Abstract
Peritoneal fibrosis is a major complication in peritoneal dialysis (PD) patients, which leads to dialysis discontinuation. Periostin, increased by transforming growth factor β1 (TGF-β1) stimulation, induces the expression of extracellular matrix (ECM) genes. Aberrant periostin expression has been demonstrated to be associated with PD-related peritoneal fibrosis. Therefore, the effect of periostin inhibition by an aptamer-based inhibitor on peritoneal fibrosis was evaluated. In vitro, TGF-β1 treatment upregulated periostin, fibronectin, α-smooth muscle actin (α-SMA), and Snail expression and reduced E-cadherin expression in human peritoneal mesothelial cells (HPMCs). Periostin small interfering RNA (siRNA) treatment ameliorated the TGF-β1-induced periostin, fibronectin, α-SMA, and Snail expression and restored E-cadherin expression in HPMCs. Similarly, the periostin-binding DNA aptamer (PA) also attenuated fibronectin, α-SMA, and Snail upregulation and E-cadherin downregulation in TGF-β1-stimulated HPMCs. In mice treated with PD solution for 4 weeks, the expression of periostin, fibronectin, α-SMA, and Snail was significantly increased in the peritoneum, whereas E-cadherin expression was significantly decreased. The thickness of the submesothelial layer and the intensity of Masson’s trichrome staining in the PD group were significantly increased compared to the untreated group. These changes were significantly abrogated by the intraperitoneal administration of PA. These findings suggest that PA can be a potential therapeutic strategy for peritoneal fibrosis in PD patients.
Keywords: peritoneal dialysis, fibrosis, periostin, aptamer, TGF-β1
Introduction
Peritoneal dialysis (PD) is a commonly used renal replacement modality to treat end-stage renal disease patients. However, long-term PD leads to peritoneal fibrosis in most patients receiving PD.1 The progression of peritoneal fibrosis invariably causes membrane failure, leading to volume control failure and loss of dialysis adequacy resulting in the discontinuation of PD.2, 3, 4 However, detailed molecular mechanisms underlying peritoneal fibrosis development and progression have not been fully elucidated. Therefore, the strategies to prevent peritoneal fibrosis and to treat established peritoneal fibrosis are limited.
The peritoneal membrane is covered by a single layer of peritoneal mesothelial cells (PMCs), which are supported by a submesothelial connective tissue area composed of fibroblasts, mast cells, and macrophages.5 Recently, PMCs were suggested to play a key role in the development and progression of peritoneal fibrosis by producing type I and type II collagen and fibronectin.6 The synthesis of these extracellular matrix (ECM) proteins by PMCs is accompanied by a complex mechanism called the epithelial-mesenchymal transition (EMT).7 EMT of PMCs and their excessive production of ECM proteins are largely attributed to the activation of the transforming growth factor β1 (TGF-β1) pathway.8 PD fluid is known to increase the production of TGF-β1 by PMCs,9 and the high glucose level in the peritoneal dialysate has been shown to be a primary cause of this upregulation of TGF-β1 in PMCs.10
Periostin is an ECM protein and is upregulated by the activation of the TGF-β1 pathway in several cell types.11 It functions as a ligand of αV/β3 and αV/β5 integrin to activate integrin-related intracellular signaling pathways, resulting in the increased transcription of ECM genes, such as collagen and fibronectin.12, 13, 14, 15 Recently, periostin levels in the PD fluid were demonstrated to be upregulated in patients with encapsulated peritoneal sclerosis, indicating the possibility that periostin contributes to peritoneal fibrosis in patients undergoing PD.16
Aptamers are single-stranded oligonucleotides that bind to specific target molecules. Once bound to a specific target, some aptamers are capable of inhibiting its activity.17 Monoclonal antibodies have been applied in the clinical field to suppress the activity of specific proteins. However, using antibodies to treat diseases is accompanied by some critical limitations, such as low bio-stability, the induction of undesirable immune responses, and high production costs. In contrast, aptamers are produced chemically and are relatively bio-stable, resulting in lower production costs and more convenient storage and distribution compared with antibodies.17, 18 They are also less immunogenic and therefore are associated with a lower risk of developing hypersensitivity reactions. Due to these advantages, aptamers have been proposed as promising drug candidates to inhibit specific target molecules in various diseases.19, 20, 21
Previously, a benzyl-d(U)TP-modified DNA aptamer, that binds selectively to periostin, was shown to disrupt the interaction with its receptor integrins in breast cancer cells.21 In this study, the effects of this periostin-binding DNA aptamer (PA) on TGF-β1-induced EMT and ECM accumulation were evaluated using human PMCs (HPMCs) and a murine model of the PD.
Results
TGF-β1 Upregulates Periostin Expression and Mediates EMT in HPMCs
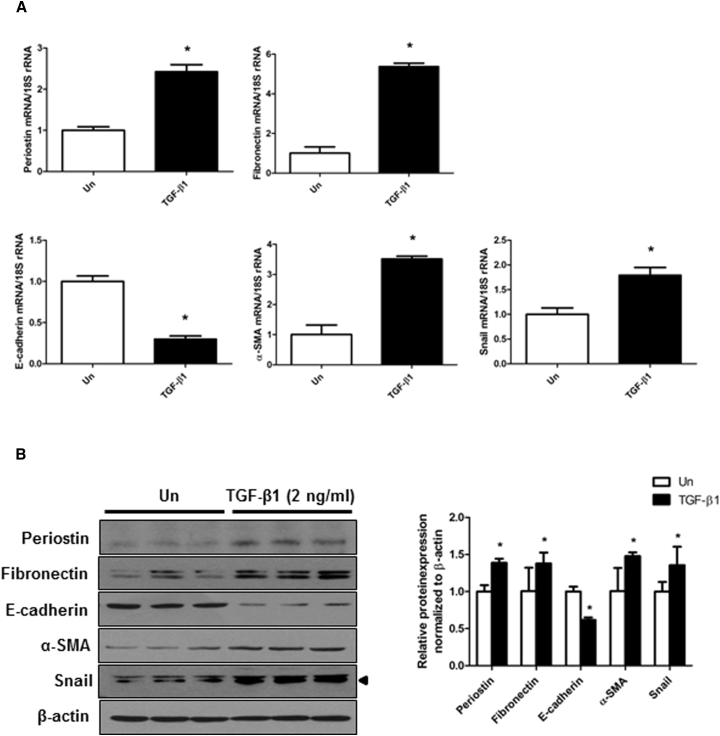
First, to verify that periostin is expressed during the EMT process of HPMCs, the expression of periostin was evaluated in HPMCs treated with TGF-β1. HPMCs treated with TGF-β1 (2 ng/mL) showed significantly increased expression of periostin mRNA and protein. This increase in periostin mRNA expression was accompanied by the upregulation of fibronectin, α-smooth muscle actin (α-SMA), and Snail and downregulation of E-cadherin mRNA expression. The protein levels of fibronectin, α-SMA, Snail, and E-cadherin showed a similar expression pattern as the corresponding mRNA levels in HPMCs (Figure 1). These results indicate that TGF-β1 administration induces EMT in HPMCs along with a significant upregulation of periostin.
