Abstract
Alhough the glucagon-like peptide-1 (GLP-1) system is critical to energy balance control and is a target for obesity pharmacotherapies, the receptor-population-mediating effects of endogenous GLP-1 signaling are not fully understood. To address this, we developed a novel adeno-associated virus (AAV-GLP-1R) that utilizes short hairpin RNA to chronically knock down GLP-1 receptors (GLP-1R) in rats. As pharmacological studies highlight the hindbrain nucleus tractus solitarius (NTS) as a brain region important for GLP-1R-mediated effects on energy balance, AAV-GLP-1R was injected into the NTS to examine the role of endogenous NTS GLP-1R signaling in energy balance control. Chow intake and meal size were significantly increased following chronic NTS GLP-1R knockdown. In addition, NTS GLP-1R knockdown significantly increased self-administration of palatable food under both fixed and progressive ratio schedules of reinforcement. Collectively, these data demonstrate that endogenous NTS GLP-1R signaling is required for the control of food intake and motivation to feed, and provide a new strategy to investigate the importance of distinct GLP-1R populations in the control of a variety of functions.
Introduction
The neuropeptide glucagon-like peptide-1 (GLP-1), produced by preproglucagon-expressing L cells of the intestine and neurons in the hindbrain nucleus tractus solitarius (NTS), is important for the control of energy balance and glycemia (see (Hayes et al, 2010a; Holst, 2007) for review). In fact, current FDA-approved pharmacotherapies targeting the GLP-1 system are efficacious for the treatment of type-2 diabetes mellitus and obesity (Blonde et al, 2006; Buse et al, 2009; Tella and Rendell, 2015). GLP-1 receptors (GLP-1R) expressed in both the periphery and central nervous system (CNS) are involved in mediating the metabolic effects of these exogenous GLP-1R agonists (Kanoski et al, 2011; Secher et al, 2014; Sisley et al, 2014) and thus it is important to understand the contributions of discrete GLP-1R populations to energy balance control.
Exogenous activation of GLP-1R in many nuclei distributed throughout the brain (eg, NTS, nuclei of the hypothalamus, ventral tegmental area, nucleus accumbens, hippocampus, and lateral parabrachial nucleus; Merchenthaler et al, 1999) can trigger reductions in food intake and body weight loss (Alhadeff et al, 2012, 2014a; Hayes et al, 2011; Hsu et al, 2015; McMahon and Wellman, 1998; Schick et al, 2003; Secher et al, 2014). However, aside from a recent report demonstrating the requirement of vagal GLP-1R for food intake control (Krieger et al, 2016), the endogenous role of distinct GLP-1R subpopulations in the daily control of energy balance has not been explored. GLP-1R in the NTS are of particular interest with regard to food intake control, given the importance of NTS neurotransmitter/neuroendocrine processing for energy balance regulation (Grill and Hayes, 2012). Indeed, exogenous stimulation of NTS GLP-1R results in potent reductions in food intake (Hayes et al, 2011) and more recently has been shown to reduce motivated and appetitive aspects of feeding (Alhadeff and Grill, 2014b; Richard et al, 2015). In addition, acute pharmacological blockade of NTS GLP-1R attenuates the intake-suppressive effects of a gastric preload (Hayes et al, 2009), suggesting a role for endogenous NTS GLP-1R in the control of food intake and satiation.
Here to assess the role of endogenous NTS GLP-1R signaling in the daily control of food intake and body weight regulation, we developed a novel adeno-associated virus (AAV) that utilizes short hairpin RNA to chronically knock down GLP-1R (AAV-GLP-1R). After injecting AAV-GLP-1R or a control virus (AAV-CONTROL) into the NTS of rats, food intake, body weight, meal patterns, and operant responding for food were analyzed. Our data suggest that endogenous NTS GLP-1R signaling is required for the control of food intake, meal size, and the motivation to work for palatable food.
Materials and methods
Subjects
Adult male Sprague-Dawley rats (250–265 g upon arrival, Charles River Laboratories, Wilmington, MA; Taconic Laboratories, Hudson, NY) were individually housed in hanging, wire-bottom metal cages and had ad libitum access to pelleted chow (Purina Rodent Chow, 5001) and water unless otherwise noted on a 12 h light/12 h dark cycle. All procedures conformed to and received approval from the institutional standards of the University of Pennsylvania Animal Care and Use Committee.
Viral Production
RNA sequences were screened in vitro (OriGene Technologies, Rockland, MD) for their ability to reduce GLP-1R expression according to previously published methods (Mietlicki-Baase et al, 2015) to create a short hairpin RNA (shRNA) targeting the GLP-1R transcript. Briefly, a plasmid designed to express GLP-1Rs (NM_053816; OriGene) was transiently transfected alone or in combination with an shRNA to reduce GLP-1R expression in a rat immortalized hypothalamic neuronal cell line (R-19; Cedarlane Laboratories, Burlington, NC). The most robust knockdown of GLP-1Rs following transient transfection was obtained using the following shRNA sequence: 5′-GATCGGGTTGCTGGTGGAAGGCGTGTATCTGTACTCAAGAGGTACAGTACACGCCTTCCACCAGCAACCTTTTTT-3′. Our in vitro studies demonstrated an 88.9% knockdown of GLP-1R expression following a 3-day incubation with this AAV-shRNA in the R19 rat neuronal cell line transfected to overexpress the GLP-1R (Figure 1a). To knockdown GLP-1R expression in vivo, this shRNA sequence was cloned and packaged into an adeno-associated virus (AAV) downstream of the U6 promoter and co-expressing GFP downstream of the CB7 promoter (AAV1.U6.shRGlp1r07.CB7.EGFP.SV40; here referred to as: AAV-GLP-1R; serotype 1; titer=5.22e12) by the Viral Core at the University of Pennsylvania. A GFP-expressing AAV1 downstream of the Cb7 promoter (AAV-CONTROL; titer=5.22e12) was used as a control.
