Abstract
Lung fibrosis is associated with inflammation, apoptosis and oxidative damage. The transcription factor nuclear factor erythroid 2-related factor-2 (Nrf2) prevents damage to cells from oxidative stress by regulating the expression of antioxidant proteins. Sulforaphane (SFN), an Nrf2 activator, additionally regulates excessive oxidative stress by promoting the expression of endogenous antioxidants. The present study investigated if SFN protects against lung injury induced by bleomycin (BLM). The secondary aim of the present study was to assess if this protection mechanism involves upregulation of Nrf2 and its downstream antioxidants. Pulmonary fibrosis was induced in C57/BL6 mice by intratracheal instillation of BLM. BLM and age-matched control mice were treated with or without a daily dose of 0.5 mg/kg SFN until sacrifice. On days 7 and 28, mice were assessed for induction of apoptosis, inflammation, fibrosis, oxidative damage and Nrf2 expression in the lungs. The lungs were investigated with histological techniques including haematoxylin and eosin staining, Masson's trichrome staining and terminal deoxynucleotidyl transferase UTP nick end labeling. Inflammatory, fibrotic and apoptotic processes were confirmed by western blot analysis for interleukin-1β, tumor necrosis factor-α, transforming growth factor-β and caspase-3 protein expressions. Furthermore, protein levels of 3-nitro-tyrosine, 4-hydroxynonenal, superoxide dismutase 1 and catalase were investigated by western blot analysis. It was demonstrated that pulmonary fibrosis induced by BLM significantly increased apoptosis, inflammation, fibrosis and oxidative stress in the lungs at days 7 and 28. Notably, SFN treatment significantly attenuated the infiltration of the inflammatory cells, collagen accumulation, epithelial cell apoptosis and oxidative stress in the lungs. In addition, SFN treatment increased expression of the Nrf2 gene and its downstream targets. In conclusion, these results suggested that SFN treatment of pulmonary fibrosis mouse models may attenuate alveolitis, fibrosis, apoptosis and lung oxidative stress by increasing the expression of antioxidant enzymes, including NAPDH quinone oxidoreductase, heme oxygenase-1, superoxide dismutase and catalase, via upregulation of Nrf2 gene expression. Thus, the results from the present study may facilitate the development of therapies for BLM-toxicity and pulmonary fibrosis.
Keywords: sulforaphane, nuclear factor erythroid2-related factor-2, fibrosis, bleomycin, oxidative damage
Introduction
Pulmonary fibrosis is a fatal and progressive disease characterized by injury to alveolar epithelial cells, infiltration of inflammatory cells, enhanced proliferation of fibroblasts, aberrant accumulation of the extracellular matrix and abnormality in lung architectural remodeling (1,2). Currently, glucocorticosteroids, which inhibit the expression of pro-inflammatory cytokines, are the primary treatment option for pulmonary fibrosis (3). However, glucocorticosteroids cannot reverse or delay the course of the disease (4). Therefore, identification of novel and effective treatment options that have the potential to improve the mortality rate are urgently required.
Recent evidence suggested that oxidative stress, due to the imbalance between the functions of oxidants and antioxidant proteins, has an important role in the progression of lung fibrosis, by activating redox-sensitive signaling pathways, modification of immune cell function, and activation of fibroblasts (5,6). Previous studies have additionally suggested that reactive oxygen species (ROS) cause the activation of growth-regulatory cytokines, including transforming growth factor (TGF)-β, a critical pro-fibrosis cytokine (7). Felton et al (8) previously analyzed the effects of N-acetylcysteine, an exogenous antioxidant, and demonstrated that it dramatically decreases lung damage in the presence of TGF-β1 by reducing intracellular ROS production. Additionally, the activity of the intracellular antioxidant enzyme catalase (CAT) has been linked with decreased lung fibrosis in a mouse model (9). Therefore, based on these studies, it was hypothesized that a strategy to upregulate the expression of endogenous multiple antioxidants maybe an efficient approach to prevent or treat lung fibrosis (10).
The transcription factor nuclear factor erythroid2-related factor-2 (Nrf2) has a primary role in regulating cellular antioxidant responses (11). Upon exposure to oxidative burden, the Nrf2-antioxidant signaling pathway is activated to stimulate transcription of various antioxidant defense enzymes, including NADPH quinone oxidoreductase (NQO1), heme oxygenase-1 (HO-1), superoxide dismutase (SOD), and CAT (12). Upregulation of the expression of these antioxidant enzymes promotes antioxidative response, detoxification, and anti-inflammation. It has been reported that Nrf2 and its downstream antioxidants enzymes serve critical roles in a lung fibrosis model (13), whereas deletion of the Nrf2 gene increases susceptibility to bleomycin (BLM) due to reduced antioxidant activity (14). BLM, an anti-cancer medicine, can induce oxidative stress and DNA damage, and can induce pulmonary fibrosis in clinical treatment. Intratracheal instillation of BLM has been used to establish pulmonary fibrotic animal models for many years (15,16).
Sulforaphane (SFN), a dietary organosulfur compound, is isolated from cruciferous vegetables and has an indirect antioxidant activity. It upregulates the expression of endogenous antioxidant proteins against oxidative stress and damage via Nrf2 activation (17). Previous studies have demonstrated that the antitoxic and antioxidant properties of SFN in experimental models most likely involves activation of the Nrf2 gene (18,19). However, it is unclear if SFN alleviates lung fibrosis, resulting in upregulation of the Nrf2 gene and its downstream antioxidant targets.
Therefore, the present study aimed to investigate if SFN may prevent the development of BLM-induced lung fibrosis in a mouse model, and to identify the potential mechanisms of action.
Materials and methods
Animals
C57/BL6 male mice (age, 8–10 weeks; weight, ~20 g; n=60) were purchased from the Animal Center of Jilin University (Changchun, China) and housed in the animal testing center of the Second Hospital of Jilin University. They were housed at a constant temperature of 22°C in 50–60% humidity and a 12 h light/dark cycle, and were fed standard rodent feed and tap water. The experimental protocol was approved by the Animal Care and Use Committee of Jilin University.