Figure 1.
Expression of Periostin and EMT-Related Molecules in HPMCs Exposed to TGF-β1
(A) Periostin, fibronectin, E-cadherin, α-SMA, and Snail mRNA levels in HPMCs treated with or without TGF-β1 (2 ng/mL) (n = 12). The TGF-β1 significantly induced fibronectin, α-SMA, and Snail mRNA expression and significantly reduced E-cadherin mRNA expression in HPMCs. The periostin mRNA expression was also significantly increased by TGF-β1. (B) A representative western blot analysis of periostin, fibronectin, E-cadherin, α-SMA, Snail, and β-actin protein expression in HPMCs treated with or without TGF-β1 (2 ng/mL) (a representative of four blots). The protein expression of periostin, fibronectin, α-SMA, and Snail significantly increased and that of E-cadherin significantly decreased in the TGF-β1-stimulated cells (n = 12). All error bars represent SD of the mean. Un, untreated. *p < 0.01 versus untreated.
Periostin Knockdown Abrogates TGF-β1-Induced EMT in HPMCs
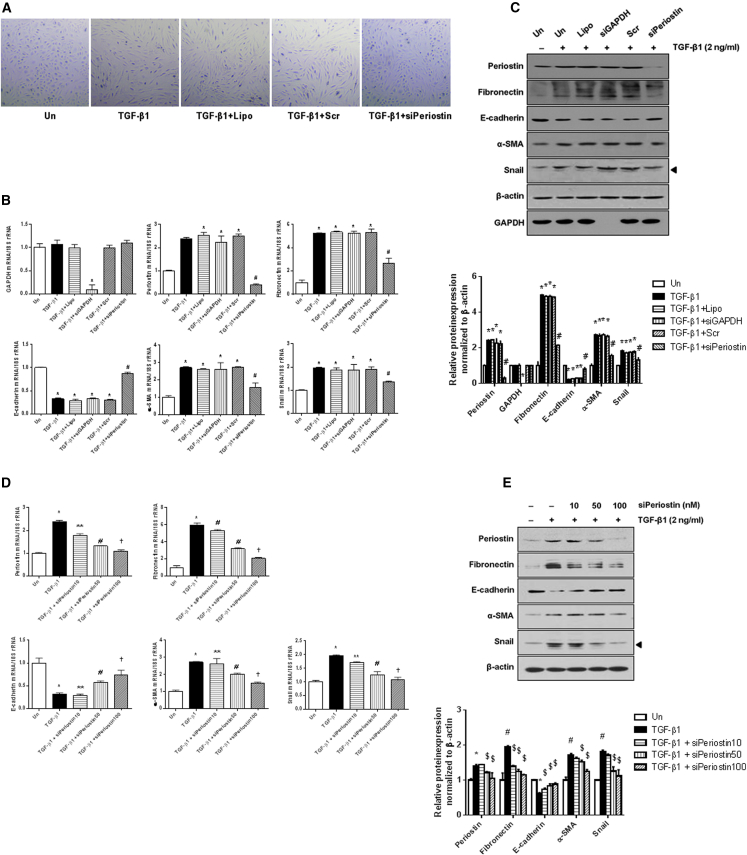
To evaluate the role of periostin in the EMT process of HPMCs, periostin expression was knocked down by small interfering RNA (siRNA) transfection in TGF-β1-stimulated HPMCs. As shown in Figure 2A, TGF-β1 (2 ng/mL) induced fibroblast-like cell morphological changes characterized by thin spindle-like cell shapes, in contrast to the cuboidal shape of unstimulated HPMCs. Interestingly, periostin siRNA (100 nM) administration markedly ameliorated the TGF-β1-induced cell morphological changes. This intriguing result prompted the examination of whether periostin knockdown normalized the expression of mesenchymal markers triggered by TGF-β1 in HPMCs. As expected, periostin knockdown by siRNA administration effectively inhibited periostin expression induced by TGF-β1 treatment. The expression of fibronectin, α-SMA, and Snail mRNA also significantly decreased in HPMCs treated with TGF-β1 and periostin siRNA compared to HPMCs treated with TGF-β1 alone, and the decreased E-cadherin mRNA level in TGF-β1-stimulated HPMCs was significantly restored by periostin siRNA transfection. Regarding the protein levels, periostin siRNA significantly attenuated the increases in periostin, fibronectin, α-SMA, and Snail expression and the decrease in E-cadherin expression induced by TGF-β1 (Figure 2). Together, these results suggest that periostin upregulation is an essential factor in the EMT process of TGF-β1-stimulated HPMCs and that periostin inhibition can mitigate the pathologic EMT of HPMCs.
Figure 2.
Changes in Cell Morphology and mRNA and Protein Expression of EMT-Related Molecules following Periostin siRNA Transfection
(A) Cell morphology of untreated and TGF-β1- (2 ng/mL) stimulated HPMCs with or without periostin siRNA (100 nM) transfection. The TGF-β1 treatment provoked fibroblast-like cell morphological changes in HPMCs, and periostin siRNA transfection reversed these TGF-β1-induced cell morphological changes (×100). (B) mRNA levels in TGF-β1-stimulated HPMCs treated with or without periostin siRNA (n = 12). The periostin siRNA significantly attenuated TGF-β1-induced increases in fibronectin, α-SMA, and Snail mRNA expression and restored the downregulated E-cadherin mRNA level in HPMCs treated with TGF-β1. The positive control GAPDH siRNA transfection significantly downregulated GAPDH mRNA level. The negative control scramble siRNA transfection did not affect the expression of mRNA levels. (C) A representative western blot analysis of protein expression in TGF-β1-stimulated HPMCs with or without periostin siRNA transfection (a representative of four blots). The periostin siRNA transfection significantly mitigated the increases in periostin, fibronectin, α-SMA, and Snail protein expression and the decrease in E-cadherin protein expression in TGF-β1-stimulated HPMCs. The positive control GAPDH siRNA transfection significantly downregulated GAPDH protein level. The negative control scramble siRNA transfection did not affect the expression of protein levels (n = 12). (D) mRNA levels in TGF-β1-stimulated HPMCs transfected with different doses of periostin siRNA (n = 12). The periostin siRNA significantly attenuated TGF-β1-induced increases in fibronectin, α-SMA, and Snail mRNA expression and restored the downregulated E-cadherin mRNA level in a dose-dependent manner. (E) A representative western blot analysis of protein expression in TGF-β1-stimulated HPMCs with different doses of periostin siRNA transfection (a representative of four blots). The periostin siRNA transfection significantly mitigated the TGF-β1-induced increases in periostin, fibronectin, α-SMA, and Snail protein expression and the decrease in E-cadherin protein expression in a dose-dependent manner (n = 12). All error bars represent SD of the mean. Un, untreated; Lipo, lipofectamine; siGAPDH, GAPDH siRNA; Scr, negative control scramble siRNA; siPeriostin, periostin siRNA. *p < 0.01 versus untreated; #p < 0.01 versus TGF-β1.
