenomenon of secondary dermatoses arising in radiation fields, akin to the Wolf isotopic response that describes the development of secondary dermatoses in the same site as a prior skin eruption (most often herpes zoster).9 Koebnerization of LP occurring outside the context of radiation is well described, and some groups suggest localized LP confined to the radiation field may represent an isomorphic or Koebnerlike reaction from radiation injury.6 Although Koebnerization remains a possibility, the observed latency periods on the order of months and subsequent generalization of disease as seen in our patient go against this theory and better support postradiation LP as an isoradiotopic response.
Traditionally, CD8+ cytotoxic T cells and natural killer cells have been held responsible for the keratinocyte damage at the dermoepidermal junction of skin affected by lichen planus.10 With this basement membrane damage, more CD8+ cells are allowed to infiltrate, leading to a vicious cycle and chronic disease.10 Pretel et al7 propose a mechanism for postradiation LP as an isoradiotopic response suggesting an immunomodulatory property of radiotherapy. The theory suggests that radiation increases expression of proinflammatory molecules such as major histocompatibility complex, adhesion molecules (eg, intercellular adhesion molecule-1 and E-selectin), and cytokines.7 This proinflammatory milieu together with adhesion molecules may encourage the transendothelial migration of leukocytes, such as CD8+ cytotoxic T cells, leading to the histologic bandlike lymphocytic infiltrate of LP. Furthermore, this immunomodulation process is reported to last up to 6 months after the end of treatment,7 which is consistent with the timing of the current and previously reported cases. However, more basic science studies are needed to correlate pathogenesis to clinical disease and further distinguish isoradiotopic response of postradiation LP from anti-inflammatory effects of low-dose radiotherapy used to treat LP.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Shurman D., Reich H.L., James W.D. Lichen planus confined to a radiation field: the “isoradiotopic” response. J Am Acad Dermatol. 2004;50(3):482–483. doi: 10.1016/s0190-9622(03)02144-3. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen T., Kwan J.M., Ahmed A.R. Relationship between radiation therapy and bullous pemphigoid. Dermatology (Basel, Switzerland) 2014;229(2):88–96. doi: 10.1159/000362208. [DOI] [PubMed] [Google Scholar]
- 3.Shon W., Wada D.A., Kalaaji A.N. Radiation-induced pemphigus or pemphigoid disease in 3 patients with distinct underlying malignancies. Cutis. 2016;97(3):219–222. [PubMed] [Google Scholar]
- 4.Badri T., Hammami H., Lachkham A., Benmously-Mlika R., Mokhtar I., Fenniche S. Radiotherapy-induced pemphigus vulgaris with autoantibodies targeting a 110 kDa epidermal antigen. Int J Dermatol. 2011;50(12):1475–1479. doi: 10.1111/j.1365-4632.2011.04889.x. [DOI] [PubMed] [Google Scholar]
- 5.Vergilis-Kalner I.J., Sharma V., Sethi A. Lichen planus arising in radiation therapy treatment sites. Cutis. 2008;82(5):353–355. [PubMed] [Google Scholar]
- 6.Kim J.H., Krivda S.J. Lichen planus confined to a radiation therapy site. J Am Acad Dermatol. 2002;46(4):604–605. doi: 10.1067/mjd.2002.119654. [DOI] [PubMed] [Google Scholar]
- 7.Pretel M., Espana A. Lichen planus induced by radiotherapy. Clin Exp Dermatol. 2007;32(5):582–583. doi: 10.1111/j.1365-2230.2007.02449.x. [DOI] [PubMed] [Google Scholar]
- 8.Eichbaum M., Harms W., Bolz S., Schneeweiss A., Sohn C. Generalized lichen ruber planus–induced by radiotherapy of the breast? Onkologie. 2006;29(11):521–523. doi: 10.1159/000096048. [DOI] [PubMed] [Google Scholar]
- 9.Wolf R., Wolf D., Ruocco E., Brunetti G., Ruocco V. Wolf's isotopic response. Clin Dermatol. 2011;29(2):237–240. doi: 10.1016/j.clindermatol.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Ammar M., Mokni M., Boubaker S., El Gaied A., Ben Osman A., Louzir H. Involvement of granzyme B and granulysin in the cytotoxic response in lichen planus. J Cutan Pathol. 2008;35(7):630–634. doi: 10.1111/j.1600-0560.2007.00892.x. [DOI] [PubMed] [Google Scholar]