Abstract
Cockayne syndrome (CS) is a rare autosomal recessive disorder characterized principally by progressive growth failure, neurologic abnormality and premature aging. Mutations of excision repair cross-complementation group 6 (ERCC6) and ERCC8 are predominantly responsible for CS, of which mutation of ERCC6 accounts for approximately two thirds of cases. The current report describes two siblings with severe neurologic abnormality and premature aging. Whole exome sequencing identified two novel mutations in ERCC6 that had not been previously reported. One was a nonsense mutation at codon 612 in exon 9 (c.1834C>T, p.Arg612Ter), and the other a missense mutation at codon 975 in exon 16 (c.2923C>T, p.Arg975Trp). Cosegregation analysis revealed c.1834C>T was paternal and c.2923C>T was maternal. A healthy baby with no mutated alleles was delivered based on prenatal diagnosis performed by genetic testing of amniocytes for the causative mutation. The present study will enrich the clinical and genetic spectrum of CS in China and world wide, and provides more evidence for future genotype-phenotype studies.
Keywords: progeroid syndrome, cockayne syndrome B, molecular diagnosis, excision repair cross-complementation group 6, prenatal diagnosis
Introduction
Cockayne syndrome (CS) is a rare autosomal recessive multisystem disorder characterized by poor growth, neurological abnormality and a short life span (1). It occurs at a rate of about 2.7 per million births in western Europe (2). Typical features of CS include severe growth failure, mental retardation, microcephaly, cutaneous photosensitivity, dental decay and deep sunken eyes (3). A total of four subtypes have been described according to the time of onset and rate of the progression: Moderate type I CS, with the first symptoms appearing from the end of the first year of life and mortality occurring prior to the age of 20; early-onset and/or severe type II CS; late-onset type III CS; and the most severe cerebro-oculo-facio-skeletal (COFS) syndrome, with disease onset at the prenatal stage (1,4). These subtypes of CS share a large overlapping spectrum of severity.
CS is clinically diagnosed by reduced recovery of RNA and DNA synthesis in fibroblasts following ultraviolet (UV) irradiation (5), involving defective nucleotide-excision repair (NER) as the molecular pathogenesis (6). The transcription-coupled NER (TCR) system allows RNA polymerase II-blocking lesions to be rapidly removed from the transcribed strand of active genes. Mutations in the excision repair cross-complementation group 6 (ERCC6) and ERCC8 genes are primarily responsible for CS, of which a mutation in ERCC6, the CS type B (CSB) gene, accounts for two thirds of all cases (3). Although clinical symptoms are indistinguishable between CSA and CSB patients, CSA mutations have not been previously reported in severe CS subgroups.
Chinese patients with CS patients have rarely been reported, rendering it difficult for clinicians to make a clear diagnosis based on the clinical symptoms of CS. Based on whole exome sequencing (WES), the present study identified two novel mutations causing compound heterozygous ERCC6 in a Chinese family with two brothers suffering from premature aging. CSB was diagnosed according to clinical characteristics and genetic analysis. Depending on the diagnosis, this study successfully performed a molecular prenatal diagnosis of CSB on amniotic fluid sampling obtained from the family.
Materials and methods
Ethical approval
The present study was conducted in accordance with the guiding principles of the Declaration of Helsinki. Informed written consent for the collection of samples, images, tests and inclusion of data were obtained from the parents and grandparents of the patients. Written consents on behalf of the patients were obtained from their parents as the patients did not understand consent and lacked the ability to write. Use of all human materials used in this study were approved by the Institutional Review Ethics Board of Xijing Hospital, Fourth Military Medical University (Xi'an, China).
Genomic DNA isolation and WES
Genomic DNA was isolated from peripheral blood samples using standard methods. WES of genomic DNA from the two patients was subsequently performed to identify potential disease-causing gene mutations shared by the two patients. Sequencing was performed using a Genome Analyzer HiSeq2000 system (Illumina, Inc., San Diego, CA, USA) following enrichment of exonic and adjacent intronic sequences using a NimbleGen 44M capture platform (SeqCap EZ Human Exome Library v2.0; NimbleGen; Roche Sequencing, Madison, WI, USA).