Figure 1.
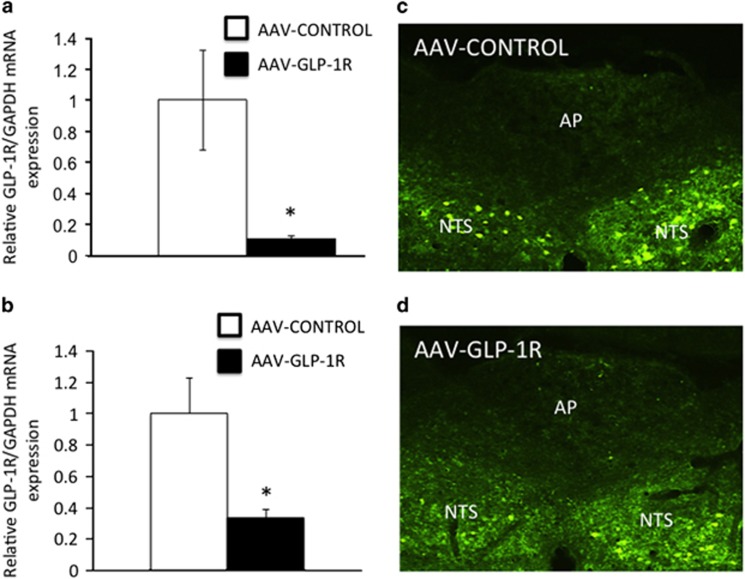
(a) rtPCR from in vitro studies demonstrated ~88% knockdown of GLP-1R expression in rat R19 neurons overexpressing the GLP-1R following 3-day transfection with AAV-GLP-1R compared with AAV-CONTROL. (b) Representative real-time PCR (rtPCR) reveals ~66% suppression of GLP-1R mRNA in micropunched NTS tissue in AAV-GLP-1R- vs AAV-CONTROL-treated rats. (c) Representative image of GFP tagged-AAV-CONTROL injection placement in the NTS. (d) Representative image of GFP tagged-AAV-GLP-1R injection placement in the NTS. AP, area postrema. Data expressed as means±SEM, *p<0.05.
AAV-GLP-1R and AAV-CONTROL Delivery to the NTS
Rats received intramuscular anesthesia (ketamine (90 mg/kg; Butler Animal Health Supply, Dublin, OH), xylazine (2.7 mg/kg; Anased, Shenandoah, IA), and acepromazine (0.64 mg/kg; Butler Animal Health Supply), and subcutaneous analgesia (2.0 mg/kg Metacam; Boehringer Ingelheim Vetmedica, St Joseph, MO)). Rats were subsequently positioned in a stereotaxic device and bilateral cannulae were positioned above the caudomedial NTS according to the following coordinates based off of a rat atlas (Paxinos and Watson, 2005) and preliminary studies: ±0.5 mm lateral from midline, 0.9 mm anterior to occipital, and 6.8 mm ventral from skull surface using a 15° angle (negative slope in the anterior to posterior direction), with the injector aimed 2.0 mm below the guide cannula. Animals (matched for body weight and food intake) received 200 nl bilateral injections of AAV-GLP-1R or AAV-CONTROL in the NTS via a micropump (PHD 2000; Harvard Apparatus, Holliston, MA) with a Hamilton syringe connected to an injector (Plastics One, Roanoke, VA). Injectors were left in place for 1 min and then removed. Guide cannulae were removed from the brain and the head incisions were sutured.
Tissue Collection
At the completion of experiments rats were anesthetized, killed by decapitation and brains were rapidly removed, flash-frozen in isopentane, and stored at −80 °C until processing. Coronal sections at the level of the NTS were slide-mounted and viewed under a fluorescence microscope (Nikon 80i) until GFP-expressing cells were visualized in the caudomedial NTS (at the level of the area postrema). Bilateral micropunches (1 × 1 × 1 mm) were taken from this region and kept frozen for qPCR analysis. Postmortem analyses indicated that the viral spread was predominately contained within the dorsal vagal complex (DVC), between −14.6 and −13.2 mm posterior from bregma. Importantly, within the DVC, the GLP-1R expressed in the NTS (and not the area postrema or dorsal motor nucleus of the vagus) are the functionally relevant GLP-1R population for feeding behavior (Hayes et al, 2011). Data from rats with targeted virus injections outside of the NTS were excluded from all analyses.
RNA Isolation and Real-Time PCR
Total RNA was extracted from micropunches using TRIzol (Invitrogen, Life Technologies, Grant Island, NY) and the RNeasy kit (Qiagen, Germantown, MD). cDNA was synthesized from using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Life Technologies, Grant Island, NY). TaqMan gene expression kits and PCR reagents (Applied Biosystems) were used to quantify relative mRNA levels of GLP-1R (Glp1r, Rn00562406) relative to rat GAPDH (Gapdh, Rn01775763_g1). Relative mRNA expression was calculated using the comparative Ct method as previously described (Hayes et al, 2010b).
Behavioral Analyses
Food intake and body weight
Beginning 1 week prior to AAV delivery, rats (n=9 AAV-GLP-1R, n=10 AAV-CONTROL) were monitored daily for chow intake (accounting for spillage) and body weight throughout the duration of the experiment.