Confirmation of DNA Aptamer Binding to Periostin in HPMCs
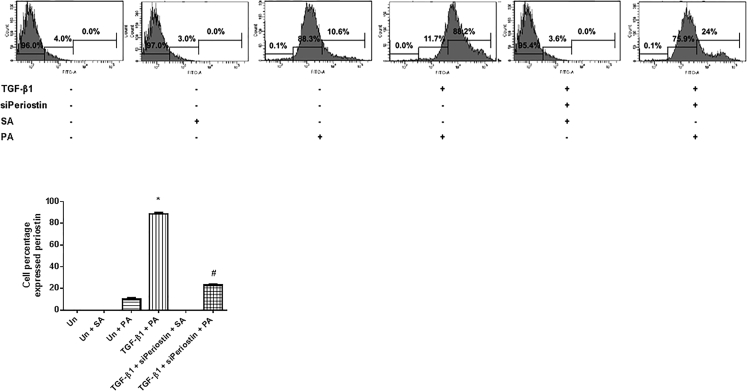
After confirming that periostin played a role in EMT induction in HPMCs, it was determined whether a PA could affect the EMT process in HPMCs. First, fluorescence-activated cell sorting (FACS) analysis was conducted to show that the PA attached specifically to periostin in HPMCs. A fluorescein isothiocyanate (FITC)-tagged PA was used to evaluate the amount of the PA that attached to periostin expressed in HPMCs. Compared to the negative control, the FITC-tagged PA-treated cells showed an increase in the FITC-positive fraction of the total HPMC count (10.6%). The FITC-positive fraction further increased to 88.2% in TGF-β1- (10 ng/mL) stimulated HPMCs treated with the FITC-tagged PA (200 nM), which was significantly abrogated by periostin siRNA (100 nM) transfection (24.0%). These findings suggest that the PA specifically binds to periostin expressed by HPMCs (Figure 3).
Figure 3.
Specific Attachment of PA to Periostin Expressed in HPMCs
FACS analysis was performed in HPMCs treated with or without TGF-β1 (10 ng/mL), periostin siRNA (100 nM), or FITC-labeled PA (200 nM). The FITC-positive fraction was significantly increased in TGF-β1-stimulated HPMCs treated with the FITC-labeled PA, which was significantly abrogated by periostin siRNA transfection. All error bars represent SD of the mean. Un, untreated; siPeriostin, periostin siRNA; SA, negative control scramble aptamer; PA, periostin-binding DNA aptamer. *p < 0.01 versus untreated; #p < 0.01 versus TGF-β1.
PA Ameliorates TGF-β1-Induced EMT in HPMCs
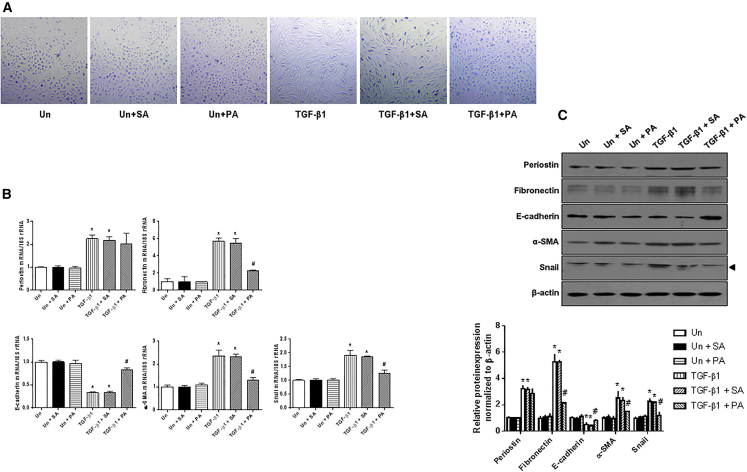
To determine whether blocking periostin signaling with the PA could affect the EMT process, the changes in cell morphology and the expression of EMT-related molecules were examined after treatment with the PA in TGF-β1-stimulated HPMCs. The spindle-shaped feature of HPMCs exposed to TGF-β1 (2 ng/mL) was significantly alleviated by the PA treatment (Figure 4), which was similar to the findings observed in TGF-β1-stimulated HPMCs transfected with periostin siRNA (Figure 2). When the cells were examined for periostin expression, the increases in periostin mRNA and protein expression following TGF-β1 stimulation were not significantly changed by the PA treatment. In contrast, the upregulated fibronectin, α-SMA, and Snail expression and the downregulated E-cadherin expression in TGF-β1-stimulated HPMCs were significantly attenuated by the PA treatment. These results indicate that the PA effectively mitigates the TGF-β1-induced EMT process in HPMCs by inhibiting periostin-related pathway activation rather than periostin expression.
Figure 4.
Changes in Cell Morphology and mRNA and Protein Expression following PA Treatment
(A) Morphologic features of control and TGF-β1- (2 ng/mL) stimulated HPMCs with or without PA treatment (200 nM). The PA inhibited the fibroblast-like cell morphological changes in HPMCs exposed to TGF-β1 (×100). (B) mRNA levels in TGF-β1-stimulated HPMCs treated with or without PA (n = 12). The PA significantly attenuated TGF-β1-induced fibronectin, α-SMA, and Snail mRNA expression and significantly restored the decrease in E-cadherin mRNA expression in HPMCs stimulated with TGF-β1. The negative control scramble aptamer treatment did not affect the expression of mRNA levels. (C) A representative western blot analysis of protein expression in HPMCs treated with or without PA (a representative of four blots). The PA significantly mitigated the increases in fibronectin, α-SMA, and Snail protein expression and the decrease in E-cadherin protein expression in TGF-β1-stimulated HPMCs. The negative control scramble aptamer treatment did not affect the expression of protein levels (n = 12). All error bars represent SD of the mean. Un, untreated; SA, negative control scramble aptamer; PA, periostin-binding DNA aptamer. *p < 0.01 versus untreated; #p < 0.01 versus TGF-β1.