Mice were divided randomly into four groups (n=15/group): Control, SFN, BLM and BLM/SFN. To generate a pulmonary fibrosis model, experimental mice were administered a single dose of 5 mg/kg body weight BLM (Nippon Kayaku, Co., Ltd., Tokyo, Japan) by intratracheal instillation, and control mice received saline. The SFN and BLM/SFN group mice received a subcutaneous injection of SFN (5 mg/kg body weight; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) each day. The dose of SFN used in the present study was in accordance with a previously published study (18). Mice in the control group received an equal volume of vehicle (1% dimethyl sulfoxide in PBS). Finally, mice from each group were sacrificed at days 7 or 28 after initial BLM or saline administration, and samples were collected.
Histopathological examination
Following anesthesia, the thorax was opened and lungs were isolated from all mice. The left lungs were fixed in 10% buffered formalin, following which they were dehydrated in a series of graded alcohol. Subsequently, they were washed with xylene, embedded into paraffin blocks and sliced into 3-µm sections. To examine basic structural alterations and collagen content, slices were stained with hematoxylin & eosin (H&E) and Masson's trichrome. Pulmonary inflammation and fibrosis was evaluated using the Szapiel score (20).
Nrf2 expression was assessed by immunohistochemical staining. Tissue sections were dewaxed and then incubated with target retrieval solution (Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) in a microwave for 15 min at 98°C for antigen retrieval, followed by treatment with 3% hydrogen peroxide for 15 min and then 5% bovine serum albumin (Sigma-Aldrich) for 2 h, at room temperature. Sections were subsequently incubated with anti-Nrf2 primary antibody (1:100; ab31163; Abcam, Cambridge, MA, USA) overnight at 4°C and then incubated with a horseradish peroxidase (HRP) conjugated goat anti-rabbit secondary antibody (1:1,000; SA00001-2; Wuhan Sanying Biotechnology, Wuhan, China) for 1 h at room temperature. Sections were subsequently stained with 3,3-diaminobenzidine, and counterstained with hematoxylin. Staining was observed under a Nikon Eclipse E600 microscope (Nikon Corporation, Tokyo, Japan).
Assay for hydroxyproline content
Pulmonary collagen content was analyzed with a Hydroxyproline assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Lung tissue was hydrolyzed by incubating with NaOH at 95–100°C for 20 min. Following neutralization with HCl, the tissue was diluted with distilled water. Subsequently, hydroxyproline content in the tissue was assessed by recording the absorbance at a wavelength of 550 nm. The results were calculated as µg hydroxyproline/g wet lung tissue.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). RNA concentrations and purity were quantified using a NanoDrop 2000™ Spectrophotometer (Thermo Fisher Scientific, Inc.). A total of 3 µg RNA was used for cDNA synthesis according to the manufacturer's protocol, using a Revert Aid First Strand cDNA Synthesis kit (Gene Copoeia, Inc., Rockville, MD, USA). Primers for Nrf2, NQO1, HO-1, SOD1, CAT and β-actin amplification were purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The primers were as follows: Nrf2 forward, TCC TAT GCG TGA ATC CCA AT, and reverse, GCG GCT TGA ATG TTT GTC TT; NQO1 forward, GTT TCT GTG GCT TCC AGG TC, and reverse, CGT TTC TTC CAT CCT TCC AG; HO-1 forward, GGG CTG TGA ACT CTG TCC AA, and reverse, GGT GAG GGA ACT GTG TCA GG; SOD1 forward, TTC TCG TCT TGC TCT CTC TGG, and reverse, GTT CAC CGC TTG CCT TCT; CAT forward, CCT CGT TCA GGA TGT GGT TT, and reverse, CCT CGT TCA GGA TGT GGT TT; β-actin forward, GTG CTA TGT TGC TCT AGA CTT CG, and reverse, ATG CCA CAG GAT TCC ATA CC. qPCR was performed in a 20 µl reaction buffer containing 10 µl Fast Start Universal SYBR®-Green Master mix (Roche Diagnostics, Basel, Switzerland), 1 µl 10 mM primer pairs, 1 µl cDNA and 8 µl ddH2O, using the Roche Light Cycler® 480 Real-Time PCR system. The thermocycling conditions were as follows: Initial step of 95°C for 5 min, then 40 cycles of 95°C for 30 sec, 58°C for 30 sec and 72°C for 60 sec. The comparative cycle time (Cq) method was used to determine fold differences between samples, values were normalized to the endogenous reference (β-actin) using the 2ΔΔCq method (21).
Western blot analysis
For preparation of total protein samples, lung tissues were homogenized in ice-cold radioimmunoprecipitation assay lysis buffer (Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China), and protein concentrations were determined using a bicinchoninic acid protein assay kit (Beijing Dingguo Changsheng Biotechnology Co., Ltd.). Equal amounts (10 µg) of protein samples were separated by 10% SDS-PAGE (15% SDS-PAGE was used for studies of IL-1β) and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA). Membranes were blocked with 5% (w/v) skimmed milk for >1 hat room temperature and subsequently incubated overnight at 4°C with the following primary antibodies: Anti-Nrf2 (1:1,000; ab31163), anti-HO-1 (1:1,000; ab13243), anti-NQO1 (1:1,000; ab34173), anti-SOD1 (1:2,000; ab16831), anti-CAT (1:1,000; ab16731), anti-3-nitro-tyrosine (3-NT) (1:1,000; ab110282), anti-4-hydroxynonenal (4-HNE) (1:1,000; ab46545), anti-tumor necrosis factor (TNF)-α (1:200; ab6671), anti-TGF-β (1:1,000; ab64715), anti-caspase-3 (1:1,000; #9665), anti-IL-1β (1:300; sc-7884) and anti-β-actin (1:1,000; 66009-1-Ig). All antibodies were purchased from Abcam, except for anti-caspase-3 (Cell Signaling Technology, Inc. Danvers, MA, USA), anti-IL-1β (Santa Cruz Biotechnology, Inc. Dallas, TX, USA) and anti-β-actin (Wuhan Sanying Biotechnology). After three washes with Tris-buffered saline (pH 7.4) containing 0.1% Tween-20, membranes were incubated with the appropriate HRP-conjugated secondary antibodies for 1 h at room temperature. The secondary antibodies were as follows: Goat anti-mouse (1:1,000; SA00001-1) and goat anti-rabbit (1:1,000; SA00001-2) both from Wuhan Sanying Biotechnology. Proteins were visualized using an Enhanced Chemiluminescence kit (EMD Millipore), and the band density values were normalized to β-actin expression. Densitometry was performed using Quantity One 4.52 software (Bio-Rad Laboratories, Inc., Hercules, CA, USA), and ≥6 blots were repeated to obtain densitometric quantification.