Meal patterns
A separate cohort of rats (n=8 AAV-GLP-1R, n=8 AAV-CONTROL) was housed in a custom automated feedometer consisting of hanging wire-bottom cages with an access hole to a food cup with powdered chow that rested on an electronic scale. The feedometer was connected to software (LabView) that records the weight of the food cup every 10 s. Cumulative food intake, meal size and meal number data were subsequently analyzed at 0.5, 1, 1.5, 2, 3, 4, 5, 6, 8, 12, and 24 h relative to dark-cycle onset over 3 days immediately before virus injection, as well as over 3 days 2 weeks post-virus injections. Data are expressed as average cumulative intakes, meal sizes, and meal numbers over the 3-day test period. A meal was defined as any intake ⩾0.25 g; ⩾10 min must have elapsed for feeding bouts to be considered two separate meals (Alhadeff et al, 2014c; Mietlicki-Baase et al, 2013).
Operant responding
A third cohort of rats (n=8) maintained ad libitum on chow and injected with AAV-GLP-1R was trained to press a lever to receive a 45 mg sucrose pellet as follows. Rats received 1 h daily operant sessions: five sessions under a fixed ratio (FR)-1 schedule of reinforcement (1 lever press required for one sucrose pellet (reinforcer)), three sessions of FR-3 (3 lever presses required for 1 reinforcer), and three sessions of FR-5 (5 lever presses required for 1 reinforcer). Next, rats underwent 5 consecutive days of progressive ratio (PR) sessions, where the effort (number of lever presses) required to obtain each reinforcer increased exponentially throughout the session as previously described (Alhadeff et al, 2014a; Kanoski et al, 2014), using the formula F(i)=5e0.2i−5, where F(i) is the number of lever presses required to obtain the next reinforcer at i=pellet number. Under this function, the number of presses required to obtain each pellet is according to the following sequence: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 693, 737, 901 (…). The PR session ended when a 20 min period elapsed without the rat earning a pellet. The numbers of lever presses and/or numbers of reinforcers earned were averaged for each rat across days within each training condition (ie, average FR-1 responding, average FR-3 responding, average FR-5 responding, and average PR responding). These values were correlated with the mRNA expression of NTS GLP-1R expression relative to GAPDH (rtPCR methods described above).
A final cohort of rats (n=17) was restricted to 20–25 g of lab chow per rat per day in their home cages for the pre-surgery training phase. Initially, rats were allowed to self-administer 45 mg sucrose pellets on a FR-1 schedule of reinforcement. Once a rat achieved at least 20 sucrose pellets in a single operant session, the response requirement was increased to an FR-5 schedule of reinforcement. Following 20 daily sucrose self-administration sessions, rats matched for FR-5 responding received NTS injections of AAV-GLP-1R (n=9) or AAV-CONTROL (n=8). Immediately after surgery, rats were returned to their home cages with food and water available ad libitum for the remainder of the experiment. Following 7 days of recovery, rats were placed back into the operant conditioning chambers and allowed to self-administer sucrose on an FR-1 schedule of reinforcement. After three days of responding on an FR-1 schedule, the response requirement was increased to FR-5. Rats were allowed to respond for sucrose on an FR-5 schedule for 12 days before they were tested on a PR schedule of reinforcement as described above. Number of lever presses and reinforcers earned from the final day of FR-5 as well as the PR test were analyzed.
Statistical Analyses
Data for each experiment were analyzed separately with unpaired t-tests, one-way ANOVA with post hoc Newman–Keuls analyses, or Pearson's correlation using Statistica (version 7; StatSoft, Tulsa, OK) and expressed as means±SEM. Alpha levels were set to α=0.05 for all analyses.
Results
In vivo Quantification and Histological Confirmation of NTS GLP-1R Viral Infection
Real-time rtPCR performed on NTS-enriched micropunches of AAV-transfected tissue revealed a 66.5% reduction in GLP-1R mRNA (relative to GAPDH) in NTS tissue transfected by AAV-GLP-1R compared with tissue transfected by AAV-CONTROL (Figure 1b). Figure 1c (AAV-GLP-1R) and Figure 1d (AAV-CONTROL) are representative images showing NTS cells expressing the GFP-tagged AAV-GLP-1R and GFP-tagged AAV-CONTROL transfection in the NTS. GAPDH expression was not significantly different between AAV-GLP-1R- and AAV-CONTROL-treated rats (data not shown).
NTS GLP-1R Knockdown Increases Chow Intake But Not Body Weight
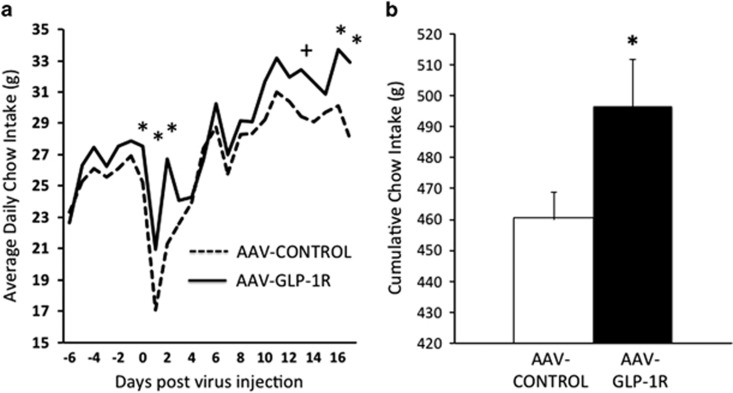
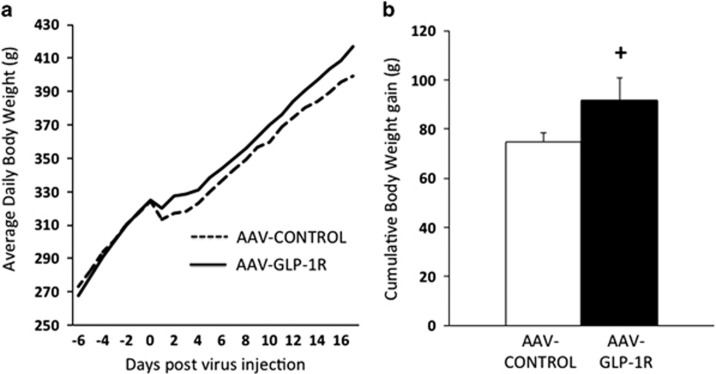
NTS AAV-GLP-1R rats maintained on standard chow showed a significant increase in daily food intake on some, but not all days post-virus injection (Figure 2a), and showed a significant increase in cumulative food intake post-virus injection (Figure 2b), compared with AAV-CONTROL rats. Average daily body weights of rats treated with AAV-GLP-1R and AAV-CONTROL in the NTS were not significantly different (Figure 3a), although there was a non-significant trend (p=0.095) toward an increase in cumulative body weight gain in AAV-GLP-1R animals (Figure 3b).