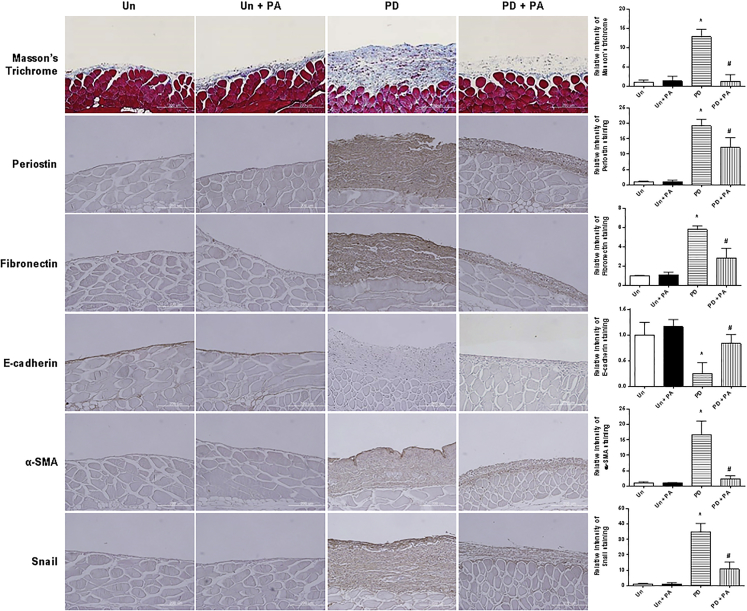
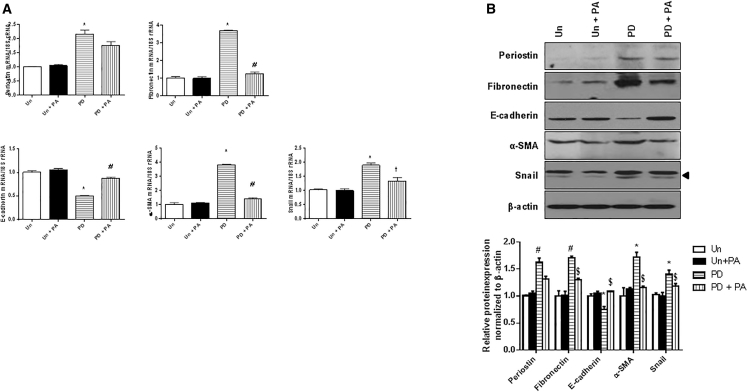
PA Prevents Peritoneal Fibrosis in PD Mouse Models
To investigate the in vivo effect of the PA on the development of peritoneal fibrosis, the PA was administered to a mouse model of the PD. The peritoneal morphology and EMT-related molecules were examined in mice treated with the PD solutions for 4 weeks with or without the intraperitoneal PA administration. Masson’s trichrome staining and immunohistochemical staining of the peritoneum revealed significantly higher ECM accumulation and periostin expression in mice treated with the PD solution relative to untreated mice. Aptamer treatment significantly abrogated the ECM accumulation in the PD mice. Periostin expression was also attenuated by the PA treatment in the PD mice. The changes in the expression of EMT-related molecules in the peritoneum of the PD mice were also significantly ameliorated by the administration of the PA (Figure 5). Furthermore, fibronectin, α-SMA, and Snail mRNA and protein expression levels were significantly higher and E-cadherin expression was significantly lower in mice treated with the PD solution compared to untreated mice, and these changes were significantly attenuated by the PA treatment (Figure 6). These findings suggest that the PA can effectively prevent the PD-induced peritoneal fibrosis.
Figure 5.
Masson’s Trichrome Staining and Immunohistochemical Staining of Periostin, Fibronectin, E-cadherin, α-SMA, and Snail in the Peritoneum of Untreated, Untreated + PA, PD, and PD + PA Mice
Masson’s trichrome staining and immunohistochemical staining of the peritoneum revealed significantly higher ECM accumulation and periostin expression in mice treated with PD solution relative to control mice, and PA treatment significantly abrogated ECM accumulation and periostin expression in PD mice. The changes in the expression of EMT-related molecules in the peritoneum of the PD mice were also significantly ameliorated by the administration of the PA (×200). For each group, 12 sections were evaluated. All error bars represent SD of the mean. Un, untreated; PD, peritoneal dialysis; PA, periostin-binding DNA aptamer. *p < 0.01 versus untreated; #p < 0.01 versus PD.
Figure 6.
Expression of Periostin and EMT-Related Molecules in the Peritoneum of Untreated, Untreated + PA, PD, and PD + PA Mice
(A) Fibronectin, α-SMA, and Snail mRNA expression was significantly higher and E-cadherin expression was significantly lower in mice treated with PD solution compared to control mice, and these changes were significantly attenuated by PA treatment (n = 12). (B) A representative western blot analysis of periostin, fibronectin, E-cadherin, α-SMA, Snail, and β-actin protein expression in the peritoneum (a representative of four blots). The protein expression levels of EMT-related molecules showed a similar pattern as the corresponding mRNA expression levels (n = 12). All error bars represent SD of the mean. Un, untreated; PD, peritoneal dialysis; PA, periostin-binding DNA aptamer. *p < 0.01 versus untreated; #p < 0.01 versus PD.
Discussion
In the present study, periostin was found to play a role in TGF-β1-induced EMT in cultured HPMCs. The inhibition of periostin function by the PA resulted in the abrogation of TGF-β1-induced EMT. In addition, the intraperitoneal administration of the PA successfully inhibited peritoneal fibrosis in an animal model of the PD.
In PD, long-term dialysate exposure results in fibrotic changes in the peritoneum, decreasing the efficiency of solute diffusion and salt removal from the peritoneal capillary vessels to the peritoneal space, a condition termed ultrafiltration failure.22, 23 This ultrafiltration failure is a major factor that limits the widespread use of the PD as a long-term renal replacement modality. The results of this study indicate the possible effectiveness of the intraperitoneal administration of a PA in the PD patients to prevent ultrafiltration failure. Moreover, due to the advantages of aptamers compared to protein- or small-molecule-based treatment options, such as lower manufacturing costs and fewer side effects, the current findings demonstrate that treatment with the PA is a practical and effective strategy to inhibit PD-induced peritoneal fibrosis and has potential clinical applications.
Continual exposure to dialysate solutions as well as recurrent peritonitis episodes cause peritoneal inflammation and injury, eventually leading to peritoneal fibrosis.24 PMCs have been implicated as one of the major cellular elements in the pathogenesis of this peritoneal fibrosis. In the past, resident peritoneal fibroblasts and infiltrating inflammatory cells have been considered the key players promoting peritoneal fibrosis.25 However, recent studies have shown that the EMT of mesothelial cells is an important process in the induction of fibrosis and subsequent deterioration of peritoneal function.6, 24, 26 It has also been demonstrated to be a key process in embryonic development, malignant cell metastasis, and the promotion of organ fibrosis.27 In the case of peritoneal fibrosis, PMCs have been found to lose their epithelial cytokeratins and gain the expression of mesenchymal cellular molecules upon exposure to dialysate.28 In the present study, TGF-β1 treatment of cultured HPMCs significantly downregulated E-cadherin expression and clearly upregulated α-SMA and Snail expression, which was accompanied by a significant increase in fibronectin expression. A similar finding was also found in the peritoneal tissue of the PD mice. These findings suggest that EMT is involved in the course of the PD-induced peritoneal fibrosis and that PMCs play a significant role in this process, which is in accordance with the results of previous studies.29, 30
TGF-β1 is a well-known profibrotic cytokine that plays key pathological roles, including EMT promotion, in peritoneal fibrosis in patients undergoing the PD.31 TGF-β1 activates Smad2 and Smad3, which consequently induces a molecular transition that results in the downregulation of the intracellular adhesion molecule E-cadherin and upregulation of mesenchymal-associated molecules, such as Snail, α-SMA, and fibronectin. Clinical studies have shown that the levels of TGF-β1 detected in the PD fluid correlated with the risk of technical failure in the PD patients.32, 33 Furthermore, overexpression of TGF-β1 by the transfection of adenovirus vectors into the peritoneal cavity led to peritoneal fibrosis, angiogenesis, and ultrafiltration failure in an animal model of the PD.34 In addition, stimulation of PMCs in vitro with dialysate-containing culture media resulted in an increase in TGF-β1 mRNA and protein expression. Based on these findings, TGF-β1 has been considered a key inducer of fibrotic changes in the peritoneum of patients undergoing the PD, and, therefore, to simulate the PD environment associated with EMT and fibrosis, cultured HPMCs were treated with TGF-β1 for the in vitro experiments in this study.