Terminal deoxynucleotidyl transferase UTP nick-end labeling (TUNEL) staining
TUNEL staining was performed on formalin-fixed, paraffin-embedded lung sections using an In Situ Cell Death Detection kit, POD (Roche Diagnostics) according to the manufacturer's protocol. Positively stained apoptotic cells were counted randomly in five microscopic fields from at least three slides of each mouse, under a light microscope. The percentage of TUNEL staining represented the number of TUNEL positive cells in a field with a total of 100 nuclei.
Statistical analysis
Data were collected from >4 animals/group and are presented as the mean ± standard deviation. Comparisons between groups were assessed by one-way analysis of variance, followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
SFN alleviates BLM-induced alveolar epithelial cell apoptosis
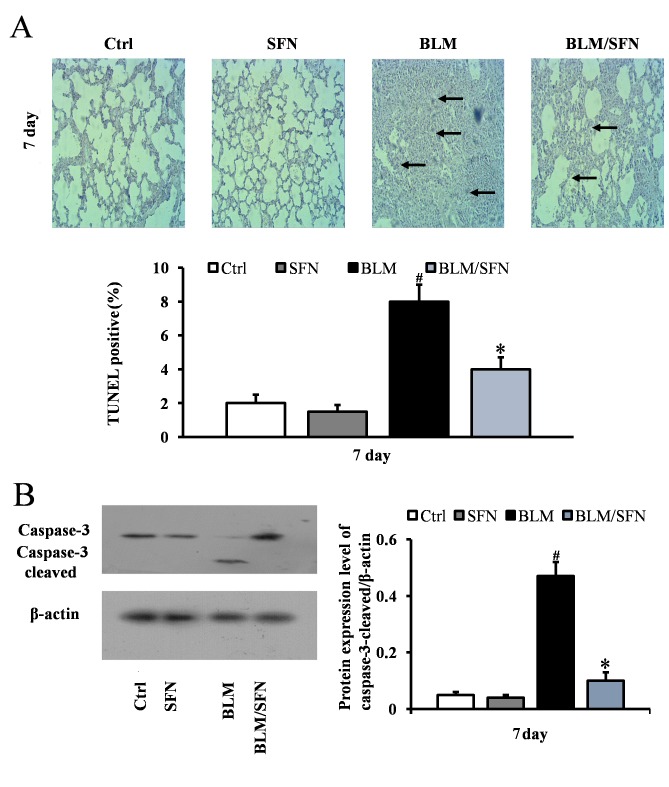
In the early stages of the pulmonary fibrosis induced by BLM, an increase in alveolar epithelial cell apoptosis was observed. At day 7, the BLM treatment mice had significantly increased TUNEL-positive cells compared with control mice. However, mice with combined BLM and SFN treatment exhibited a significant decrease in epithelial cell apoptosis compared with BLM treatment alone (P<0.05; Fig. 1A). Consistent with TUNEL staining results, western blot analysis additionally revealed a significant increase in the expression of cleaved caspase-3 in the BLM group at day 7. However, the effect of BLM treatment was neutralized by the presence of SFN (P<0.05; Fig. 1B).
Figure 1.
SFN alleviates BLM-induced lung epithelial apoptosis. (A) Cellular apoptosis of lung epithelial cells was analyzed by TUNEL staining at 7 days post-treatment. Apoptotic cell nuclei are stained brown and marked by arrows. The BLM treatment mice exhibited significantly increased TUNEL-positive cells, which were decreased following SFN treatment. Magnification, ×200. (B) Western blot analysis for caspase-3 and cleaved caspase-3 protein expression levels was used to demonstrate the effect of SFN on BLM-induced lung epithelial apoptosis at day 7. Data are presented as the mean ± standard deviation (n=6/group). #P<0.05 vs. control group; *P<0.05 vs. BLM group. BLM, bleomycin; TUNEL, terminal deoxynucleotidyl transferase UTP nick-end labeling; Ctrl, control; SFN, sulforaphane.
SFN attenuates BLM-induced alveolitis and lung fibrosis
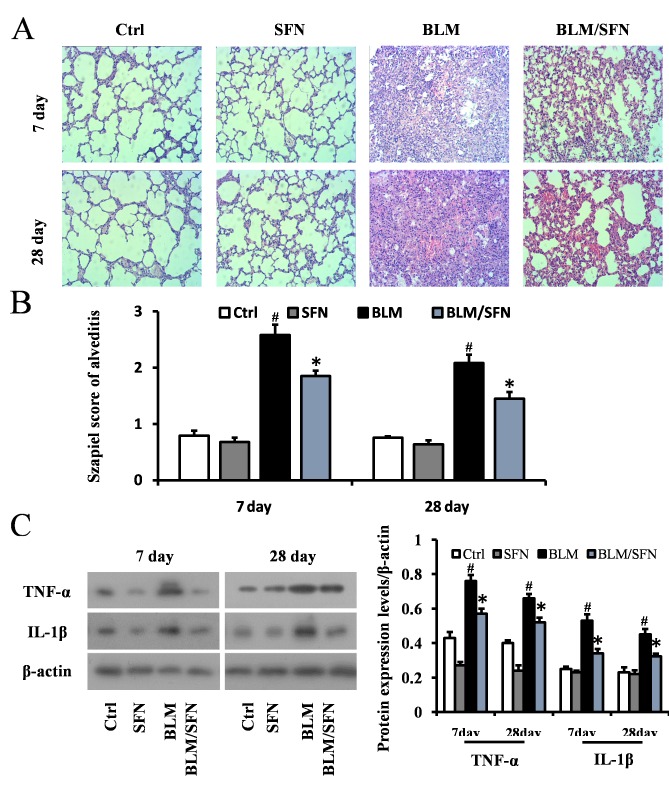
Histological examination using H&E staining of mouse lungs from the BLM treated group revealed significant progressive lesions in lung structure. This included excessive infiltration of inflammatory cells, thickened pulmonary alveolar septa and a large number of collapsed alveoli (Fig. 2A and B). However, combined SFN and BLM markedly alleviated the degree of alveolitis at days 7 and 28. In the cases of the control and SFN treated groups, the lungs had healthy histology. Protein expression levels of the inflammatory cytokines IL-1β and TNF-α were examined in lung tissues at days 7 and 28 by western blot analysis (Fig. 2C). In the fibrosis model (BLM treatment alone), the expression levels of IL-1β and TNF-α were elevated compared with the control group at days 7 and 28. However, SFN treatment in the fibrosis model significantly downregulated the expression of TNF-α and IL-1β compared with the BLM treatment alone at the two time points (P<0.05).
