Abstract
Myocardial ischemia/reperfusion (I/R) injury is a major pathological process in coronary heart disease and cardiac surgery, and is associated with aberrant microRNA (miR) expression. Previous studies have demonstrated that inhibition of miR-15a expression may ameliorate I/R-induced myocardial injury. In the present study, the potential role and underlying mechanism of miR-15a in hypoxia/reoxygenation-induced apoptosis of cardiomyocytes was investigated. Myocardial I/R was simulated in cultured H9c2 cells by 24 h hypoxia followed by 24 h reoxygenation. Using recombinant lentivirus vectors, the inhibition of miR-15a was indicated to significantly reduce cardiomyocyte apoptosis and release of lactate dehydrogenase and malondialdehyde. Conversely, upregulated miR-15a expression was pro-apoptotic. Mothers against decapentaplegic homolog 7 (SMAD7) was identified by bioinformatics analysis as a potential target of miR-15a. Luciferase reporter assays and western blotting for endogenous SMAD7 protein indicated that miR-15a inhibited SMAD7 expression via its 3′-untranslated region. Nuclear levels of nuclear factor-κB (NF-κB) p65 were increased by miR-15a expression and decreased by miR-15a inhibition, which is consistent with the possibility that the inhibition of SMAD7 by miR-15a results in NF-κB activation. These findings suggested that the therapeutic effects of miR-15a inhibition on I/R injury may potentially be explained by its ability to release SMAD-7-dependent NF-κB inhibition. This may provide evidence for miR-15a as a potential therapeutic target for the treatment of cardiac I/R injury.
Keywords: microRNA-15a, hypoxia/reoxygenation, mothers against decapentaplegic homolog 7
Introduction
Coronary heart disease, particularly acute myocardial infarction, is one of the leading causes of human morbidity and mortality. Myocardial ischemia/reperfusion (I/R) has been identified as a major pathological process that contributes to further damage to myocardial tissues in patients with coronary heart disease, including patients who require extracorporeal circulation surgery, off-pump coronary artery bypass surgery or percutaneous coronary intervention (1). The underlying mechanisms of myocardial I/R injury have been demonstrated to involve intracellular overload of calcium, excessive production of free radicals, impaired function of mitochondria and the endoplasmic reticulum, and exaggerated inflammation (2,3). All of these mechanisms induce cardiomyocyte apoptosis and may eventually lead to the development of heart failure (4).
MicroRNAs (miRNAs/miRs) are a family of single-stranded, small non-protein-coding RNAs of ~22 nt that regulate gene expression post-transcriptionally by degrading or inhibiting target miRNAs (5,6). Many previous studies indicated that miRNAs serve significant roles in cardiovascular diseases, including myocardial I/R injury (7), myocardial hypertrophy (8), arrhythmia (9) and heart failure (10). For example, miR-499 inhibits cardiomyocyte apoptosis by suppressing calcineurin-mediated dephosphorylation of dynamin-related protein 1 via targeting of α-and β-isoforms of the calcineurin catalytic subunit (11), whereas overexpression of miR-320 increases the infarction size in mouse hearts and enhances cardiomyocyte apoptosis by targeting heat shock protein 20 (12). Additionally, miR-214 is upregulated during I/R injury, and knockdown of miR-214 decreases cardiac contractility and increases cardiomyocyte apoptosis in response to I/R injury (13). The cardioprotective effect of miR-214 is attributed to the suppression of sodium/calcium exchanger 1 and inhibition of the downstream Ca2+ signaling pathway (13). Furthermore, transduction of an miR-125b-expressing lentivirus into mouse hearts attenuates I/R-induced apoptosis by decreasing the levels of p53, B-cell lymphoma 2 (Bcl2)-antagonist/killer-1, Bcl2-associated X protein, Fas and tumor necrosis factor receptor-associated factor 6 (14).