ERCC6 sequencing
The mutation in ERCC6 identified by WES was verified by Sanger sequencing in two orientations of the polymerase chain reaction (PCR) product. The primers used are as follows: Forward, 5′-CAGCGTTTACTACTTGCCAG-3′ and reverse, 5′-CCACTTGGAAATCTCCCTT-3′ for c.1834C>T; forward, 5′-CTGGGAGTGACAGGTAGTGA-3′ and reverse, 5′-CAACTCACAGTAAGACATCTAAGC-3′ for c.2923C>T. Primers were designed to amplify other protein-coding exons of the ERCC6 gene and their flanking intronic sequences as previously reported (7). Direct sequencing of the PCR products was performed using an ABI PRISM® 310 Genetic Analyzer system (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The base-pair number of the mutation site was determined according to the GenBank mRNA reference sequence NM_000124.
Prenatal diagnosis
Fetal DNA was obtained from amniocytes by amniocentesis at 6 months of gestation and further used for genetic testing of the ERCC6 mutations by Sanger sequencing.
Results
Case presentation
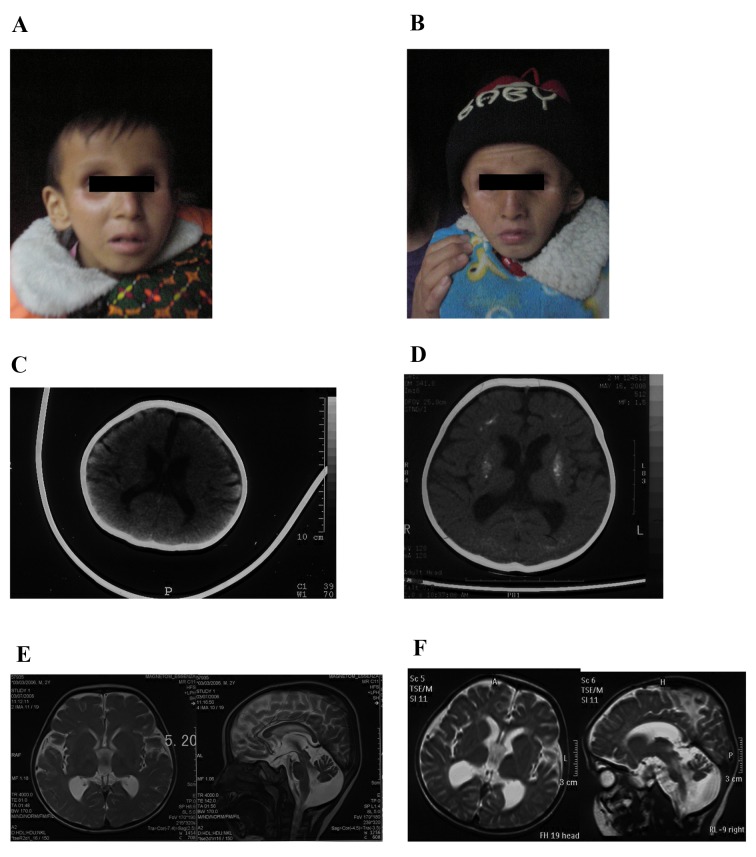
The two patients were brothers born at full-term by spontaneous vaginal delivery to non-consanguineous healthy Chinese parents. The birth weight and height were not available; however, they were typical according to the mother. The proband (III-2; Fig. 1A) was healthy during the neonatal period. Development was delayed and regression was observed. He was able to hold his head steady at 9 months, and roll over and sit erect without support when over 1 year old. He was able to speak certain simple words at ~18 months. However, at 2 years old, he lost the ability to speak and climb, and to sit or stand without support. He was able to walk landing on the toes, with support. Clinic features at 3 years old included cutaneous photosensitivity that was observed at ~6 months old and subsequently disappeared until 4 years old, bird-like features with deep-set eyes, a beaked nose, large and protruding ears, a small lower jaw, dedentition, low muscle tone of lower limbs, a head circumference of 43.5 cm, dysphagia, joint contracture and a pear-shaped chest. The proband additionally exhibited thinning hair, healthy hearing ability and cataracts. The proband died at the age of 9 years and 4 months.