Figure 2.
SFN attenuates BLM-induced lung alveolitis. (A) Hematoxylin and eosin staining of lung tissues at 7 and 28 days following SFN treatment. BLM induced lung alveolitis, which was attenuated by SFN treatment. Magnification, ×200. (B) The Szapiel alveolitis score was lower in the BLM/SFN group, compared with the BLM group at day 7 and 28. (C) Western blot analysis for TNF-α and IL-1β protein expression levels was used to investigate the effect of SFN on BLM-induced lung alveolitis at day 7 and 28 post-treatment. Data are presented as the mean ± standard deviation (n=6/group). #P<0.05 vs. control group; *P<0.05 vs. BLM group. BLM, bleomycin; SFN, sulforaphane; TNF-α; tumor necrosis factor-α, IL-1β, interleukin-1β; Ctrl, control.
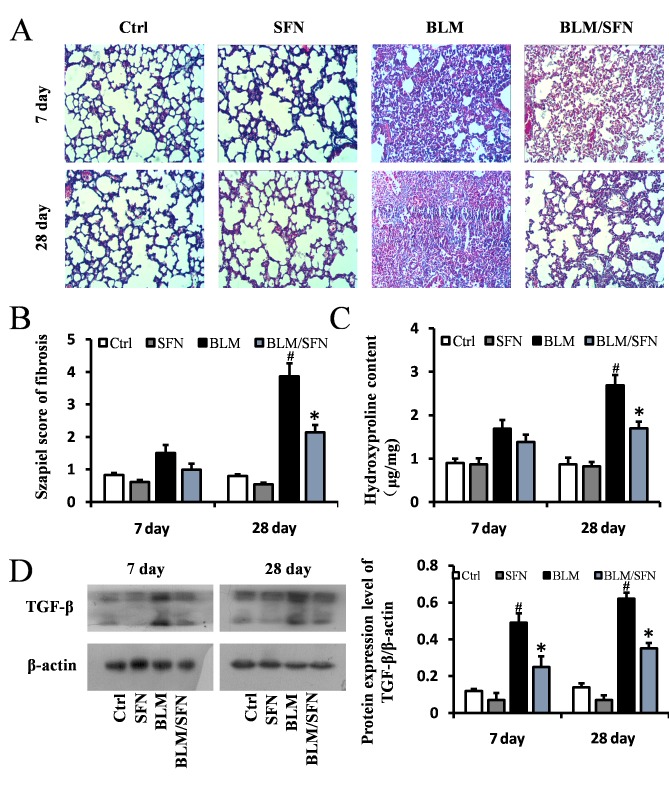
Furthermore, lung fibrosis was examined by Masson's staining, where blue staining represented collagen matrix. BLM treatment predominantly induced collagen accumulation in the interstitial areas at day 28 (Fig. 3A and B), and its effects were significantly inhibited by SFN treatment at day 28 (P<0.05). Simultaneously, hydroxyproline content of lung samples on days 7 and 28 in the four groups were examined. Hydroxyproline content in the lung fibrosis model (BLM treatment alone) was increased compared with control mice; however, combined BLM and SFN treatment significantly suppressed this effect at day 28 (P<0.05; Fig. 3C). To further examine the protective effect of SFN on pulmonary fibrosis, protein expression levels of TGF-β, a critical pro-fibrotic mediator, were assessed by western blot analysis. Expression levels of TGF-β were markedly increased in the lungs of mice from the BLM group compared with the control group. However, SFN treatment significantly reduced this increase at days 7 and 28 (Fig. 3D).
Figure 3.
SFN prevents BLM-induced lung fibrosis. (A) Masson's trichrome staining of lung tissues from various treatment groups on days 7 and 28 post-treatment. BLM induced marked lung fibrosis and collagen deposition at day 28, which was alleviated by SFN treatment. Magnification, ×200. (B) The Szapiel fibrosis score was lower in the BLM/SFN group, compared with the BLM group at day 28. (C) Hydroxyproline content was quantified by on days 7 and 28. The hydroxyproline content of BLM group was higher than the control group at day 28, which was reduced by SFN treatment. (D) Western blot analysis for TGF-β was used to demonstrate the effect of SFN on BLM-induced lung fibrosis at day 7 and 28 post-treatment. Data are presented as the mean ± standard deviation (n=6/group). #P<0.05 vs. control group; *P<0.05 vs. BLM group. BLM, bleomycin; SFN, sulforaphane; TGF-β, transforming growth factor-β; Ctrl, control.