Previously, strategies to inhibit TGF-β1 were evaluated to prevent organ fibrosis in various disease conditions.35 The administration of TGF-β1 antibodies has been considered as an option for TGF-β1 inhibition and has shown promising anti-fibrotic results in some animal models.36, 37, 38 Moreover, substances having an inhibitory effect on TGF-β1, such as decorin and pirfenidone [5-methyl-1-phenyl-2(1H)-pyridone], have been tested for their efficacies in various diseases, such as systemic sclerosis and glomerulosclerosis.39, 40 Regarding peritoneal fibrosis, in vitro studies on PMCs transfected with TGF-β1 small hairpin RNA (shRNA)-expressing vectors demonstrated the amelioration of ECM protein synthesis in these cells.41 However, in addition to promoting pathological fibrotic changes in disease states, TGF-β1 is also involved in various physiological functions, such as wound healing and immune reactions.42 Furthermore, because TGF-β1 is located upstream of the TGF-β1-induced fibrosis signaling cascade, inhibition of TGF-β1 blocks numerous downstream cell signaling pathways critical for cell survival.31 For these reasons, the clinical applications of anti-TGF-β1 therapies have been limited. Therefore, recent investigations have focused on molecular targets farther downstream of the cell-signaling cascade to induce anti-fibrotic effects. It should be noted that the inhibition of periostin, a terminally located protein in the TGF-β1-induced fibrosis pathway, successfully attenuated the PD-related fibrosis process in the peritoneum.
Treatment with the PA resulted in significant abrogation of EMT and fibrosis. Previous studies have found that periostin acts as a ligand and exerts intracellular signaling through the activation of integrin αV/β3 and αV/β5.43 The activation of integrin is known to involve key cell signaling pathways that contribute to EMT and fibrosis, such as Akt and p38.44 The periostin-specific binding property of the PA has been previously shown to inhibit the periostin-integrin interaction, thereby suppressing the integrin signaling pathways.21 This is supported by the results for the periostin protein levels in this study, which were not changed following the PA treatment despite its anti-fibrotic effects. Further investigations on the cellular pathways that are affected by the PA are needed to confirm this conclusion. In addition, because periostin expression itself is also known to be regulated by integrin activation,43 a higher dose of the PA could ultimately downregulate the expression of periostin. The possibility of periostin level attenuation with a higher PA dose treatment could explain the discrepancy found between in vivo and in vitro experiments in this study. Treatment with the PA significantly attenuated the immunohistochemical staining of periostin in the peritoneum of the PD mice, while a clear amelioration of periostin level was not found in cell studies. The PA treatment could have had different dose effects between the experiments.
In conclusion, the results of this study suggest that periostin acts as a key mediator of peritoneal fibrosis in the PD patients, and treatment with the PA can be considered an effective treatment modality for peritoneal fibrosis in these patients.
Materials and Methods
Primary Culture of HPMCs
Primary HPMCs were isolated in accordance with the process reported by Stylianou et al.45 using human omenta specimens from patients who had undergone abdominal surgery and agreed to provide consent. The specimens were washed three times using sterile PBS and incubated in a solution containing 0.05% trypsin and 0.02% EDTA for 20 min at 37°C, with continuous shaking. After an incubation period, free HPMC-containing suspension was centrifuged at 100 g for 10 min at 4°C. The supernatant was removed, and the pellet was washed once. Subsequently, the pellet was re-suspended into M199 media supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, 100 mg/mL streptomycin, and 26 mM NaHCO3 and seeded on cell culture dishes. The cells were cultured in the same media at 37°C in humidified 5% CO2 in air. The media were exchanged 24 hr after seeding and then every 3 days.
Treatment of HPMCs
Subconfluent HPMCs were FBS restricted for 24 hr. Next, the media were exchanged to FBS-free M199 media for the control group and media with TGF-β1 (2 ng/mL) (R&D Systems) for the intervention group. HPMCs were harvested for RNA and protein analyses 72 hr after media change. For periostin knockdown experiments, periostin siRNA and negative control scramble siRNA was purchased from Dharmacon. Positive control GAPDH siRNA was purchased from Bioneer. Periostin siRNA was transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. Briefly, 6 μL of Lipofectamine 2000 was diluted in 1 mL of Opti-MEM I Reduced Serum Medium (Invitrogen), incubated for 15 min at room temperature (RT), and mixed with periostin siRNA. After a 15 min incubation at RT, the mixture was added to each well of HPMCs, which were plated at a density of 5 × 105 cells/well into 6-well plates the day before, and the medium was changed after 24 hr. The doses of TGF-β1 and periostin siRNA for this study were determined based on preliminary experimental results.
PA
To select periostin-specific aptamers, a modified DNA SELEX procedure was used.21, 46 The selected PA and negative control scramble aptamer were constructed by Aptamer Sciences Inc. as previously reported.21 The sequence of the aptamer used is shown in Table 1. This aptamer was prepared in three forms: Cy3-labeled aptamer, FITC-labeled aptamer, and polyethylene glycol (PEG)-conjugated aptamer. Cy3-labeled PA and FITC-labeled PA were prepared by labeling Cy3 and FITC to the 5′ end of the aptamer, respectively. Cy3-labled PA was used for in vitro studies. FITC-labeled PA was used to evaluate the periostin-specific aptamer attachment in the FACS analysis studies. PEG-conjugated PA was prepared by conjugating 40 kDa PEG to the 5′ end. In addition, the PEG-conjugated PA contained an inverted deoxythymidine (dT) to the 3′ end to increase in vivo biological stability. The PEG-conjugated PA was applied for animal studies.
Table 1.
Periostin-Binding DNA Aptamer Sequence
| Sequence | |
|---|---|
| Cy3-labled PA | Cy3-ACGAGYYGYCGCAYGYGCGGYYCAGYCYGGYCCYYCAGCACCGYACAACAA |
| FITC-labeled PA | FITC-ACGAGYYGYCGCAYGYGCGGYYCAGYCYGGYCCYYCAGCACCGYACAACAA |
| PEG-conjugated PA | PEG-ACGAGYYGYCGCAYGYGCGGYYCAGYCYGGYCCYYCAGCACCGYACAACAA-idT |
Y, benzyl-d(U)TP; idT, inverted dT.
FACS Analysis of HPMCs
HPMCs were centrifuged at 500 g for 5 min and washed twice in an isotonic, calcium, and magnesium free-PBS buffer to remove residual growth factors in the medium. A final concentration of 1 × 107 cells/mL was re-suspended and incubated with 200 nmol/L FITC-labeled PA containing binding buffer (PBS including 5 mM MgCl2). Samples of 100 μL were used per assay (1 × 106 cells) into each 1.5 mL tube for staining. Flow cytometric analysis of surface staining intensity was performed using an FACSVERSE System (BD Biosciences) and signals were obtained from a blue laser. Analysis of the results was conducted using FACSuite software (BD Biosciences).