Figure 1.
Clinical characteristics. Facial images of (A) the proband and (B) his elder brother. Computed tomography scans of the brain at (C) 6 months and (D) 3 years old. Magnetic resonance imaging of the brain at (E) 3 and (F) 4 years old.
The elder brother (Fig. 1B) had very similar symptoms as the proband; however, without cutaneous photosensitivity. He had earlier disease onset at 3 months old, and died at the age of 8 years and 5 months.
A computed tomography (CT) scan of the proband brain obtained at 6 months old (Fig. 1C) revealed a mildly enlarged ventricular system and sulci. Cerebral white matter and gray matter were blurred, and a mottled high-density signal was visible in the bilateral basal ganglia region due to mild calcification. No other abnormalities were indicated. A CT scan of the brain at 3 years old (Fig. 1D) revealed progressive deterioration of the disease, and diffuse and symmetric calcifications of the bilateral basal ganglia region, centrum semiovale, frontal and parietal lobes, and generally enlarged ventricular system and sulci. Magnetic resonance imaging (MRI) of the brain (Fig. 1E) demonstrated broadened cisterna magna, thinning corpus callosum, and signal contraction of white and gray matter behind the eyes. A follow-up MRI scan of the brain at four years old (Fig. 1F) revealed marked sulci and gyri enlargement, ventricular system expansion, cerebral and cerebellar atrophy, and severe calcifications of bilateral basal ganglia region.
WES analysis
A recessive file including 790 genes with 1081 homozygous or compound heterozygous variants shared by the two subjects was generated. Excluding the variants with a minor allele frequency >0.005 in dbSNP (ncbi.nlm.nih.gov/projects/SNP/) 126, 129 and 131 or 1,000 Genomes Project.15, and synonymous mutations, 58 candidate genes were selected. Of the candidate genes, 16 genes were recorded in the Human Ageing Genomic Resources (HAGR) database (genomics.senescence.info/), concerning function on gene transcription, signal pathways, cell cycles, development, or as nuclear receptor (Tables I and II). According to the description in HAGR, ERCC6 and transcription factor 3 (TCF3) were selected as the most likely candidates.
Table I.
Categorization of variants from whole exome sequencing.
| Category | Number |
|---|---|
| Detected variants | 3,619 |
| Detected genes | 2,564 |
| Shared variants | 1,081 |
| Shared genes | 790 |
| Candidate variants | 173 |
| Candidate genes | 58 |
Table II.
Age-associated candidate genes.
| Function | Genes |
|---|---|
| Transcription-associated (including nucleotide-excision repair proteins) | POU1F1, GTF2H2, SP1, STAT5A, ERCC6, TCF3, POLA1 |
| Signaling pathways | DLL3, STAT5A, PTK2 |
| Cell cycle | BUB3, CHEK2, POU1F1 |
| Nuclear receptor | NCOR1, NCOR2 |
| Development | VEGFA |
| Others | HSPD1 |
POU1F1, POU class 1 homeobox 1; GTF2H2, general transcription factor IIH subunit 2; SP1, Sp1 transcription factor; STAT5A, signal transducer and activator of transcription 5A; ERCC6, ERCC excision repair 6 chromatin remodeling factor; TCF3, transcription factor 3; POLA1, DNA polymerase α 1 catalytic subunit; delta like canonical Notch ligand 3; PTK2, protein tyrosine kinase 2; BUB3, BUB3 mitotic checkpoint protein; CHEK2, checkpoint kinase 2; NCOR, nuclear receptor corepressor; VEGFA, vascular endothelial growth factor A.