SFN protects against BLM-induced lung oxidative stress
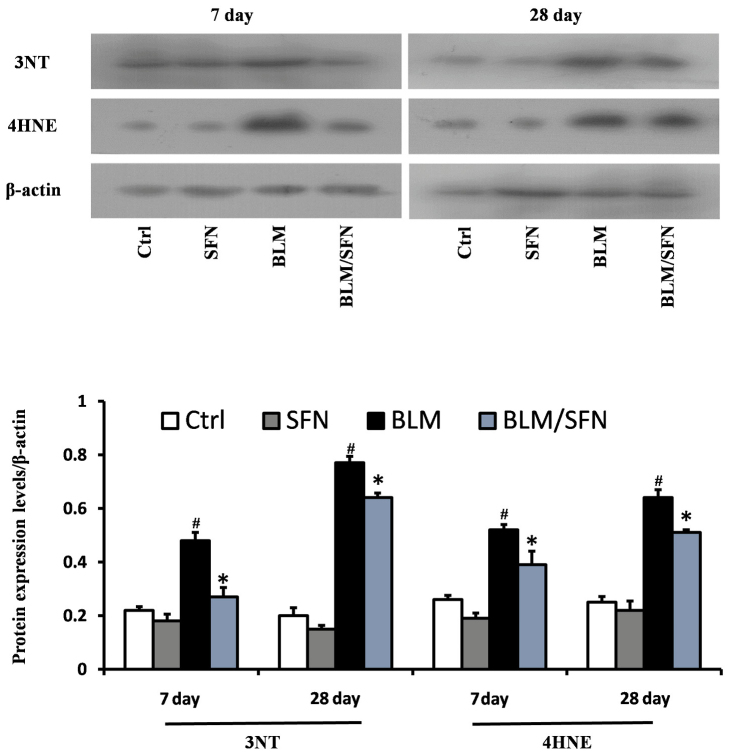
BLM treatment was demonstrated to significantly increase lung oxidative stress, as detected by accumulation of 3-NT and 4-HNE, which are indicators of nitration damage and lipid peroxidation, respectively. Western blot analysis revealed that BLM treatment significantly increased expression levels of 3-NT and 4-HNE at days 7 and 28. However, treatment with SFN partly prevented oxidative damage at the two time-points compared with BLM alone (P<0.05; Fig. 4).
Figure 4.
SFN inhibits oxidative stress of BLM-induced lung fibrosis. Western blot analysis for the oxidative damage markers 3-NT and 4-HNE was used to demonstrate the effect of SFN on BLM-induced lung oxidative stress at days 7 and 28. Compared with BLM, SFN markedly protected against BLM-induced lung oxidative stress. Data are presented as the mean ± standard deviation (n=6/group). #P<0.05 vs. control group; *P<0.05 vs. BLM group. BLM, bleomycin; SFN, sulforaphane; Ctrl, control; 3-NT, 3-nitro-tyrosine; 4-HNE, 4-hydroxynonenal.
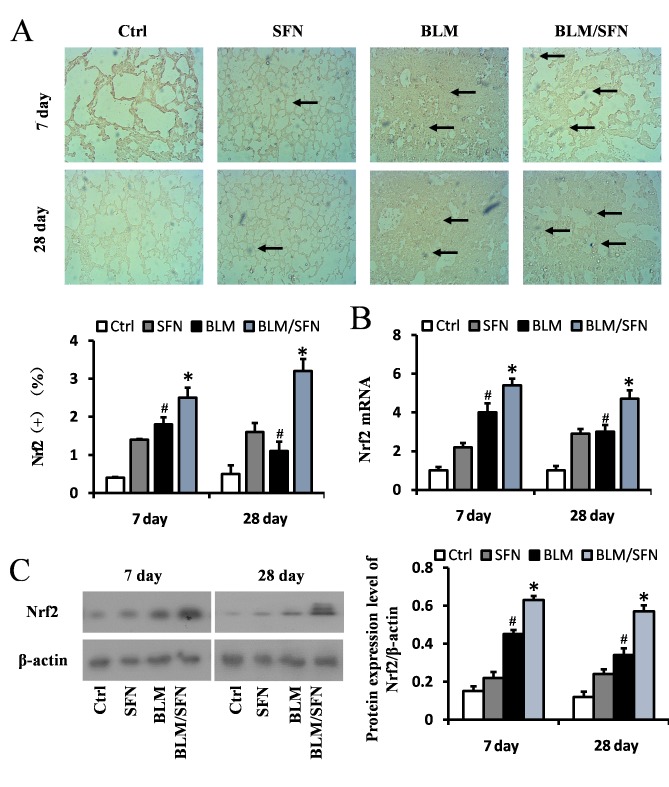
SFN upregulates the expression of Nrf2 and downstream genes in the lungs
Based on the above results, it appeared that SFN may protect against BLM-induced lung inflammation, fibrosis, apoptosis and oxidative damage. Furthermore, as oxidative stress is considered a pivotal mediator for pathological alterations in pulmonary fibrosis, the underlying mechanisms of SFN action in pulmonary fibrosis was investigated. Expression of the Nrf2 gene in the lungs was assessed by immunochemical staining (Fig. 5A), RT-qPCR (Fig. 5B) and western blot analysis (Fig. 5C). The results from all these assays indicated that Nrf2 expression levels were increased at days 7 and 28 in the lungs of BLM treated mice compared with control group mice. Notably, an additional significant increase in Nrf2 expression in the lungs of mice treated with BLM and SFN simultaneously was observed compared with BLM treatment alone, at the two time-points (P<0.05).
Figure 5.
Effects of SFN on the expression of Nrf2 in BLM-induced lung fibrosis models. (A) Immunohistochemical staining and quantification of Nrf2 (marked with black arrows) indicated that BLM increased Nrf2 expression, and this increased further following SFN treatment at days 7 and 28. (B) Quantification of Nrf2mRNA expression levels in each groups at day 7 and 28 post-treatment, as detected by reverse transcription-quantitative polymerase chain reaction. (C) Western blot analysis of the Nrf2 protein expression levels was used to demonstrate the effect of SFN on BLM-induced oxidative stress at days 7 and 28 post-treatment. Data are presented as the mean ± standard deviation (n=6/group). #P<0.05 vs. control group; *P<0.05 vs. BLM group. BLM, bleomycin; SFN, sulforaphane; Ctrl, control; Nrf2, nuclear factor erythroid 2-related factor-2.