In addition, miRNAs have well-established roles in cancer. miR-15a, which is known to suppress proliferation and increase the apoptosis of tumor cells, is involved in the pathogenesis of many types of cancer, including pituitary tumors, colorectal cancers and non-small cell lung cancer (15–17). In human liver cells, miR-15a has been demonstrated to target mothers against decapentaplegic homolog 7 (SMAD7) (18). A microarray assay additionally indicated that miR-15a is upregulated in an animal model of I/R injury (19). However, the detailed mechanisms of how miR-15a may be involved in cardiac I/R injury are not well established.
SMAD proteins were initially identified as key downstream effectors in the transforming growth factor-β1 (TGF-β1) signaling pathway (20). For example, upon phosphorylation by TGF-β type I receptor, SMAD2/SMAD4 translocates to the nucleus to promote downstream gene transcription (21). However, SMAD7 serves as an inhibitory molecule by blocking TGF-β1-induced SMAD activation and interfering with the interactions between other SMAD proteins and receptors (21). Furthermore, SMAD7 blocks nuclear factor-κB (NF-κB) activation by increasing inhibitor of NF-κB (IκB) production (22). A recent study indicated that upregulated levels of SMAD7 decreases NF-κB protein levels and attenuates hypoxia/reoxygenation (H/R)-induced cardiomyocyte apoptosis (23).
In the present study, the expression of miR-15a and its role in myocardial injury and apoptosis induced by H/R in cultured H9c2 rat cardiomyocytes were examined. The results suggested that miR-15a induces H/R-induced apoptosis of cardiomyocytes by targeting SMAD7; therefore, inhibition of miR-15a may have therapeutic benefits.
Materials and methods
Cell culture
H9c2 rat cardiomyoblasts (Cell Bank of the Chinese Academy of Sciences, Shanghai, China) were cultured at 37°C in 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Biowest Europe, Nuaillé, France). The cells were plated at a density of 1.5×105 cells/cm2 in 6-well plates.
H/R model
Cells were subjected to hypoxia for 24 h at 37°C in 1% O2, 94% N2 and 5% CO2 using a modular incubator (Model 3131; Forma; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Subsequently, reoxygenation (5% CO2; 37°C) was performed for 24 h. Cells under normoxia served as a control. Ischemia and reperfusion were simulated using DMEM containing 1 or 10% FBS, respectively.
miRNA transfection
Transfection was performed using a lentivirus (Novobio Scientific, Inc., Shanghai, China). The sense, antisense and negative control duplex oligonucleotides of miR-15a were recombined using a BLOCK-iTTM Pol II miR RNAi Expression Vector kit (Invitrogen; Thermo Fisher Scientific, Inc.). The lentivirus expression vector additionally expressed green fluorescent protein for assessment of infection efficiency. Recombinant lentiviruses were packaged and produced in 293T cells (Novobio Scientific, Inc.).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA, including miRNA, was extracted using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Reverse transcriptase and oligo (dT) primers were employed to synthesize cDNA from 5 µg total RNA in a volume of 12 µl, and then the reaction mixture was finally adjusted to 20 µl for RT-qPCR. RT-qPCR analysis was performed on a Rotor-Gene 3000 real-time cDNA detection system (Qiagen, Inc., Valencia, CA, USA) to examine the expression of miR-15a with SYBR®−Green (Invitrogen; Thermo Fisher Scientific, Inc.). qPCR was performed under the following conditions: Initiation at 95°C for 2 min, followed by 40 cycles of denaturation at 95°C for 10 sec; annealing at 60°C for 30 sec; and extension at 70°C for 45 sec. The quantitation cycle (Cq) value was set within the exponential phase of PCR and normalized against a U6 housekeeping gene (24). The following primer sequences were used: rno-miR-15a-5p-RT, CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAAACCATT (reverse primer); rno-miR-15a-5p-F, AGCTGGGTAGCAGCACATAATGGTTT (forward primer).