ERCC6 mutation inheritance in the family
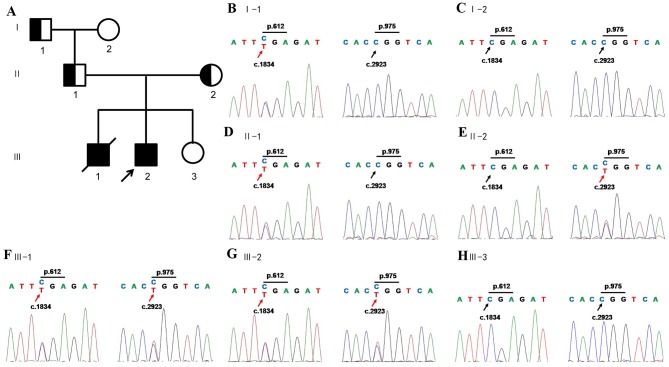
The variations of TCF3 were proved to be a false positive of WES by Sanger sequencing. ERCC6 contained compound heterozygous mutations according to WES results; one was a nonsense mutation in exon9 c.1834C>T, p.Arg612X, leading to a truncated protein, and the other was a missense mutation in exon16, c.2923C>T, p.Arg975Trp, causing amino acid transition of arginine to tryptophan. The compound heterozygous mutations were confirmed by Sanger sequencing of amplicons of patients. By the following co-segregation of the two ERCC6 alleles in the pedigree the present study demonstrated that the c.1834C>T allele was paternal, and the c.2923C>T allele was maternal (Fig. 2). Sequencing of other exons was additionally performed and no other variations were detected (data not shown).
Figure 2.
ERCC6 mutation inheritance in the family. (A) The pedigree of the family. Squares, males; circles, females; half-shaded shapes, carrier; fully shaded shape, affected individual. The black arrow indicates the proband. ERCC6 mutations in (B) I-1, (C) I-2, (D) II-1, (E) II-2, (F) III-1, (G) III-2 and (H) III-3.
Structure-function correlations of the ERCC6 mutation
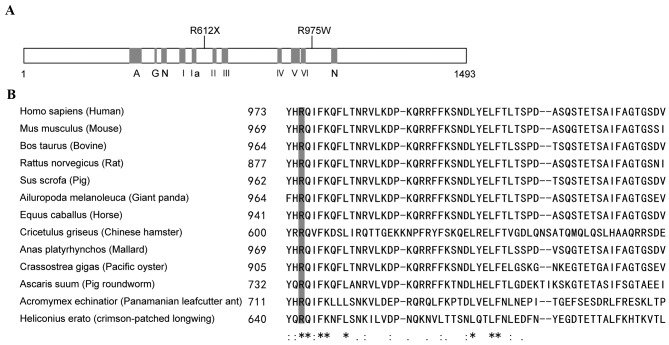
ERCC6 encodes a 1493-amino acid protein which contains seven helicase-like motifs that are divided into a helicase ATP-binding domain and a helicase C-terminal domain. The nonsense mutation, p.R612X, caused protein truncation at motif II (Fig. 3A). The other mutation, an amino acid substitution of Arg with Trp at codon 975, which was highly conserved in mammals (Fig. 3A and B), was characterized as ‘damaging’ using SIFT (sift.jcvi.org/) and Polyphen2 (http://genetics.bwh.harvard.edu/pph2/) online prediction tools.
Figure 3.
Linear map and conservation of the mutations in ERCC6. (A) Linear map of the mutations in ERCC6. (B) Multiple sequence alignment of ERCC6 among homologous genes in mammals, reptile, nematode, oyster and insects demonstrating evolutionary conservation of residue 975 arginine. A, acidic domain; I–VI, helicase motifs; N, nuclear localization signal.
ERCC6 prenatal diagnosis
Based on the molecular characterization of the probands, a prenatal diagnosis on a 6-month old fetus of the family was performed. Analysis of ERCC6 exons 9 and 16 revealed that the fetus did not inherit any of the mutations present in the proband (Fig. 2). A follow-up study of the baby 2 years after birth did not reveal any CS symptoms.