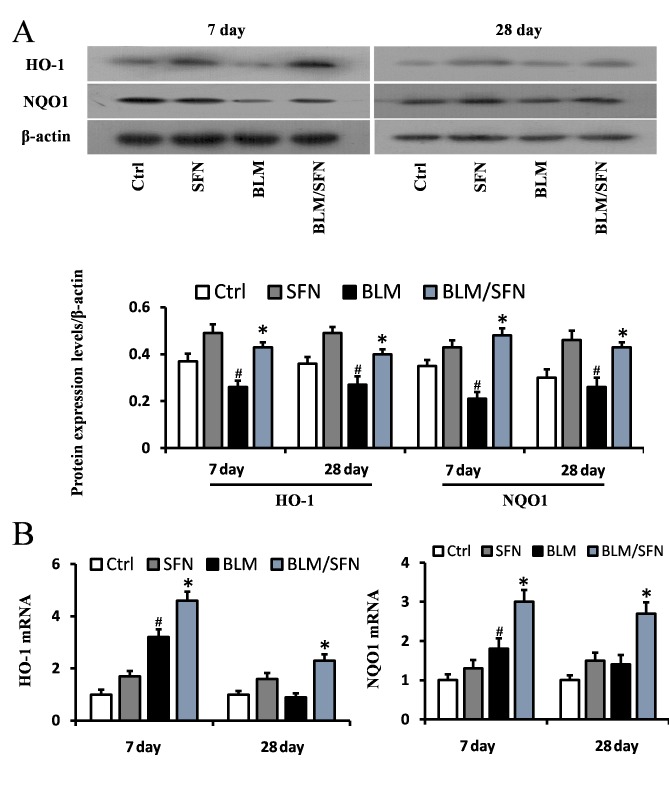
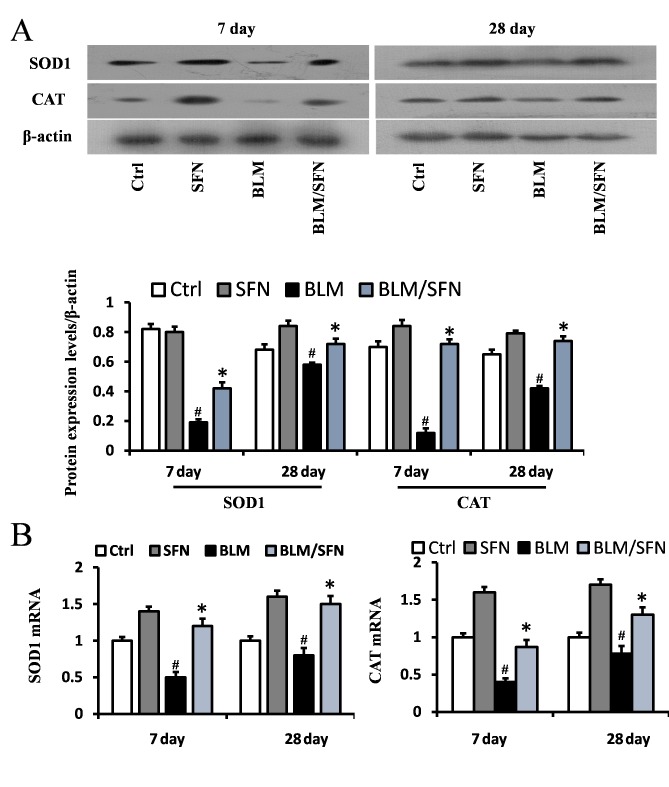
In addition, the transcriptional activity of Nrf2 was investigated by examining the mRNA and protein expression levels of its downstream antioxidant target genes, including NQO1 and HO-1 (Fig. 6), and SOD1 and CAT (Fig. 7). In the BLM group, the protein expression levels of HO-1, NQO1, SOD1 and CAT were significantly decreased in the lungs at days 7 and 28. However, the mRNA expression levels of NQO1 and HO-1 were increased at day 7, and the mRNA expression levels of SOD1 and CAT were decreased at days 7 and day 28, compared with the control. Notably, the protein levels and the mRNA levels of HO-1, NQO1, SOD1 and CAT were enhanced following the addition treatment of SFN to BLM. Therefore, it was hypothesized that SFN may inhibit pulmonary fibrosis by activating the expression of Nrf2 and its downstream genes.
Figure 6.
Effects of SFN on the protein and gene expression of HO-1 and NQO1 in BLM-induced models. (A) Western blot analysis for HO-1 and NQO1 was used to investigate the effect of SFN on BLM induced models at days 7 and 28 post-treatment. BLM reduced the protein levels of HO-1 and NQO1 at days 7 and 28, and this was reversed by SFN treatment. (B) Quantification of mRNA expression levels of HO-1 and NQO1 in BLM induced lung tissues at day 7 and 28 post-treatment, as detected by reverse transcription-quantitative polymerase chain reaction. Data are presented as the mean ± standard deviation (n=6/group). #P<0.05 vs. control group; *P<0.05 vs. BLM group. BLM, bleomycin; SFN, sulforaphane; Ctrl, control; HO-1, heme oxygenase-1; NQO1, nicotinamide adenine dinucleotide phosphate quinone oxidoreductase.
Figure 7.
Effects of SFN on the protein and gene expressions of SOD1 and CAT in BLM-induced models. (A) Western blot analysis for SOD1 and CAT was used to investigate the effect of SFN on BLM-induced models at days 7 and 28 post-treatment. BLM reduced the protein levels of SOD1 and CAT at day 7 and 28, and this was reversed following SFN treatment. (B) Quantification of mRNA expression levels of SOD1 and CAT in BLM induced lung tissues at day 7 and 28 post-treatment, as detected by reverse transcription-quantitative polymerase chain reaction. Data are presented as the mean ± standard deviation (n=6/group). #P<0.05 vs. control group; *P<0.05 vs. BLM group. SOD1, superoxide dismutase 1; CAT, catalase; BLM, bleomycin; SFN, sulforaphane; Ctrl, control.