Western blot analysis
Cell lysates were extracted by centrifugation at 12,000 × g for 15 min at 4°C. Whole lysates isolated from cultured H9c2 cells were prepared in ice cold lysis buffer (BioTeke Corporation, Beijing, China) with protease inhibitors (Pierce; Thermo Fisher Scientific, Inc.) Protein concentration was measured by performing a bicinchoninic assay (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. Subsequently, 20 mg/well protein was separated by 10% SDS-PAGE and transferred to polyvinylidene fluoride membranes. The membranes were blocked in 5% non-fat milk and Tris-buffered saline with 0.05% Tween-20 at room temperature for 1 h prior to incubation with primary antibodies against SMAD7 (cat. no. bs-0566R; 1:1,000; BIOSS, Beijing, China), NF-κB (cat. no. AN365-1; 1:1,000; Beyotime Institute of Biotechnology, Haimen, China), GAPDH (cat. no. ab-9485; 1:2,500; Abcam, Cambridge, UK) or lamin B1 (cat. no. ab-16048; 1:1,000; Abcam) at 4°C overnight, followed by incubation with the horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (catalog no. ab-6721; 1:1,000; Abcam) for 1 h at room temperature. Bands were visualized using an Enhanced Chemiluminescence kit (EMD Millipore, Billerica, MA, USA) according to the manufacturer's instructions, with GAPDH as the control. Nuclear NF-κB was normalized to lamin B1 as a loading control. Experiments were repeated three times and the band intensity was quantified using Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Measurement of cell injury and apoptosis
Cell injury was assessed by measuring the concentrations of lactate dehydrogenase (LDH) and malonaldehyde (MDA) in the culture medium using detection kits LDH Cytotoxicity Assay and Lipid Peroxidation MDA Assay kits (Beyotime Institute of Biotechnology). Cell apoptosis was detected by Annexin V-phycoerythrin (PE)/7-aminoactinomycin D (AAD) dual staining. Briefly, following washing twice with PBS, the cells were resuspended in binding buffer. The cells were stained using an Annexin V-PE/7-AAD apoptosis kit (Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) according to the manufacturer's instructions, and then examined using a fluorescence microscope (IX51; Olympus Corporation, Tokyo, Japan). Undyed cells, cells under H/R conditions and cells transfected with lentivirus PDS019 under H/R conditions were used as a blank control, positive control and negative control, respectively. Subsequently, the cells were assayed with a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Luciferase assays
Putative miR-15a target was predicted by bioinformatics analysis using MiRanda (http://www.microrna.org), miRDB (http://www.mirdb.org/miRDB/), miRwalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/) and TargetScan (http://www.targetscan.org). Following transfection of miR-15a or anti-miR-15a into H9c2 cells, both the CL358-SMAD7-3′UTR-WT (wild-type) and CL440-SMAD7-3′UTR-MU (mutant type) vector expressing firefly luciferase, and the pRL-cmv vector expressing Renilla luciferase (GeneChem, Co., Ltd., Shanghai, China) were co-transfected using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Luciferase activity was measured with the Dual Luciferase Reporter Assay system (Promega Corporation, Madison, WI, USA) and Renilla luciferase activity served as an internal control.
Statistics and data analysis
Data are presented as the mean ± standard deviation. Student's t-test and one-way analysis of variance followed by Tukey post hoc tests were used for statistical analysis. P<0.05 was considered to indicate a statistically significant difference. SPSS software (version 19.0; IBM SPSS, Armonk, MY, USA) was employed in statistical analyses. All experiments were performed at least three times.
Results
Upregulation of miR-15a expression in response to H/R in H9c2 cells
To identify the potential role of miR-15a in H/R, myocardial I/R injury was simulated by exposing H9c2 rat cardiomyoblasts to 24 h hypoxia, followed by 24 h reoxygenation. The expression of miR-15a was subsequently measured by RT-qPCR. The results demonstrated that miR-15a expression was significantly increased compared with the normoxia group (Fig. 1). These results are consistent with microarray assays indicating that miR-15a is upregulated in an animal model of I/R injury (17). On the basis of these findings, it was hypothesized that miR-15a may serve a role in the H/R response in H9c2 cells.
Figure 1.