Figure 2.
(a) Average daily chow intake for NTS AAV-GLP-1R and AAV-CONTROL rats. (b) Cumulative chow intake (from day of virus injection) in NTS AAV-GLP-1R and AAV-CONTROL rats. Data expressed as means±SEM, +p<0.10, *p<0.05.
Figure 3.
(a) Average daily body weight for NTS AAV-GLP-1R and AAV-CONTROL rats. (b) Cumulative body weight gain (from day of virus injection) in NTS AAV-GLP-1R and AAV-CONTROL rats. Data expressed as means±SEM, +p<0.10.
NTS GLP-1R KD Increases Dark Cycle Cumulative Food Intake and Meal Size but Not Meal Number
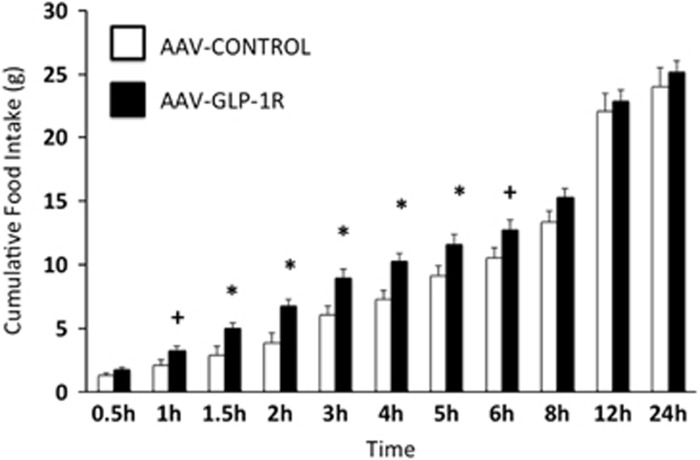
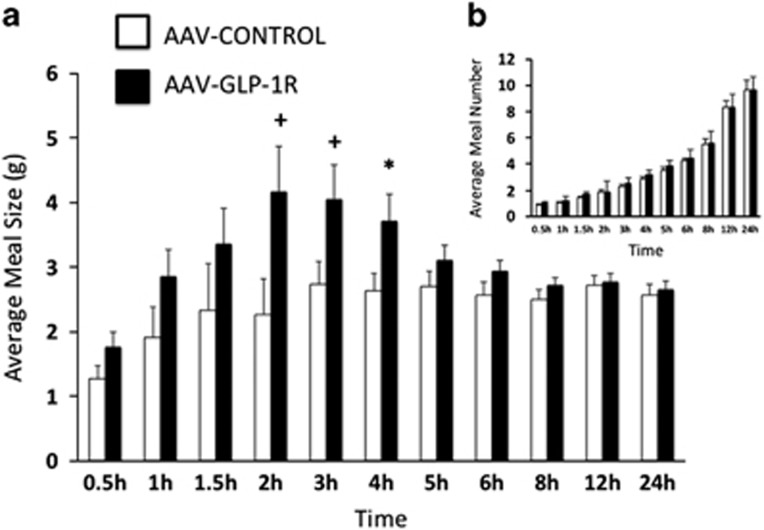
Prior to virus delivery, there were no significant differences in cumulative chow intake, meal size, or meal number between AAV-GLP-1R and AAV-CONTROL groups at any time point analyzed (3-day averages, data not shown). Two weeks post-virus delivery, AAV-GLP-1R rats had significantly elevated cumulative chow intake at 1.5, 2, 3, 4, and 5 h, and a nonsignificant trend for elevated intake at 1 and 6 h, after onset of the dark cycle (Figure 4). These increases in cumulative chow intake were mediated by increases in average meal size (Figure 5a) with no alteration in average meal number (Figure 5b). During the light cycle, there were no significant differences in cumulative chow intake or average meal number, but there was a non-significant trend for an increase in meal size (p=0.09, data not shown).
Figure 4.
Average (3-day) cumulative food intake in NTS AAV-GLP-1R and AAV-CONTROL rats, dark cycle begins at 0 h. Data expressed as means±SEM, +p<0.10, *p<0.05.
Figure 5.
(a) Average (3-day) meal size in NTS AAV-GLP-1R and AAV-CONTROL rats, dark cycle begins at 0 h. (b) Average (3-day) meal number in NTS AAV-GLP-1R and AAV-CONTROL rats. Data expressed as means±SEM, +p<0.06, *p<0.05.