Discussion
Pulmonary fibrosis is a chronic disease of variable etiology, and oxidative stress has been implicated as an important cause in promoting its pathogenesis in humans and animals (22). Emerging evidence has indicated that BLM induces lung injury as a result of its ability to generate ROS and elevate oxidative burden by disturbing the antioxidant/oxidant balance in the lungs (10). Accordingly, in the present study, levels of oxidative stress and antioxidant enzymes were investigated. It was demonstrated that oxidative and nitrogen oxidative damage was increased and the activities of the antioxidant enzymes SOD1 and CAT decreased immediately following BLM injection compared with control group mice. Of note, SFN treatment alleviated BLM-induced oxidative burden and additionally increased the levels of antioxidant enzymes. This data supported the hypothesis that upregulation of antioxidative enzymes following SFN injection is responsible for the alleviation of pulmonary fibrosis, at least in part by reducing the oxidative damage. The present study demonstrated that SFN-induced Nrf2 expression in the lungs protected against BLM-mediated damage. This was supported by a significant reduction of lung lesions, including lung epithelial cell apoptosis and excessive infiltration of inflammatory cells, reduced BLM-induced thickness of alveolar septa, and significant collagen deposition in the interstitial areas. Furthermore, administration of SFN to BLM treated mice attenuated the expression of IL-1β, TNF-α, TGF-β and cleaved caspase-3.
A previous study demonstrated that Nrf2 expression was increased in a lung fibrosis model (23). However, the precise role of Nrf2 in a mouse fibrosis model was identified by Cho et al (24) using Nrf2 knockout mice. They used BLM to induce fibrosis in Nrf2 knockout and wild-type mice and demonstrated that compared to wild-type mice, Nrf2 knockout mice exhibited an increased susceptibility, including increased lung weight, inflammation, hydroxyproline content and fibrotic score (24). Another study observed that protein expression levels of the Nrf2 transcription factor was involved in circadian regulation in the lungs of C57BL/6 mice. The study observed a significantly higher fibrotic score when Nrf2 protein expression levels were reduced, even when BLM treatment was decreased. The Nrf2 rhythmic activity affected tissue susceptibility to BLM-induced lung fibrosis in a time-of-day-dependent manner (13). Walters et al (25) identified elevated protein and mRNA levels of Nrf2, and upregulation of NQO1 and HO-1 in a pulmonary fibrosis model. Consistent with this, the present study observed significantly increased mRNA and protein expression levels of Nrf2 at days 7 and 28 in a lung fibrosis model. However, the mRNA expression levels of the Nrf2-associated downstream target genes NQO1 and HO-1 were increased at day 7 following BLM administration. This high level of Nrf2 expression in the mouse fibrosis model appeared to be protective, because Nrf2 expression was rapidly upregulated in tissues in response to oxidative stress at an early stage; however, gradually decreased following chronic oxidative stress. The protective role of Nrf2 was additionally demonstrated in previous studies investigating other disease models (26,27). For instance, Nrf2 expression in the renal tissue of multiple low-dose streptozotocin-induced type1 diabetic mice was significantly increased at 3 months; however, not at 6 months (18).
A study by Artaud-Macari et al (28) used SNF to treat primary lung fibroblasts cultured from healthy and patients with idiopathic pulmonary fibrosis (IPF). They observed that Nrf2 activation increased antioxidant defenses and reversed the myofibroblastic differentiation in IPF patient-derived fibroblasts. Furthermore, SFN treatment was demonstrated to increase the expression of Nrf2 and antioxidants proteins, and decrease ROS levels and myofibroblastic dedifferentiation in cultured fibroblasts from healthy and IPF patients. In addition, SFN treatment inhibited TGF-β1-mediated pro-fibrotic deleterious effects in IPF and healthy fibroblasts; however, not in Nrf2 ablated fibroblasts. Another study demonstrated that pretreatment of mouse fibroblasts with SFN increased their resistance to subsequent treatment with paraquat or hydrogen peroxide in an Nrf2-dependent manner (29). SFN has additionally been reported to protect against diabetes-induced aorta damage, cardiomyopathy and nephropathy (18,30,31), and attenuate hepatic fibrosis (32), via upregulation and activation of Nrf2. In the present study, the data is consistent with the observations that SFN may act as a protective agent against BLM-induced oxidative stress by alleviating pulmonary lesions.
In conclusion, SFN may protect against BLM-induced pulmonary fibrosis in mice. Fibrosis-induced and age-matched control mice were administered with a daily dose of 0.5 mg/kg SFN, which prevented the progression of lung damage. Lung damage prevention was accompanied by significant upregulation of Nrf2 expression and its downstream target antioxidant genes NQO1, HO-1, SOD1 and CAT. These results suggested that lung fibrosis maybe prevented by SFN treatment, most likely via upregulation of Nrf2-mediated protein expression. Thus, the results from this study may facilitate the development of therapies for BLM-toxicity and pulmonary fibrosis.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81370162).