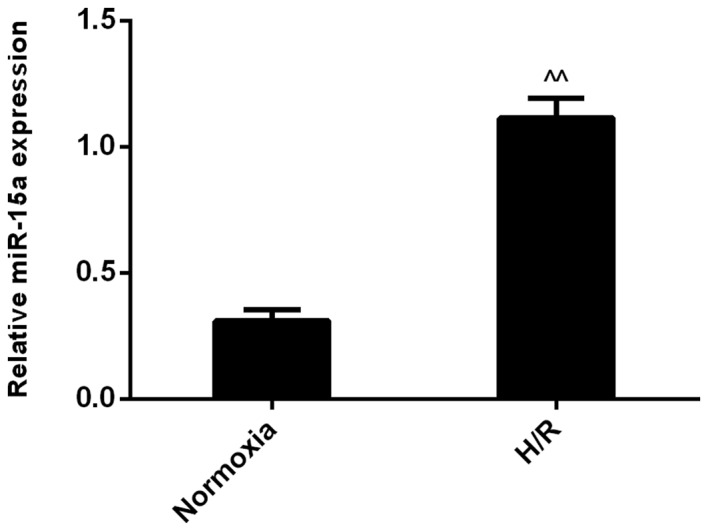
H/R modulates the expression level of miR-15a in H9c2 cells. Reverse transcription-quantitative polymerase chain reaction analysis indicated that miR-15a expression in H9c2 cells was upregulated in the H/R group, when compared with the control (normoxia) group. Results were normalized to U6 and expressed as the fold change relative to the control. Data are presented as the mean ± standard deviation from three independent experiments. ^^P<0.01 vs. normoxia. H/R, hypoxia/reoxygenation; miR-15a, microRNA-15a.
Inhibition of miR-15a protects H9c2 cells and reduces H/R-induced cell apoptosis
To directly determine whether miR-15a is involved in the H/R response in H9c2 cells, a lentivirus expressing miR-15a and anti-miR-15a was prepared. Transduction with the lentivirus expressing miR-15a significantly increased miR-15a levels in H9c2 cells under H/R conditions. Conversely, anti-miR-15a markedly decreased the levels of miR-15a (Fig. 2A).
Figure 2.
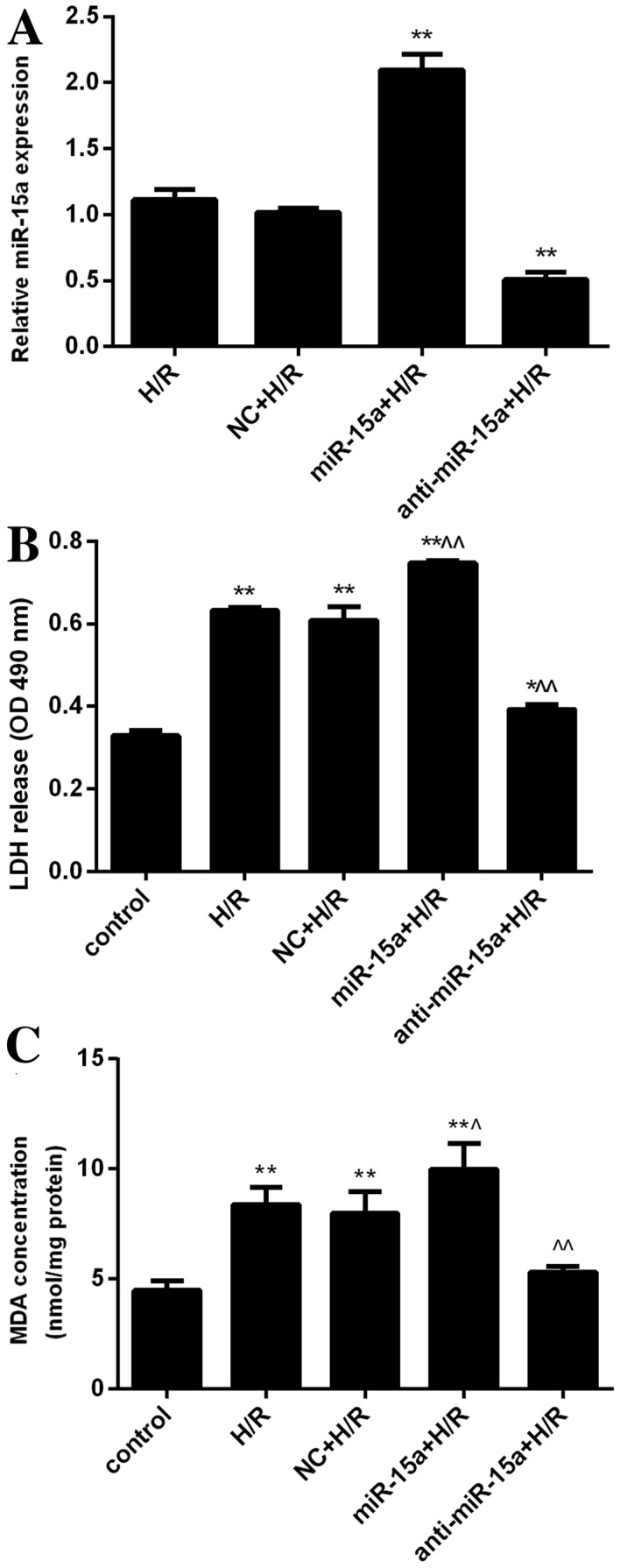
Inhibition of miR-15a is cytoprotective. (A) H9c2 cells were transfected with lentivirus expressing miR-15a or anti-miR-15a. The effect of overexpression and inhibition of miR-15a under H/R was examined. Results were normalized to U6 and expressed as the fold change relative to the control (non-transfected H/R-treated cells). **P<0.01 vs. H/R. (B) LDH release of control H9c2 cells, cells exposed to H/R, and cells exposed to H/R following transfection with a control lentivirus (NC+H/R), a miR-15a-expressing lentivirus (miR-15a+H/R) or an anti-miR-15a-expressing virus (anti-miR-15a+H/R). *P<0.05 and **P<0.01 vs. control; ^P<0.05 and ^^P<0.01 vs. H/R. (C) MDA release of the cells from panel B. *P<0.05 and **P<0.01 vs. control; ^P<0.05 and ^^P<0.01 vs. H/R. Data are presented as the mean ± standard deviation from three independent experiments. H/R, hypoxia/reoxygenation. H/R, hypoxia/reoxygenation; miR-15a, microRNA-15a; LDH, lactate dehydrogenase; OD, optical density; NC, negative control; MDA, malondialdehyde.