Knockdown of NTS GLP-1R is Correlated with an Increase in Operant Responding and the Motivation to Work for Palatable Food: Fixed and Progressive Ratio Responding
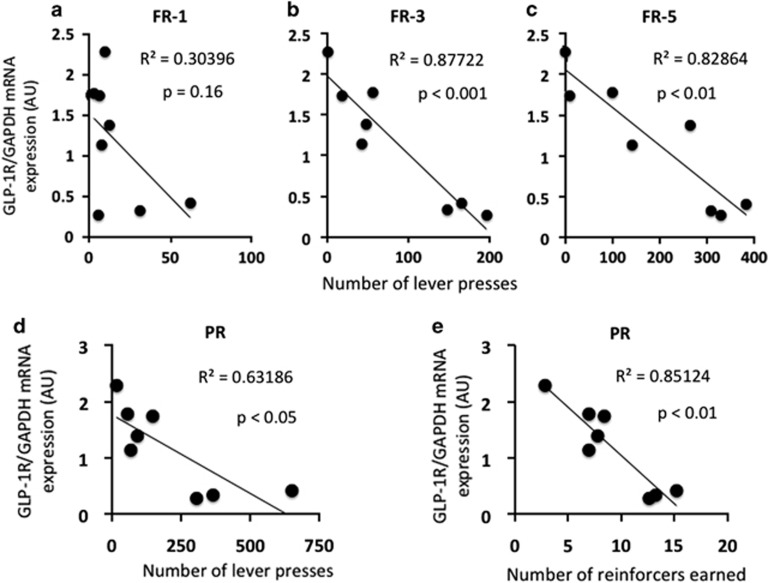
In rats that received NTS AAV-GLP-1R, the relative NTS GLP-1R mRNA expression negatively correlated with average FR-1 responding (r2=0.30, p=0.16, p=NS, Figure 6a), FR-3 responding (r2=0.88, p<0.001, Figure 6b), and FR-5 responding (r2=0.83, p<0.01, Figure 6c), suggesting that reduced endogenous NTS GLP-1R signaling is associated with higher fixed ratio responding for sucrose reinforcers. Furthermore, relative NTS GLP-1R mRNA expression negatively correlated with average PR responding [lever presses (r2=0.63, p<0.05, Figure 6d) and reinforcers earned (r2=0.85, p<0.01, Figure 6e)], suggesting that reduced endogenous NTS GLP-1R signaling is associated with a heightened motivation to work for sucrose reinforcers.
Figure 6.
Relative NTS GLP-1R mRNA expression negatively correlates with (a) FR-1 operant responding (p=NS), (b) FR-3 operant responding and (c) FR-5 operant responding. In addition, relative NTS GLP-1R mRNA expression negatively correlates with (d) number of lever presses and (e) number of reinforcers earned on a progressive ratio schedule of reinforcement.
NTS GLP-1R KD Increases Fixed and Progressive Ratio Responding for Sucrose
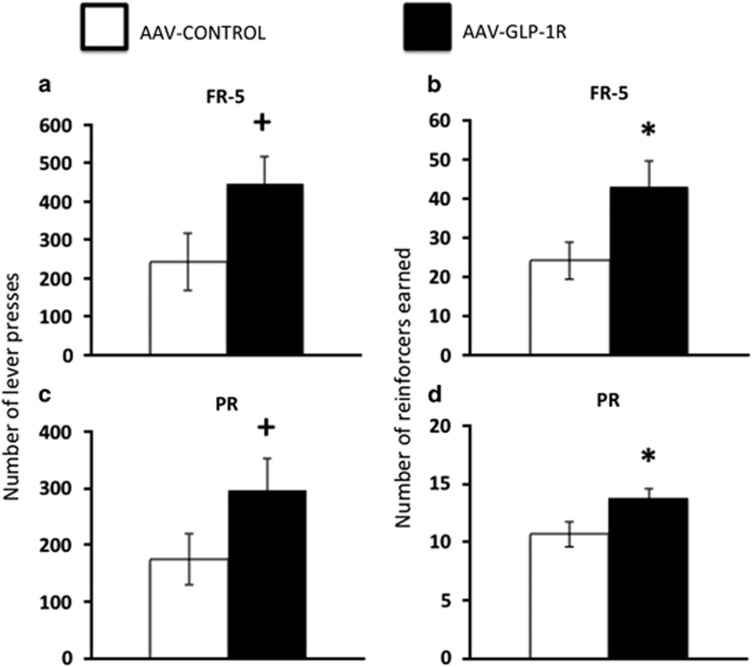
NTS AAV-GLP-1R rats maintained ad libitum on chow had significantly increased FR-5 responding for sucrose as they performed more lever presses (Figures 7a, p=0.06) and earned more reinforcers (Figure 7b, p<0.05) compared with AAV-CONTROL rats. Similarly, the same NTS AAV-GLP-1R rats demonstrated increased PR responding for sucrose as they performed more lever presses (Figure 7c, p=0.10) and earned more reinforcers (Figure 7d, p<0.05) compared with AAV-CONTROL rats. Altogether, these data demonstrate a role for endogenous NTS GLP-1R signaling in the motivation to work for palatable food.
Figure 7.
Number of lever presses (a) and reinforcers earned (b) in AAV-GLP-1R and AAV-CONTROL rats under an FR-5 schedule of reinforcement. Number of lever presses (c) and reinforcers earned (d) in AAV-GLP-1R and AAV-CONTROL rats under PR schedule of reinforcement. Data expressed as means±SEM, +p⩽0.1, *p<0.05.
Discussion
The GLP-1 system is a well-established target for obesity and type II diabetes pharmacotherapies, yet the receptor populations mediating the energy balance effects of endogenous GLP-1R signaling are unknown. To address this, we created a novel adeno-associated virus (AAV-GLP-1R) that utilizes short-hairpin RNA to chronically knockdown the GLP-1R. We injected the AAV-GLP-1R into the NTS of rats, as pharmacological data highlight the NTS as a brain region important for GLP-1R-mediated effects on food intake (Alhadeff and Grill, 2014b; Hayes et al, 2009, 2011). NTS GLP-1R knockdown significantly increased chow intake, meal size, and operant responding for sucrose reinforcers, demonstraing that endogenous NTS GLP-1R signaling is required for the normal control of food intake and motivation to feed.