Animal Experiments
The protocols for animal experiments were approved by the Committee for the Care and Use of Laboratory Animals at Yonsei University College of Medicine, Seoul, Korea. All of the animal experiments were conducted in accordance with the Principles of Laboratory Animal Care (NIH Publication no. 85-23, revised 1985).
48 male C57BL/6 mice weighing 20–25 g were used. To establish the PD model, PD catheter and ports (cat # MMP-4S-061108A; Access Technologies and Solomon Scientific) were inserted, and the wounds were observed for 1 week. At 1 week after catheter insertion, the untreated group received 2 mL of saline instillation once a day. The PD group was infused intraperitoneally with 2 mL of peritoneal dialysate daily (physioneal PD solution with 4.25% dextrose, pH 7.4; Baxter International) for 4 weeks. In the untreated + PA group, the PA was delivered at 500 μg/kg/d mixed with saline. In the PD +PA group, the PA was delivered at 500 μg/kg/d mixed with the PD fluid. The mixtures were given through the PD catheter daily in a final volume of 2 mL. The amount of the PA delivered did not include the conjugated PEG weight. Preliminary investigations evaluating the amount of the PA detected in the peritoneum, evaluated by real-time qPCR were used to select the dosage of the PA delivered (Figure S1). The primers used to detect the PA are provided in Table 2. After 4 weeks of treatment, all of the mice were sacrificed, and the parietal peritoneum was collected from each animal.
Table 2.
Primer Sequences Used for Real-Time qPCR
| Sequence (5′–3′) | ||
|---|---|---|
| Periostin (mouse and human) | sense | GGCACCAAAAAGAAATACT |
| antisense | GGAAGGTAAGAGTATAT | |
| Fibronectin (mouse) | sense | TGACAACTGCCGTAGACCTGG |
| antisense | TACTGGTTGTAGGTGTGGCCG | |
| Fibronectin (human) | sense | AACCTACGGATGACTCGTGC |
| antisense | TGAATCACATCTGAAATGACCAC | |
| E-cadherin (mouse) | sense | TTTCTACAGCATCACCGGC |
| antisense | CACTTTCAGCCAGCCTGTC | |
| E-cadherin (human) | sense | CACAGACGCGGACGATGAT |
| antisense | AGGATCTTGGCTGAGGATGGT | |
| α-SMA (mouse) | sense | CTGACAGAGGCACCACTGAA |
| antisense | CATCTCCAGAGTCCAGCACA | |
| α-SMA (human) | sense | GCCTTGGTGTGTGACAATGG |
| antisense | AAAACAGCCCTGGGAGCAT | |
| PA detection | sense | ACGAGTTGTCGCATGTGCGGT |
| antisense | TGTTGTACGGTGCTGAAGGACCA |
Real-Time qPCR
The RNA extraction methods from HPMCs and the peritoneum were described in a previous study.47 For animal samples, a total of 0.1 g peritoneum tissue for each animal was used to extract RNA. A Boehringer Mannheim cDNA Synthesis Kit (Boehringer Mannheim) was used to obtain first-strand cDNA. Using the ABI PRISM 7700 Sequence Detection System (Applied Biosystems), a total volume of 20 μL mixture in each well was used containing 10 μL of SYBR Green PCR Master Mix (Applied Biosystems), 5 μL of cDNA, and 5 pmol sense and antisense primers. The primer sequences used in this study were described in Table 2. The RNAs used for amplification were 25 ng per reaction tube. The primer concentrations were determined by preliminary experiments that analyzed the optimal concentrations of each primer. The PCR conditions were as follows: 35 cycles of denaturation for 30 min at 94.5°C, annealing for 30 s at 60°C, and extension for 1 min at 72°C. Initial heating for 9 min at 95°C and final extension for 7 min at 72°C were performed for all PCR reactions. Each sample was run in triplicate in separate tubes and a control without cDNA was also run in parallel with each assay. After real-time PCR, the temperature was increased from 60°C to 95°C at a rate of 2°C/min to construct a melting curve. The cDNA content of each specimen was determined using a comparative CT method with 2ΔΔCT. The results were given as the relative expression normalized to the expression of 18S rRNA and expressed in arbitrary units.
Western Blot Analysis
Western blotting was performed as described previously.47 For animal samples, a total of 0.2 g peritoneum tissue for each animal was used to extract protein. Blots were incubated overnight at 4°C with polyclonal antibodies to periostin (Abcam), E-cadherin (BD Bioscience), α-SMA (Abcam), fibronectin (Dako), Snail (Abcam), GAPDH (Abcam), or β-actin (Sigma Chemical). The membranes were washed three times for 10 min in 1 × PBS with 0.1% Tween-20 and incubated in buffer A containing a 1:1,000 dilution of horseradish peroxidase-linked goat anti-rabbit or anti-mouse IgG (Santa Cruz Biotechnology). TINA image software (Raytest) was used to measure the band densities, and the changes in the optical densities of bands from the treated groups relative to untreated cells or tissues were used for analysis.
Immunohistochemical Staining and Masson’s Trichrome Staining
To fix the peritoneum samples, 10% neutral-buffered formalin was used. Paraffin-embedded tissues were processed in 5 μm-thick sections for immunohistochemical staining. Tissue sections were deparaffinized, rehydrated in ethyl alcohol, and washed in tap water. Antigen retrieval was performed in 10 mM sodium citrate buffer for 20 min using a Black & Decker vegetable steamer. Slides were blocked with 10% donkey serum for 30 min at RT and then washed using PBS. Primary antibodies for fibronectin, E-cadherin, α-SMA, and Snail were diluted to the proper concentrations with 2% casein in BSA, added to the slides, and then incubated overnight at 4°C. After washing, the slides were treated with a secondary antibody for 1 hr at RT. Diaminobenzidine was added for 2 min, and hematoxylin was used to counterstain the slides. For Masson’s trichrome staining, paraffin-embedded tissues processed in 5 μm-thick sections were deparaffinized, rehydrated in ethyl alcohol, washed in tap water, and re-fixed in Bouin’s solution for 1 hr at 56°C. After rinsing in running tap water for 10 min and staining with Weigert’s iron hematoxylin working solution for 10 min, the slides were stained with Biebrich scarlet-acid fuchsin solution for 15 min and washed in tap water. The sections were differentiated in phosphomolybdic-phosphotungstic acid solution for 15 min, transferred to aniline blue solution, and stained for 10 min. After rinsing briefly in tap water, the slides were reacted with 1% acetic acid solution for 5 min. A semiquantitative score for staining intensity was assessed by examining at least five fields in each section under ×400 magnification and with digital image analysis (MetaMorph version 4.6r5, Universal Imaging).
Statistical Analysis
Statistical analyses were conducted using SPSS for Windows version 21 (IBM). Data are expressed as the mean ± SD. One-way ANOVA with post hoc testing using Bonferroni’s correction was used for multiple comparisons. A p value less than 0.05 was considered statistically significant.