To determine the effects of modulating miR-15 levels on H9c2 cell growth, the effects of the lentiviruses on LDH and MDA release into the culture media were assessed. H/R significantly increased LDH release (OD, 0.632±0.007 vs. 0.329±0.012 in normoxia condition; P<0.01; Fig. 2B). Furthermore, overexpression of miR-15a further increased LDH levels induced by H/R (OD, 0.747±0.007; P<0.01 vs. the H/R group), whereas anti-miR-15a significantly decreased LDH levels induced by H/R (OD, 0.393±0.012; P<0.01 vs. the H/R group). In addition, H/R treatment raised MDA levels (8.381±0.771 vs. 4.486±0.437 nmol/mg protein under normoxic conditions; P<0.01). MDA release was further increased by miR-15a overexpression (9.979±1.190 nmol/mg protein; P<0.05 vs. the H/R group), but was significantly suppressed by anti-miR-15a expression (5.324±0.263 nmol/mg protein, P<0.01 vs. the H/R group; Fig. 2C). These results suggested that miR-15a expression inversely correlates with H9c2 cell growth under conditions of H/R, and that inhibition of miR-15a has protective effects against H/R-induced cellular damage.
To further determine whether the regulation of cell growth by miR-15a occurs at the level of cell apoptosis, Annexin V-PE/7-AAD dual staining was performed. As expected, apoptosis levels were greater in H/R-treated cells than in control normoxia H9c2 cells (8.150±0.070% vs. 2.72±0.093%; P<0.01; Fig. 3A and B). Furthermore, miR-15a expression increased the apoptosis rate (13.250±0.105%; P<0.01 vs. the H/R group), whereas anti-miR-15a expression decreased the apoptosis rate (5.050±0.407%; P<0.01 vs. the H/R group). Taken together, these results indicated that inhibition of miR-15a protects H9c2 cells against H/R-induced apoptosis.