Discussion
CS may be diagnosed by clinical characterization and in vitro assays on fibroblasts by experienced clinicians. Chinese patients with CS have rarely been reported before; therefore, the final clinical diagnosis for our cases by clinicians was progeroid syndrome. Thus, WES or targeted approaches by next-generation sequencing for progeroid syndrome may be effective to make a clear genetic diagnosis. The present study selected WES in consideration of time and cost. The results demonstrated that WES was an effective method of genetic testing case, and may be applied to other undiagnosed inherited diseases.
The criteria for CS diagnosis was initially defined by Nance and Berry in 1992 (1). Developmental delay, growth failure and microcephaly were the mandatory criteria, with at least three out of the five minor criteria: Cutaneous photosensitivity, pigmentary retinopathy and/or cataracts, sensorineural hearing loss, dental caries and cachectic dwarfism (1). Recently, certain modifications were proposed to improve the specificity of criteria: Progressive courses of growth failure, microcephaly and sensorineural hearing loss were specifically defined, enamel hypoplasia was proposed to replace dental caries, and cachectic dwarfism was replaced by enophthalmia (distinctive facial features) (3). Brain imaging allows for a more reliable diagnosis of CS: White matter hypomyelination, atrophy of the cortex and cerebellum, and bilateral calcifications of the putamen. Patients in the present study fulfilled these clinical diagnostic criteria. In addition, the patients exhibited other CS characteristics; cutaneous photosensitivity was initially observed at disease onset and lasted ~4 years, and language ability was lost during disease progression. However, hypodontia, a type of hearing loss, which is considered a constant feature in patients with CS, was not observed at 3 years old. Brain imaging by CT and MRI scanning of the proband revealed the abnormalities mentioned above. These were detectable at the disease onset, and progressively worsened over 2 years. Based on these phenotypes, type II CS was suggested as the diagnosis for these patients. Previously, related or non-related patients with the same mutations have been reported. These patients often exhibit characteristic heterogeneities, including cataracts and deafness (4,8,9). The siblings in the present report additionally had varying degrees of cutaneous photosensitivity, suggesting that certain reported characteristics are not inevitable in CS. Further investigation of the patients carrying the same pathogenic mutations will facilitate improvement of the criteria for CS diagnosis.
ERCC6, or CSB, is a component of the NER pathway and primarily causes type II CS. There are numerous other CS subtypes and NER dysfunction diseases involving ERCC6: COFS1 (MIM# 214150) (10), UV-sensitive syndrome (UVSS) 1 (MIM# 600630) (11) and De Sanctis-Cacchione syndrome (MIM# 609413) (12). However, no clear phenotype-genotype correlations have been reported. There have been >80 different mutations reported in the CSB gene, with almost all types of mutations distributed along the whole gene sequence, including short insertions and deletions, nonsense, missense, splice and promoter mutations, and chromosomal microdeletions including CSB (4,13–16). Of these mutations, nonsense and splice mutations causing truncated proteins account for a large proportion, and 12 missense mutations have been reported (4,16). Previous studies have investigated CSB-piggyBac3 (PGBD3) fusion protein interactions with functional CSB on TCR, to elucidate genotype-phenotype correlations (4,17–19). It has been proposed that CSB-PGBD3, consisting of the first five exons of CSB and the PGBD3 transposon, may serve a deleterious role in the absence of functional CSB and trigger the classical CS phenotype. As a result, mutations downstream of intron 5 may cause CS whereas mutations upstream of intron 5 may cause milder forms of CS or UVSS (4,17). This was consistent with the biallelic mutations [NM_000124.2: Exon9 c.1834C>T (p.Arg 612X), and exon16 c.2923C>T (p. Arg975Tyr)] of the siblings in the present study. Previous reports of CS patients additionally support this hypothesis (13–16,20,21). However, certain patients do not follow this paradigm (4), suggesting that the mutation spectrum is further expanded; thus, additional evidence on correlations of protein expression levels and disease severity is required. Furthermore, structure-function correlations require investigation, as biallelic missense mutations cause severe CS (4,16).