Author Contributions
B.Y.N., J.T.P., J.P.L., and S.-W.K. conceived the project. B.Y.N., Y.E.K., S.K., J.P., J.E.U., and M.W. performed the experiments. J.H.J. and Y.K. constructed the DNA aptamers. B.Y.N., J.T.P., S.H.H., T.-H.Y., and S.-W.K. interpreted data. J.T.P., Y.E.K., and S.-W.K. wrote the manuscript. J.T.P. and S.-W.K. provided final approval for publication.
Conflicts of Interest
All authors declare no competing interests.
Acknowledgments
This work was supported by a faculty research grant from Yonsei University College of Medicine (6-2016-0079) and a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIP) (NRF-2014R1A1A1002198 and NRF-2015R1A2A2A01002308).
Footnotes
Supplemental Information includes one figure and can be found with this article online at http://dx.doi.org/10.1016/j.omtn.2017.05.001.
Supplemental Information
References
- 1.Ayuzawa N., Ishibashi Y., Takazawa Y., Kume H., Fujita T. Peritoneal morphology after long-term peritoneal dialysis with biocompatible fluid: recent clinical practice in Japan. Perit. Dial. Int. 2012;32:159–167. doi: 10.3747/pdi.2010.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bargman J.M. Advances in peritoneal dialysis: a review. Semin. Dial. 2012;25:545–549. doi: 10.1111/j.1525-139X.2012.01124.x. [DOI] [PubMed] [Google Scholar]
- 3.Devuyst O., Margetts P.J., Topley N. The pathophysiology of the peritoneal membrane. J. Am. Soc. Nephrol. 2010;21:1077–1085. doi: 10.1681/ASN.2009070694. [DOI] [PubMed] [Google Scholar]
- 4.Kawaguchi Y., Kawanishi H., Mujais S., Topley N., Oreopoulos D.G., International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. Perit. Dial. Int. 2000;20(Suppl 4):S43–S55. [PubMed] [Google Scholar]
- 5.Brulez H.F., Verbrugh H.A. First-line defense mechanisms in the peritoneal cavity during peritoneal dialysis. Perit. Dial. Int. 1995;15:S24–S33. discussion S33–S34. [PubMed] [Google Scholar]
- 6.Yáñez-Mó M., Lara-Pezzi E., Selgas R., Ramírez-Huesca M., Domínguez-Jiménez C., Jiménez-Heffernan J.A., Aguilera A., Sánchez-Tomero J.A., Bajo M.A., Alvarez V. Peritoneal dialysis and epithelial-to-mesenchymal transition of mesothelial cells. N. Engl. J. Med. 2003;348:403–413. doi: 10.1056/NEJMoa020809. [DOI] [PubMed] [Google Scholar]
- 7.Yang A.H., Chen J.Y., Lin J.K. Myofibroblastic conversion of mesothelial cells. Kidney Int. 2003;63:1530–1539. doi: 10.1046/j.1523-1755.2003.00861.x. [DOI] [PubMed] [Google Scholar]
- 8.Margetts P.J., Bonniaud P., Liu L., Hoff C.M., Holmes C.J., West-Mays J.A., Kelly M.M. Transient overexpression of TGF-beta1 induces epithelial mesenchymal transition in the rodent peritoneum. J. Am. Soc. Nephrol. 2005;16:425–436. doi: 10.1681/ASN.2004060436. [DOI] [PubMed] [Google Scholar]
- 9.Oh K.H., Margetts P.J. Cytokines and growth factors involved in peritoneal fibrosis of peritoneal dialysis patients. Int. J. Artif. Organs. 2005;28:129–134. doi: 10.1177/039139880502800208. [DOI] [PubMed] [Google Scholar]
- 10.Yao Q., Pawlaczyk K., Ayala E.R., Styszynski A., Breborowicz A., Heimburger O., Qian J.Q., Stenvinkel P., Lindholm B., Axelsson J. The role of the TGF/Smad signaling pathway in peritoneal fibrosis induced by peritoneal dialysis solutions. Nephron Exp. Nephrol. 2008;109:e71–e78. doi: 10.1159/000142529. [DOI] [PubMed] [Google Scholar]
- 11.Horiuchi K., Amizuka N., Takeshita S., Takamatsu H., Katsuura M., Ozawa H., Toyama Y., Bonewald L.F., Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J. Bone Miner. Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 12.Merle B., Garnero P. The multiple facets of periostin in bone metabolism. Osteoporos. Int. 2012;23:1199–1212. doi: 10.1007/s00198-011-1892-7. [DOI] [PubMed] [Google Scholar]
- 13.Norris R.A., Damon B., Mironov V., Kasyanov V., Ramamurthi A., Moreno-Rodriguez R., Trusk T., Potts J.D., Goodwin R.L., Davis J. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J. Cell. Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ontsuka K., Kotobuki Y., Shiraishi H., Serada S., Ohta S., Tanemura A., Yang L., Fujimoto M., Arima K., Suzuki S. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp. Dermatol. 2012;21:331–336. doi: 10.1111/j.1600-0625.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 15.Rios H., Koushik S.V., Wang H., Wang J., Zhou H.M., Lindsley A., Rogers R., Chen Z., Maeda M., Kruzynska-Frejtag A. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol. Cell. Biol. 2005;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Braun N., Sen K., Alscher M.D., Fritz P., Kimmel M., Morelle J., Goffin E., Jörres A., Wüthrich R.P., Cohen C.D., Segerer S. Periostin: a matricellular protein involved in peritoneal injury during peritoneal dialysis. Perit. Dial. Int. 2013;33:515–528. doi: 10.3747/pdi.2010.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song K.M., Lee S., Ban C. Aptamers and their biological applications. Sensors (Basel) 2012;12:612–631. doi: 10.3390/s120100612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keefe A.D., Pai S., Ellington A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apte R.S. Pegaptanib sodium for the treatment of age-related macular degeneration. Expert Opin. Pharmacother. 2008;9:499–508. doi: 10.1517/14656566.9.3.499. [DOI] [PubMed] [Google Scholar]
- 20.Held D.M., Kissel J.D., Patterson J.T., Nickens D.G., Burke D.H. HIV-1 inactivation by nucleic acid aptamers. Front. Biosci. 2006;11:89–112. doi: 10.2741/1782. [DOI] [PubMed] [Google Scholar]
- 21.Lee Y.J., Kim I.S., Park S.A., Kim Y., Lee J.E., Noh D.Y., Kim K.T., Ryu S.H., Suh P.G. Periostin-binding DNA aptamer inhibits breast cancer growth and metastasis. Mol. Ther. 2013;21:1004–1013. doi: 10.1038/mt.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margetts P.J., Churchill D.N. Acquired ultrafiltration dysfunction in peritoneal dialysis patients. J. Am. Soc. Nephrol. 2002;13:2787–2794. doi: 10.1681/ASN.V13112787. [DOI] [PubMed] [Google Scholar]
- 23.Williams J.D., Craig K.J., Topley N., Von Ruhland C., Fallon M., Newman G.R., Mackenzie R.K., Williams G.T., Peritoneal Biopsy Study Group Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 2002;13:470–479. doi: 10.1681/ASN.V132470. [DOI] [PubMed] [Google Scholar]