Figure 3.
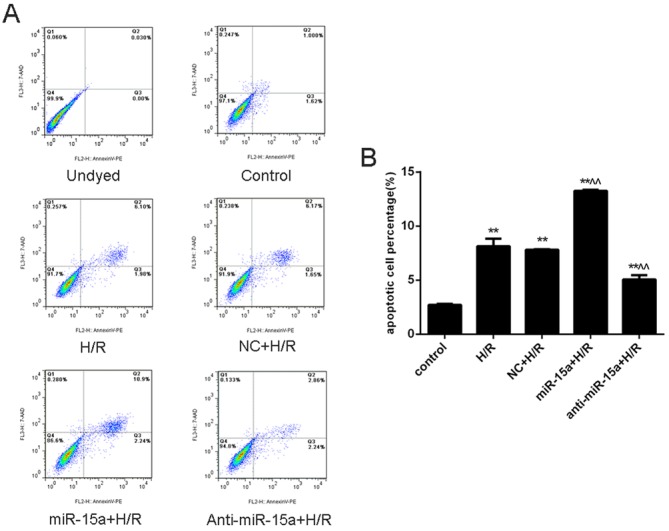
Effect of miR-15a on H/R-induced cardiomyocyte apoptosis. (A) Cell apoptosis was detected using Annexin V-PE/7-AAD dual staining. (B) Apoptotic cell percentage. Data are presented as the mean ± standard deviation from three independent experiments. *P<0.05 and **P<0.01 vs. control; ^P<0.05 and ^^P<0.01 vs. H/R. miR-15a, microRNA-15a; H/R, hypoxia/reoxygenation; NC, negative control; PE, phycoerythrin; 7-AAD, 7-aminoactinomycin D.
SMAD7 is a target of miR-15a whose regulation correlates inversely with NF-κB translocation
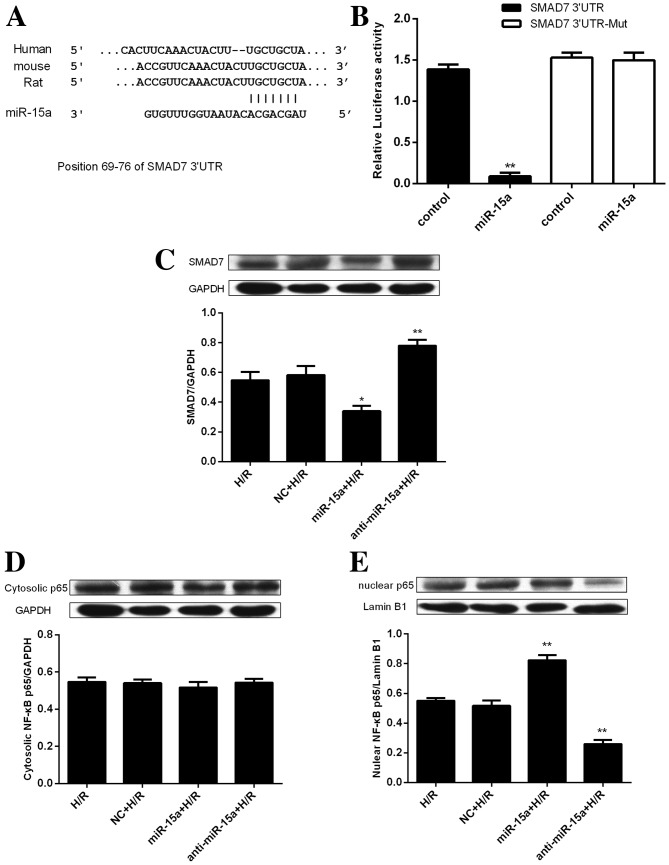
To further elucidate the mechanism of miR-15a in myocardial I/R injury, bioinformatics analysis was performed using MiRanda (http://www.microrna.org), miRDB (http://www.mirbd.org/miRDB/), miRwalk (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/) and TargetScan (http://www.targetscan.org). The results demonstrated that the SMAD7 3′-untranslated region (UTR) has a conserved binding site for miR-15a (Fig. 4A). To confirm that SMAD7 is a target of miR-15a in H9c2 cells, a firefly luciferase reporter plasmid was constructed by cloning the 3′-UTR SMAD7 segment harboring the miR-15a binding sequence (SMAD7 3′-UTR) or a mutated miR-15a binding site (SMAD7 3′-UTR-Mut). The current results indicated that miR-15a overexpression significantly reduced the activity of the wild-type luciferase reporter, but not the mutant luciferase reporter (Fig. 4B).