AAV-mediated knockdown of GLP-1R restricted to the caudomedial NTS increased daily and cumulative chow intake, suggesting that NTS GLP-1R signaling is required for food intake control. These data complement and extend results from pharmacological studies showing that exogenous NTS GLP-1R stimulation reduces food intake (Hayes et al, 2011), and acute blockade of NTS GLP-1R attenuates the intake-suppressive effects of a gastric preload (Hayes et al, 2009). Unlike previous studies that utilized food-deprivation and preload paradigms, we showed here that chronic reduction of NTS GLP-1R signaling was sufficient to increase food intake under normal ad libitum-fed conditions. It is interesting to note that increases in food intake were observed despite no significant differences in average body weight or body weight gain in NTS AAV-GLP-1R vs AAV-CONTROL-treated rats. It is possible that changes in energy expenditure account for the lack of body weight phenotype, especially given that exogenous hindbrain (ie, fourth ICV) GLP-1R agonist injection decreases core temperature and activity (Hayes et al, 2008); however, until explicitly investigated this remains speculative. In addition, it is possible that maintenance on the standard, low-fat chow diet precluded excess body weight gain, and/or that the magnitude of GLP-1R knockdown was not sufficient to achieve a body weight phenotype. Thus, future studies are warranted to investigate the effects of NTS GLP-1R knockdown in animals maintained on calorie-dense palatable diets and with various magnitudes and time-course durations of NTS GLP-1R knockdown.
Meal-pattern analyses revealed that NTS GLP-1R knockdown increased mid-dark cycle food intake, even in the absence of increases in 24 h food intake. These significant increases in food intake during the rats' normal feeding period likely contributed to the significant increases in daily and cumulative food intake that were observed over time. Our data also indicate that NTS GLP-1R knockdown increased food intake by increasing meal size, with no effect on meal number. These results are in line with a large literature demonstrating that the NTS (Grill, 2010; Grill and Hayes, 2012) and GLP-1 (Dossat et al, 2013; Mietlicki-Baase et al, 2013; Punjabi et al, 2011; Scott and Moran, 2007; Williams et al, 2009) both have well-established roles in the control of satiation and meal size. However, previous data show that pharmacological activation of NTS GLP-1R reduces food intake specifically via a reduction in meal number (Hayes et al, 2011). This apparent discrepancy is likely due to methodological differences (eg, acute knockdown virus injection vs repeated pharmacological injections in awake animals), and/or differences in pharmacokinetics or pharmacodynamics between exogenous GLP-1R ligands and endogenous GLP-1R signaling. Given that the virus we used in the current studies produces a chronic knockdown of GLP-1R, the animals remain undisturbed (eg, no agonist/antagonist injection that may cause off-target effects; eg, stress) through the automatic measurements of meal patterns. Thus, we argue that the results presented here are the best representation of the effects of endogenous NTS GLP-1R signaling on meal patterns to date. That NTS knockdown of endogenous GLP-1R produced a specific increase in meal size suggests that effects on meal size are physiologically relevant and involve a potential interaction between NTS GLP-1R signaling and the processing of gastrointestinal satiation signaling.
GLP-1R signaling has received increasing attention for its role in food reward and the motivation to feed (Alhadeff et al, 2012; Dickson et al, 2012; Dossat et al, 2011). Recent pharmacological studies have extended the sites of action for GLP-1R-mediated effects on food reward beyond the mesolimbic system to include the NTS (Alhadeff and Grill, 2014b; Richard et al, 2015). However, until now the role of endogenous GLP-1R signaling in the control of food reward has remained unexplored. Thus, we measured the motivation to work for palatable food by analyzing FR and PR operant responding in animals injected with AAV-GLP-1R or AAV-CONTROL in the NTS. Rats receiving NTS delivery of AAV-GLP-1R showed a significantly increased FR and PR responding for palatable food compared with AAV-CONTROL-injected rats. NTS GLP-1R mRNA levels were inversely correlated with FR-3, FR-5, and PR responding for sucrose reward, further demonstrating that endogenous NTS GLP-1R signaling contributes to the motivation to work for food under ad libitum-fed conditions. These data are the first to highlight the NTS as a region where endogenous GLP-1R signaling affects food reward. It would be useful for future studies to further explore this hypothesis by determining whether the effects of reduced endogenous NTS GLP-1R signaling extend to other food reward paradigms such as conditioned place preference and reinstatement of operant responding for palatable foods.
Endogenous GLP-1 is produced by cells in the intestine and in the NTS and is released upon food ingestion (Holst, 2007). Given the very short half-life of intestinally derived GLP-1 (Vilsboll et al, 2003), we speculate that the observed phenotypic effects following NTS GLP-1R KD are mediated by reduced GLP-1R binding of GLP-1 synthesized by and released from NTS neurons. Although in the current studies, AAV-GLP-1R knockdown was targeted to the NTS and was sufficient to affect chow intake, meal size, and food reward, it is likely that NTS GLP-1R-expressing neurons engage other brain regions to exert effects on these feeding behaviors. Importantly, as AAV-1 serotypes are able to be transported to neuronal processes (Castle et al, 2014), we cannot rule out the possible contribution of GLP-1R knockdown in distal CNS nuclei innervated by NTS neurons. However, there is no convincing evidence that AAV1 serotypes are transsynaptically transfecting distant cell types (Aschauer et al, 2013; Castle et al, 2014). Thus, the effects observed here are likely attributed to knockdown of GLP-1R on NTS cells. Indeed, although it is well known that NTS neurons project to a variety of regions throughout the hindbrain, midbrain, and forebrain (Norgren, 1978; Rinaman, 2010), the target projection sites of NTS GLP-1R-expressing neurons and the downstream circuits mediating the effects described here remain unknown. In addition, it is entirely possible that our observed effects on food intake and motivation are not directly due to reduced NTS GLP-1R signaling, but rather are mediated by interactions between NTS GLP-1R and other feeding-related peptides and signaling pathways. GLP-1 signaling pathways are known to interact with other hormonal systems such as leptin (Kanoski et al, 2015; Williams et al, 2006; Zhao et al, 2012), ghrelin (Chelikani et al, 2006), and amylin (Bello et al, 2010) to exert effects on food intake and energy balance control. Disruption of these peptide interactions by our NTS GLP-1R KD manipulation may in part mediate the food intake phenotypes in the current study. These mechanistic questions are critical to understanding how endogenous NTS GLP-1R signaling mediates food intake and motivation and should be investigated in future studies.