- 24.Lai K.N., Tang S.C., Leung J.C. Mediators of inflammation and fibrosis. Perit. Dial. Int. 2007;27(Suppl 2):S65–S71. [PubMed] [Google Scholar]
- 25.Jörres A. Effect of peritoneal dialysis on peritoneal cell biology: peritoneal fibroblasts. Perit. Dial. Int. 1999;19(Suppl 2):S348–S352. [PubMed] [Google Scholar]
- 26.Liu Y., Dong Z., Liu H., Zhu J., Liu F., Chen G. Transition of mesothelial cell to fibroblast in peritoneal dialysis: EMT, stem cell or bystander? Perit. Dial. Int. 2015;35:14–25. doi: 10.3747/pdi.2014.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grande M.T., López-Novoa J.M. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat. Rev. Nephrol. 2009;5:319–328. doi: 10.1038/nrneph.2009.74. [DOI] [PubMed] [Google Scholar]
- 28.López-Cabrera M. Mesenchymal conversion of mesothelial cells is a key event in the pathophysiology of the peritoneum during peritoneal dialysis. Adv. Med. 2014;2014:473134. doi: 10.1155/2014/473134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee H.B., Ha H. Mechanisms of epithelial-mesenchymal transition of peritoneal mesothelial cells during peritoneal dialysis. J. Korean Med. Sci. 2007;22:943–945. doi: 10.3346/jkms.2007.22.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selgas R., Bajo A., Jiménez-Heffernan J.A., Sánchez-Tomero J.A., Del Peso G., Aguilera A., López-Cabrera M. Epithelial-to-mesenchymal transition of the mesothelial cell--its role in the response of the peritoneum to dialysis. Nephrol. Dial. Transplant. 2006;21(Suppl 2):ii2–ii7. doi: 10.1093/ndt/gfl183. [DOI] [PubMed] [Google Scholar]
- 31.Böttinger E.P., Bitzer M. TGF-beta signaling in renal disease. J. Am. Soc. Nephrol. 2002;13:2600–2610. doi: 10.1097/01.asn.0000033611.79556.ae. [DOI] [PubMed] [Google Scholar]
- 32.Lin C.Y., Chen W.P., Fu L.W., Yang L.Y., Huang T.P. Persistent transforming growth factor beta 1 expression may predict peritoneal fibrosis in CAPD patients with frequent peritonitis occurrence. Adv. Perit. Dial. 1997;13:64–71. [PubMed] [Google Scholar]
- 33.Gangji A.S., Brimble K.S., Margetts P.J. Association between markers of inflammation, fibrosis and hypervolemia in peritoneal dialysis patients. Blood Purif. 2009;28:354–358. doi: 10.1159/000232937. [DOI] [PubMed] [Google Scholar]
- 34.Margetts P.J., Kolb M., Galt T., Hoff C.M., Shockley T.R., Gauldie J. Gene transfer of transforming growth factor-beta1 to the rat peritoneum: effects on membrane function. J. Am. Soc. Nephrol. 2001;12:2029–2039. doi: 10.1681/ASN.V12102029. [DOI] [PubMed] [Google Scholar]
- 35.Akhurst R.J., Hata A. Targeting the TGFβ signalling pathway in disease. Nat. Rev. Drug Discov. 2012;11:790–811. doi: 10.1038/nrd3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miyajima A., Chen J., Lawrence C., Ledbetter S., Soslow R.A., Stern J., Jha S., Pigato J., Lemer M.L., Poppas D.P. Antibody to transforming growth factor-beta ameliorates tubular apoptosis in unilateral ureteral obstruction. Kidney Int. 2000;58:2301–2313. doi: 10.1046/j.1523-1755.2000.00414.x. [DOI] [PubMed] [Google Scholar]
- 37.Sharma K., Jin Y., Guo J., Ziyadeh F.N. Neutralization of TGF-beta by anti-TGF-beta antibody attenuates kidney hypertrophy and the enhanced extracellular matrix gene expression in STZ-induced diabetic mice. Diabetes. 1996;45:522–530. doi: 10.2337/diab.45.4.522. [DOI] [PubMed] [Google Scholar]
- 38.Ziyadeh F.N., Hoffman B.B., Han D.C., Iglesias-De La Cruz M.C., Hong S.W., Isono M., Chen S., McGowan T.A., Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc. Natl. Acad. Sci. USA. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denton C.P., Merkel P.A., Furst D.E., Khanna D., Emery P., Hsu V.M., Silliman N., Streisand J., Powell J., Akesson A., Cat-192 Study Group. Scleroderma Clinical Trials Consortium Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: a multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007;56:323–333. doi: 10.1002/art.22289. [DOI] [PubMed] [Google Scholar]
- 40.Cho M.E., Smith D.C., Branton M.H., Penzak S.R., Kopp J.B. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2007;2:906–913. doi: 10.2215/CJN.01050207. [DOI] [PubMed] [Google Scholar]
- 41.Liu F., Liu H., Peng Y., Yuan F., Liu Y., Duan S. Inhibition of transforming growth factor beta (TGFbeta1) expression and extracellular matrix secretion in human peritoneal mesothelial cells by pcDU6 vector-mediated TGFbeta1 shRNA and by pcDNA3.1(-)-mediated antisense TGFbeta1 RNA. Adv. Perit. Dial. 2005;21:41–52. [PubMed] [Google Scholar]
- 42.Wrzesinski S.H., Wan Y.Y., Flavell R.A. Transforming growth factor-beta and the immune response: implications for anticancer therapy. Clin. Cancer Res. 2007;13:5262–5270. doi: 10.1158/1078-0432.CCR-07-1157. [DOI] [PubMed] [Google Scholar]
- 43.Morra L., Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459:465–475. doi: 10.1007/s00428-011-1151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mael-Ainin M., Abed A., Conway S.J., Dussaule J.C., Chatziantoniou C. Inhibition of periostin expression protects against the development of renal inflammation and fibrosis. J. Am. Soc. Nephrol. 2014;25:1724–1736. doi: 10.1681/ASN.2013060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stylianou E., Jenner L.A., Davies M., Coles G.A., Williams J.D. Isolation, culture and characterization of human peritoneal mesothelial cells. Kidney Int. 1990;37:1563–1570. doi: 10.1038/ki.1990.150. [DOI] [PubMed] [Google Scholar]
- 46.Davies D.R., Gelinas A.D., Zhang C., Rohloff J.C., Carter J.D., O’Connell D., Waugh S.M., Wolk S.K., Mayfield W.S., Burgin A.B. Unique motifs and hydrophobic interactions shape the binding of modified DNA ligands to protein targets. Proc. Natl. Acad. Sci. USA. 2012;109:19971–19976. doi: 10.1073/pnas.1213933109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang S.W., Adler S.G., Lapage J., Natarajan R. p38 MAPK and MAPK kinase 3/6 mRNA and activities are increased in early diabetic glomeruli. Kidney Int. 2001;60:543–552. doi: 10.1046/j.1523-1755.2001.060002543.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.