Figure 4.
miR-15a targets SMAD7. (A) Conserved miR-15a binding site in 3′-UTR of SMAD7. The miR-15a seed match is indicated by vertical lines. (B) Luciferase reporter assays were performed by co-transfection of H9c2 cells with luciferase reporters containing a wild-type or mutant SMAD7 3′UTR sequence together with a control vector or an miR-15a-expression vector. Luciferase activity was normalized to Renilla activity. **P<0.01 vs. control. Representative western blot images and quantification of protein expression levels of (C) SMAD7, (D) cytosolic NF-κB p65 and (E) nuclear NF-κB p65 in H9c2 cells that were non-transfected or were transfected with a NC, miR-15a or anti-miR-15a. Data are presented as the mean ± standard deviation from three independent experiments. *P<0.05 and **P<0.01 vs. H/R. SMAD7, mothers against decapentaplegic homolog 7; miR-15a, microRNA-15a; 3′-UTR, 3′-untranslated region; H/R, hypoxia/reoxygenation; NC, negative control; NF-κB, nuclear factor-κB; Mut, mutant.
To verify the ability of miR-15a to regulate the expression of SMAD7, the levels of endogenous SMAD7 protein in H/R-treated H9c2 cells were assessed following expression of miR-15a or anti-miR-15a. Overexpression of miR-15a significantly downregulated SMAD7, whereas anti-miR-15a expression upregulated SMAD7 (Fig. 4C). These results demonstrated the miR-15a regulates SMAD7 and that inhibition of miR-15a may release its repression of SMAD7 under conditions of H/R.
Based on a previous study demonstrating that SMAD7 protects against apoptosis by inhibiting NF-κB activation (23), the effects of miR-15a on the SMAD7/NF-κB p65 signaling pathway were investigated by western blot. Protein expression levels of cytosolic NF-κB p65 remained unaltered following miR-15a or anti-miR-15a transfection (Fig. 4D). However, nuclear NF-κB p65 protein expression levels were significantly increased by miR-15a ectopic expression and inhibited by anti-miR-15a expression (Fig. 4E). Therefore, the present results suggested that miR-15a targeting of SMAD7 in H/R-induced H9c2 cells correlates with activation of NF-κBp65, whereas inhibition of miR-15a releases SMAD7 and ameliorates cell injury, potentially via restoration of SMAD7-dependent NF-κB p65 inhibition.
Discussion
Myocardial apoptosis serves a critical role in myocardial I/R injury (25); however, the mechanisms underlying the associated pathological alterations remain unclear. At the cellular level, I/R injury often results in myocardial apoptosis, and multiple miRNAs are known to be involved in the pathological process. In the current study, the potential role of miR-15a in myocardial I/R injury was investigated in vitro, and a miR-15a-controlled apoptotic signaling pathway involving SMAD7 and NF-κB p65 was identified.
The miRNA-15 family members, which include miR-15a, miRNA-15b, miRNA-16, miRNA-195, miRNA-424 and miRNA-497, were initially suggested to contribute to the inhibition of cardiomyocyte proliferation by repressing several cell cycle regulators (26). Hullinger et al (19) demonstrated that the levels of miR-15 family members were increased following ischemia-induced cardiomyocyte cell death, and that inhibition of miR-15 family members reduced the infarct size following I/R injury. In addition, miR-15a and miR-15b were demonstrated to be upregulated in an animal model of I/R injury (27). Furthermore, miRNA-15b was indicated to impair mitochondrial function by repressing ADP-ribosylation factor-like protein 2 (28). Liu et al (29) revealed that miRNA-15b may enhance H/R-induced apoptosis of cardiomyocytes by targeting the anti-apoptotic factor Bcl2. The present study demonstrated that the expression of miR-15a is sensitive to H/R in H9c2 cells. Following 24 h hypoxia and 24 h reoxygenation, the expression of miR-15a increased significantly compared with the expression in the normoxia control group, which is consistent with previous observations suggesting that the miR-15 family is involved in cardiac I/R injury.