As previously mentioned, GLP-1R agonists are currently used to treat obesity and type-II diabetes. As these agonists enter the brain (Goke et al, 1995; Secher et al, 2014) and act on central GLP-1R to affect food intake (Kanoski et al, 2011; Sisley et al, 2014), the current data may have clinical implications with regard to the central sites of action involved in mediating therapeutic effects of these drugs. In future research, our strategy for GLP-1R knockdown could be applied to a variety of brain and/or peripheral sites to determine the receptor populations necessary for GLP-1R-mediated effects on food intake, energy balance, and glycemia. As new potential applications for GLP-1 pharmacotherapies emerge in the literature (eg, addiction (Egecioglu et al, 2013; Shirazi et al, 2013; Skibicka, 2013; Sorensen et al, 2015; Suchankova et al, 2015), neurodegenerative diseases (Bao et al, 2015; Holscher, 2014)), it is our hope that this tool can be broadly used to elucidate the relevant receptor populations involved in a wide variety of GLP-1R-mediated physiological functions.
FUNDING AND DISCLOSURE
This work was funded by NIH-F31NS084633 (ALA), DK21397 (HJG), DK096139 (MRH), DA037897 (HDS), and DA039393 (HDS). HJG and MRH have both received research funding from Novo Nordisk. HJG is also on the Novo Nordisk Global Obesity Advisory Board. MRH has received research funding from Zealand Pharma and the Dairy Research Institute; however, the support from these companies has no relevance to the current manuscript. The authors declare no conflict of interest.
Acknowledgments
We thank Dr Zhi Yi Ong, Hallie Wald, Carlos Couce, Diana Bongiorno, and Lauren McGrath for technical assistance.
References
- Alhadeff AL, Baird JP, Swick JC, Hayes MR, Grill HJ (2014. a). Glucagon-like peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology 39: 2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Grill HJ (2014. b). Hindbrain nucleus tractus solitarius glucagon-like peptide-1 receptor signaling reduces appetitive and motivational aspects of feeding. Am J Physiol Regul Integr Comp Physiol 307: R465–R470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Hayes MR, Grill HJ (2014. c). Leptin receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake. Am J Physiol Regul Integr Comp Physiol 307: R1338–R1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhadeff AL, Rupprecht LE, Hayes MR (2012). GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology 153: 647–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschauer DF, Kreuz S, Rumpel S (2013). Analysis of transduction efficiency, tropism and axonal transport of AAV serotypes 1, 2, 5, 6, 8 and 9 in the mouse brain. PLoS ONE 8: e76310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao Y, Jiang L, Chen H, Zou J, Liu Z, Shi Y (2015). The neuroprotective effect of liraglutide is mediated by glucagon-like peptide 1 receptor-mediated activation of cAMP/PKA/CREB pathway. Cell Physiol Biochem 36: 2366–2378. [DOI] [PubMed] [Google Scholar]
- Bello NT, Kemm MH, Ofeldt EM, Moran TH (2010). Dose combinations of exendin-4 and salmon calcitonin produce additive and synergistic reductions in food intake in nonhuman primates. Am J Physiol Regul Integr Comp Physiol 299: R945–R952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blonde L, Klein EJ, Han J, Zhang B, Mac SM, Poon TH et al (2006). Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab 8: 436–447. [DOI] [PubMed] [Google Scholar]
- Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH et al (2009). Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet 374: 39–47. [DOI] [PubMed] [Google Scholar]
- Castle MJ, Gershenson ZT, Giles AR, Holzbaur EL, Wolfe JH (2014). Adeno-associated virus serotypes 1, 8, and 9 share conserved mechanisms for anterograde and retrograde axonal transport. Hum Gene Ther 25: 705–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelikani PK, Haver AC, Reidelberger RD (2006). Ghrelin attenuates the inhibitory effects of glucagon-like peptide-1 and peptide YY(3-36) on food intake and gastric emptying in rats. Diabetes 55: 3038–3046. [DOI] [PubMed] [Google Scholar]
- Dickson SL, Shirazi RH, Hansson C, Bergquist F, Nissbrandt H, Skibicka KP (2012). The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. J Neurosci 32: 4812–4820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Diaz R, Gallo L, Panagos A, Kay K, Williams DL (2013). Nucleus accumbens GLP-1 receptors influence meal size and palatability. Am J Physiol Endocrinol Metab 304: E1314–E1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dossat AM, Lilly N, Kay K, Williams DL (2011). Glucagon-like Peptide 1 receptors in nucleus accumbens affect food intake. J Neurosci 31: 14453–14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egecioglu E, Engel JA, Jerlhag E (2013). The glucagon-like peptide 1 analogue Exendin-4 attenuates the nicotine-induced locomotor stimulation, accumbal dopamine release, conditioned place preference as well as the expression of locomotor sensitization in mice. PLoS ONE 8: e77284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goke R, Larsen PJ, Mikkelsen JD, Sheikh SP (1995). Distribution of GLP-1 binding sites in the rat brain: evidence that exendin-4 is a ligand of brain GLP-1 binding sites. Eur J Neurosci 7: 2294–2300. [DOI] [PubMed] [Google Scholar]
- Grill HJ (2010). Leptin and the systems neuroscience of meal size control. Front Neuroendocrinol 31: 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill HJ, Hayes MR (2012). Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab 16: 296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Bradley L, Grill HJ (2009). Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology 150: 2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, De Jonghe BC, Kanoski SE (2010. a). Role of the glucagon-like-peptide-1 receptor in the control of energy balance. Physiol Behav 100: 503–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Leichner TM, Zhao S, Lee GS, Chowansky A, Zimmer D et al (2011). Intracellular signals mediating the food intake suppressive effects of hindbrain glucagon-like-peptide-1 receptor activation. Cell Metab 13: 320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Grill HJ (2008). Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149: 4059–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK et al (2010. b). Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11: 77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C (2014). Central effects of GLP-1: new opportunities for treatments of neurodegenerative diseases. J Endocrinol 221: T31–T41. [DOI] [PubMed] [Google Scholar]