Prenatal diagnosis for CS has been performed using various methods (9). In the present study, sequencing of the ERCC6 amplicons for the causative mutations from DNA of amniocytes was performed; the fetus did not inherit pathogenic alleles from the parents. Follow-up studies for the baby over 2 years additionally confirmed the prenatal diagnosis. Although tetramer assays of cultured amniocytes are the primary method for CS clinical confirmation, in a family with known mutations, direct testing for the causative mutations will be efficient and reliable.
In conclusion, CS is a rare multisystem disorder that encompasses a wide spectrum of clinical phenotypes, from the most severe prenatal subtype to the mildest late-onset subtype, or UVSS. To date, ~130 CS patients have been genetically confirmed and reported in the literature (4,13–16). The present study reported two novel causative mutations on ERCC6 loci, and the clinical characteristics are described. These results add to clinical and molecular data for elucidating genotype-phenotype correlations in CS; however, further investigations are required.
Acknowledgements
The authors would like to thank Professor J. Fan (Fourth Military Medical University, Xi'an, China) for editing this manuscript. The present study was supported by the China Postdoctoral Science Foundation (grant no. 2014M562545) and the Key Innovation Project of Shaanxi Province (grant no. 2013FWPT-06).
Glossary
Abbreviations
- CS
cockayne syndrome
- ERCC
excision repair cross-complementation group
- COFS
cerebro-oculo-facio-skeletal
- NER
nucleotide-excision repair
- WES
whole exome sequencing
- TCR
transcription-coupled nucleotide-excision repair
- PCR
polymerase chain reaction
References
- 1.Nance MA, Berry SA. Cockayne syndrome: Review of 140 cases. Am J Med Genet. 1992;42:68–84. doi: 10.1002/ajmg.1320420115. [DOI] [PubMed] [Google Scholar]
- 2.Kleijer WJ, Laugel V, Berneburg M, Nardo T, Fawcett H, Gratchev A, Jaspers NG, Sarasin A, Stefanini M, Lehmann AR. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst) 2008;7:744–750. doi: 10.1016/j.dnarep.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Laugel V. Cockayne syndrome: The expanding clinical and mutational spectrum. Mech Ageing Dev. 2013;134:161–170. doi: 10.1016/j.mad.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Laugel V, Dalloz C, Durand M, Sauvanaud F, Kristensen U, Vincent MC, Pasquier L, Odent S, Cormier-Daire V, Gener B, et al. Mutation update for the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. Hum Mutat. 2010;31:113–126. doi: 10.1002/humu.21154. [DOI] [PubMed] [Google Scholar]
- 5.Rapin I, Lindenbaum Y, Dickson DW, Kraemer KH, Robbins JH. Cockayne syndrome and xeroderma pigmentosum. Neurology. 2000;55:1442–1449. doi: 10.1212/WNL.55.10.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cleaver JE, Lam ET, Revet I. Disorders of nucleotide excision repair: The genetic and molecular basis of heterogeneity. Nat Rev Genet. 2009;10:756–768. doi: 10.1038/nrg2663. [DOI] [PubMed] [Google Scholar]
- 7.Laugel V, Dalloz C, Stary A, Cormier-Daire V, Desguerre I, Renouil M, Fourmaintraux A, Velez-Cruz R, Egly JM, Sarasin A, Dollfus H. Deletion of 5′ sequences of the CSB gene provides insight into the pathophysiology of Cockayne syndrome. Eur J Hum Genet. 2008;16:320–327. doi: 10.1038/sj.ejhg.5201991. [DOI] [PubMed] [Google Scholar]
- 8.Colella S, Nardo T, Mallery D, Borrone C, Ricci R, Ruffa G, Lehmann AR, Stefanini M. Alterations in the CSB gene in three Italian patients with the severe form of Cockayne syndrome (CS) but without clinical photosensitivity. Hum Mol Genet. 1999;8:935–941. doi: 10.1093/hmg/8.5.935. [DOI] [PubMed] [Google Scholar]