References
- 1.Todd NW, Luzina IG, Atamas SP. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair. 2012;5:11. doi: 10.1186/1755-1536-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez IE, Eickelberg O. New cellular and molecular mechanisms of lung injury and fibrosis in idiopathic pulmonary fibrosis. Lancet. 2012;380:680–688. doi: 10.1016/S0140-6736(12)61144-1. [DOI] [PubMed] [Google Scholar]
- 3.Shi K, Jiang J, Ma T, Xie J, Duan L, Chen R, Song P, Yu Z, Liu C, Zhu Q, Zheng J. Dexamethasone attenuates bleomycin-induced lung fibrosis in mice through TGF-β, Smad3 and JAK-STAT pathway. Int J Clin Exp Med. 2014;7:2645–2650. [PMC free article] [PubMed] [Google Scholar]
- 4.Barratt S, Millar A. Vascular remodelling in the pathogenesis of idiopathic pulmonary fibrosis. QJM. 2014;107:515–519. doi: 10.1093/qjmed/hcu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bocchino M, Agnese S, Fagone E, Svegliati S, Grieco D, Vancheri C, Gabrielli A, Sanduzzi A, Avvedimento EV. Reactive oxygen species are required for maintenance and differentiation of primary lung fibroblasts in idiopathic pulmonary fibrosis. PLoS One. 2010;5:e14003. doi: 10.1371/journal.pone.0014003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinnula VL, Myllärniemi M. Oxidant-antioxidant imbalance as a potential contributor to the progression of human pulmonary fibrosis. Antioxid Redox Signal. 2008;10:727–738. doi: 10.1089/ars.2007.1942. [DOI] [PubMed] [Google Scholar]
- 7.Liu RM, Vayalil PK, Ballinger C, Dickinson DA, Huang WT, Wang S, Kavanagh TJ, Matthews QL, Postlethwait EM. Transforming growth factor β suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Free Radic Biol Med. 2012;53:554–563. doi: 10.1016/j.freeradbiomed.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felton VM, Borok Z, Willis BC. N-acetylcysteine inhibits alveolar epithelial-mesenchymal transition. Am J Physiol Lung Cell Mol Physiol. 2009;297:L805–L812. doi: 10.1152/ajplung.00009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odajima N, Betsuyaku T, Nagai K, Moriyama C, Wang DH, Takigawa T, Ogino K, Nishimura M. The role of catalase in pulmonary fibrosis. Respir Res. 2010;11:183. doi: 10.1186/1465-9921-11-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: A possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sykiotis GP, Habeos IG, Samuelson AV, Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr Metab Care. 2011;14:41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, Sagami A, Taguchi K, Bechtold D, Loudon A, et al. The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes Dev. 2014;28:548–560. doi: 10.1101/gad.237081.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kikuchi N, Ishii Y, Morishima Y, Yageta Y, Haraguchi N, Itoh K, Yamamoto M, Hizawa N. Nrf2 protects against pulmonary fibrosis by regulating the lung oxidant level and Th1/Th2 balance. Respir Res. 2010;11:31. doi: 10.1186/1465-9921-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: Decisive role of Bax, Nrf2, NF-κB, Muc5ac, TNF-α and IL-1β. Chem Biol Interact. 2015;237:151–165. doi: 10.1016/j.cbi.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Mouratis MA, Aidinis V. Modeling pulmonary fibrosis with bleomycin. Curr Opin Pulm Med. 2011;17:355–361. doi: 10.1097/MCP.0b013e328349ac2b. [DOI] [PubMed] [Google Scholar]
- 17.Fahey JW, Talalay P. Antioxidant functions of sulforaphane: A potent inducer of Phase II detoxication enzymes. Food Chem Toxicol. 1999;37:973–979. doi: 10.1016/S0278-6915(99)00082-4. [DOI] [PubMed] [Google Scholar]
- 18.Cui W, Bai Y, Miao X, Luo P, Chen Q, Tan Y, Rane MJ, Miao L, Cai L. Prevention of diabetic nephropathy by sulforaphane: Possible role of Nrf2 upregulation and activation. Oxid Med Cell Longev. 2012;2012:821936. doi: 10.1155/2012/821936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noh JR, Kim YH, Hwang JH, Choi DH, Kim KS, Oh WK, Lee CH. Sulforaphane protects against acetaminophen-induced hepatotoxicity. Food Chem Toxicol. 2015;80:193–200. doi: 10.1016/j.fct.2015.03.020. [DOI] [PubMed] [Google Scholar]
- 20.Szapiel SV, Elson NA, Fulmer JD, Hunninghake GW, Crystal RG. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am Rev Respir Dis. 1979;120:893–899. doi: 10.1164/arrd.1979.120.4.893. [DOI] [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Cheresh P, Kim SJ, Tulasiram S, Kamp DW. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta. 2013;1832:1028–1040. doi: 10.1016/j.bbadis.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walters DM, Kleeberger SR. Mouse models of bleomycin-induced pulmonary fibrosis. Curr Protoc Pharmacol Chapter. 2008;5:Unit 5 46. doi: 10.1002/0471141755.ph0546s40. [DOI] [PubMed] [Google Scholar]
- 24.Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 25.Walters DM, Cho HY, Kleeberger SR. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: A potential role for Nrf2. Antioxid Redox Signal. 2008;10:321–332. doi: 10.1089/ars.2007.1901. [DOI] [PubMed] [Google Scholar]
- 26.Jiang X, Bai Y, Zhang Z, Xin Y, Cai L. Protection by sulforaphane from type 1 diabetes-induced testicular apoptosis is associated with the up-regulation of Nrf2 expression and function. Toxicol Appl Pharmacol. 2014;279:198–210. doi: 10.1016/j.taap.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Zhang Z, Sun W, Tan Y, Liu Y, Zheng Y, Liu Q, Cai L, Sun J. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid Med Cell Longev. 2014;2014:123963. doi: 10.1155/2014/123963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Artaud-Macari E, Goven D, Brayer S, Hamimi A, Besnard V, Marchal-Somme J, Ali ZE, Crestani B, Kerdine-Römer S, Boutten A, Bonay M. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2013;18:66–79. doi: 10.1089/ars.2011.4240. [DOI] [PubMed] [Google Scholar]
- 29.Higgins LG, Kelleher MO, Eggleston IM, Itoh K, Yamamoto M, Hayes JD. Transcription factor Nrf2 mediates an adaptive response to sulforaphane that protects fibroblasts in vitro against the cytotoxic effects of electrophiles, peroxides and redox-cycling agents. Toxicol Appl Pharmacol. 2009;237:267–280. doi: 10.1016/j.taap.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Miao X, Bai Y, Sun W, Cui W, Xin Y, Wang Y, Tan Y, Miao L, Fu Y, Su G, Cai L. Sulforaphane prevention of diabetes-induced aortic damage was associated with the up-regulation of Nrf2 and its down-stream antioxidants. Nutr Metab (Lond) 2012;9:84. doi: 10.1186/1743-7075-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bai Y, Cui W, Xin Y, Miao X, Barati MT, Zhang C, Chen Q, Tan Y, Cui T, Zheng Y, Cai L. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol. 2013;57:82–95. doi: 10.1016/j.yjmcc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Oh CJ, Kim JY, Min AK, Park KG, Harris RA, Kim HJ, Lee IK. Sulforaphane attenuates hepatic fibrosis via NF-E2-related factor 2-mediated inhibition of transforming growth factor-β/Smad signaling. Free Radic Biol Med. 2012;52:671–682. doi: 10.1016/j.freeradbiomed.2011.11.012. [DOI] [PubMed] [Google Scholar]