- Holst JJ (2007). The physiology of glucagon-like peptide 1. Physiol Rev 87: 1409–1439. [DOI] [PubMed] [Google Scholar]
- Hsu TM, Hahn JD, Konanur VR, Lam A, Kanoski SE (2015). Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology 40: 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Alhadeff AL, Fortin SM, Gilbert JR, Grill HJ (2014). Leptin signaling in the medial nucleus tractus solitarius reduces food seeking and willingness to work for food. Neuropsychopharmacology 39: 605–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR (2011). Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 152: 3103–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanoski SE, Ong ZY, Fortin SM, Schlessinger ES, Grill HJ (2015). Liraglutide, leptin and their combined effects on feeding: additive intake reduction through common intracellular signalling mechanisms. Diabetes Obes Metab 17: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JP, Arnold M, Pettersen KG, Lossel P, Langhans W, Lee SJ (2016). Knockdown of GLP-1 Receptors in Vagal Afferents Affects Normal Food Intake and Glycemia. Diabetes 65: 34–43. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Wellman PJ (1998). PVN infusion of GLP-1-(7-36) amide suppresses feeding but does not induce aversion or alter locomotion in rats. Am J Physiol 274(1 Pt 2): R23–R29. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Lane M, Shughrue P (1999). Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403: 261–280. [DOI] [PubMed] [Google Scholar]
- Mietlicki-Baase EG, Ortinski PI, Rupprecht LE, Olivos DR, Alhadeff AL, Pierce RC et al (2013). The food intake-suppressive effects of glucagon-like peptide-1 receptor signaling in the ventral tegmental area are mediated by AMPA/kainate receptors. Am J Physiol Endocrinol Metab 305: E1367–E1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietlicki-Baase EG, Reiner DJ, Cone JJ, Olivos DR, McGrath LE, Zimmer DJ et al (2015). Amylin modulates the mesolimbic dopamine system to control energy balance. Neuropsychopharmacology 40: 372–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R (1978). Projections from the nucleus of the solitary tract in the rat. Neuroscience 3: 207–218. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (2005) The rat brain in stereotaxic coordinates5th edn. Academic Press: San Diego, CA, USA. [Google Scholar]
- Punjabi M, Arnold M, Geary N, Langhans W, Pacheco-Lopez G (2011). Peripheral glucagon-like peptide-1 (GLP-1) and satiation. Physiol Behav 105: 71–76. [DOI] [PubMed] [Google Scholar]
- Richard JE, Anderberg RH, Goteson A, Gribble FM, Reimann F, Skibicka KP (2015). Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS ONE 10: e0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L (2010). Ascending projections from the caudal visceral nucleus of the solitary tract to brain regions involved in food intake and energy expenditure. Brain Res 1350: 18–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V (2003). Peptides that regulate food intake: glucagon-like peptide 1-(7-36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol 284: R1427–R1435. [DOI] [PubMed] [Google Scholar]
- Scott KA, Moran TH (2007). The GLP-1 agonist exendin-4 reduces food intake in nonhuman primates through changes in meal size. Am J Physiol Regul Integr Comp Physiol 293: R983–R987. [DOI] [PubMed] [Google Scholar]
- Secher A, Jelsing J, Baquero AF, Hecksher-Sorensen J, Cowley MA, Dalboge LS et al (2014). The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 124: 4473–4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirazi RH, Dickson SL, Skibicka KP (2013). Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS ONE 8: e61965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisley S, Gutierrez-Aguilar R, Scott M, D'Alessio DA, Sandoval DA, Seeley RJ (2014). Neuronal GLP1R mediates liraglutide's anorectic but not glucose-lowering effect. J Clin Invest 124: 2456–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibicka KP (2013). The central GLP-1: implications for food and drug reward. Front Neurosci 7: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen G, Reddy IA, Weikop P, Graham DL, Stanwood GD, Wortwein G et al (2015). The glucagon-like peptide 1 (GLP-1) receptor agonist exendin-4 reduces cocaine self-administration in mice. Physiol Behav 149: 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Caparelli EC, Momenan R et al (2015). The glucagon-like peptide-1 receptor as a potential treatment target in alcohol use disorder: evidence from human genetic association studies and a mouse model of alcohol dependence. Translational psychiatry 5: e583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tella SH, Rendell MS (2015). Glucagon-like polypeptide agonists in type 2 diabetes mellitus: efficacy and tolerability, a balance. Ther Adv Endocrinol Metab 6: 109–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilsboll T, Agerso H, Krarup T, Holst JJ (2003). Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab 88: 220–224. [DOI] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW (2006). Leptin regulation of the anorexic response to glucagon-like peptide-1 receptor stimulation. Diabetes 55: 3387–3393. [DOI] [PubMed] [Google Scholar]
- Williams DL, Baskin DG, Schwartz MW (2009). Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150: 1680–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Kanoski SE, Yan J, Grill HJ, Hayes MR (2012). Hindbrain leptin and glucagon-like-peptide-1 receptor signaling interact to suppress food intake in an additive manner. Int J Obes (Lond) 36: 1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]