- 9.Falik-Zaccai TC, Laskar M, Kfir N, Nasser W, Slor H, Khayat M. Cockayne syndrome type II in a Druze isolate in Northern Israel in association with an insertion mutation inERCC6. Am J Med Genet A. 2008;146A:1423–1429. doi: 10.1002/ajmg.a.32309. [DOI] [PubMed] [Google Scholar]
- 10.Jaakkola E, Mustonen A, Olsen P, Miettinen S, Savuoja T, Raams A, Jaspers NG, Shao H, Wu BL, Ignatius J. ERCC6 founder mutation identified in Finnish patients with COFS syndrome. Clin Genet. 2010;78:541–547. doi: 10.1111/j.1399-0004.2010.01424.x. [DOI] [PubMed] [Google Scholar]
- 11.Horibata K, Iwamoto Y, Kuraoka I, Jaspers NG, Kurimasa A, Oshimura M, Ichihashi M, Tanaka K. Complete absence of Cockayne syndrome group B gene product gives rise to UV-sensitive syndrome but not Cockayne syndrome; Proc Natl Acad Sci USA; 2004; pp. 15410–15415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colella S, Nardo T, Botta E, Lehmann AR, Stefanini M. Identical mutations in the CSB gene associated with either Cockayne syndrome or the DeSanctis-cacchione variant of xeroderma pigmentosum. Hum Mol Genet. 2000;9:1171–1175. doi: 10.1093/hmg/9.8.1171. [DOI] [PubMed] [Google Scholar]
- 13.Ghai SJ, Shago M, Shroff M, Yoon G. Cockayne syndrome caused by paternally inherited 5Mb deletion of 10q11.2 and a frameshift mutation of ERCC6. Eur J Med Genet. 2011;54:272–276. doi: 10.1016/j.ejmg.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Gao J, Ye J, Gong Z, Gu X. Maternal origin of a de novo microdeletion spanning the ERCC6 gene in a classic form of the Cockayne syndrome. Eur J Med Genet. 2011;54:e389–e393. doi: 10.1016/j.ejmg.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Xin B, Wang H. Identification of two novel ERCC6 mutations in old order amish with cockayne syndrome. Mol Syndromol. 2013;3:288–290. doi: 10.1159/000345924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu S, Chen L, Ye L, Fei L, Tang W, Tian Y, Geng Q, Yi X, Xie J. Identification of two missense mutations of ERCC6 in three Chinese sisters with Cockayne syndrome by whole exome sequencing. PLoS One. 2014;9:e113914. doi: 10.1371/journal.pone.0113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman JC, Bailey AD, Fan HY, Pavelitz T, Weiner AM. An abundant evolutionarily conserved CSB-PiggyBac fusion protein expressed in Cockayne syndrome. PLoS Genet. 2008;4:e1000031. doi: 10.1371/journal.pgen.1000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey AD, Gray LT, Pavelitz T, Newman JC, Horibata K, Tanaka K, Weiner AM. The conserved Cockayne syndrome B-piggyBac fusion protein (CSB-PGBD3) affects DNA repair and induces both interferon-like and innate antiviral responses in CSB-null cells. DNA Repair (Amst) 2012;11:488–501. doi: 10.1016/j.dnarep.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner AM, Gray LT. What role (if any) does the highly conserved CSB-PGBD3 fusion protein play in Cockayne syndrome? Mech Ageing Dev. 2013;134:225–233. doi: 10.1016/j.mad.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xin B, Wang H. Identification of two novel ERCC6 mutations in old order amish with cockayne syndrome. Mol Syndromol. 2013;3:288–290. doi: 10.1159/000345924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shehata L, Simeonov DR, Raams A, Wolfe L, Vanderver A, Li X, Huang Y, Garner S, Boerkoel CF, Thurm A, et al. ERCC6 dysfunction presenting as progressive neurological decline with brain hypomyelination. Am J Med Genet A. 2014;164:2892–2900. doi: 10.1002/ajmg.a.36709. [DOI] [PMC free article] [PubMed] [Google Scholar]