Although previous studies have confirmed the pro-apoptotic activity of miR-15a in certain cancers, prior to the present study, it was unclear how miR-15a is involved in I/R injury. To elucidate the underlying mechanism of miR-15a in H/R-induced cardiomyocyte apoptosis, miR15a expression was regulated in H9c2 cells using a lentivirus. The presented results demonstrated that miR-15a inhibition decreased LDH and MDA release and reduced the cell apoptosis rate, whereas overexpression of miR-15a had the opposite effect. Therefore, inhibition of miR-15a may attenuate cell injuries and protect against H/R-induced cell apoptosis.
Using bioinformatics analysis, SMAD7 was identified as a potential target of miR-15a. Additionally, using luciferase assays, SMAD7 was confirmed to serve as a target of miR-15a. However, because miRNAs typically have multiple targets, other targets may contribute to the detrimental effects of miR-15a on I/R injury. As an inhibitory SMAD, SMAD7 is known to block NF-κB activation by inducing IκB expression and simultaneously inhibiting the TGF-β-induced SMAD signaling pathway. Conversely, the NF-κB subunit p65 may suppress TGF-β-SMAD signaling via upregulation of SMAD7 (30). NF-κB, which comprises five subunits including p65, RelB, c-Rel, p50 and p52, regulates genes that are associated with immune response, inflammation, cell survival and proliferation (31,32). NF-κB is intimately involved in apoptosis and possesses pro- and anti-apoptotic effects in different types of cells and with different stimuli (33–35). Among the five subunits, multiple studies demonstrated that NF-kB p65 activation in cardiomyocytes is pro-apoptotic (23,34). In resting conditions, NF-κB is localized in the cytoplasm and is bound to IκB. However, upon stimulation, IκB ubiquitination and degradation are triggered by IκB kinase-mediated IκB phosphorylation. This results in nuclear translocation of NF-κB, which activates the transcription of target genes. The current study demonstrated that the expression of miR-15a in H/R-induced H9C2 cells inhibits the expression of endogenous SMAD7 and activates the nuclear localization of NF-κB p65, whereas anti-miR-15a has the opposite effect. These results indicated that the upregulation of SMAD7 expression by miR-15a inhibition may provide a significant approach for the amelioration of H/R induced myocardial injury.
In conclusion, the present findings indicated that miR-15a inhibition protects cardiomyocytes against H/R-induced apoptosis by upregulating the expression of its target, SMAD7, and downregulating the NF-κB p65 subunit. Future studies should further elucidate the interaction between SMAD7 and NF-κB p65 in this process, how the pathway is integrated into apoptosis, and the potential interaction with the TGF-β signaling pathway. This may provide evidence for miR-15a as a potential therapeutic target for the treatment of cardiac I/R injury.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 81572248).
References
- 1.Cannon RO., III Mechanisms, management and future directions for reperfusion injury after acute myocardial infarction. Nat Clin Pract Cardiovasc Med. 2005;2:88–94. doi: 10.1038/ncpcardio0096. [DOI] [PubMed] [Google Scholar]
- 2.Wu N, Zhang X, Guan Y, Shu W, Jia P, Jia D. Hyper-cholesterolemia abrogates the cardioprotection of ischemic postconditioning in isolated rat heart: Roles of glycogen synthase kinase-3β and the mitochondrial permeability transition pore. Cell Biochem Biophys. 2014;69:123–130. doi: 10.1007/s12013-013-9778-2. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu X, Zhou X, He B, Xu C, Wu L, Cui B, Wen H, Lu Z, Jiang H. Minocycline protects against myocardial ischemia and reperfusion injury by inhibiting high mobility group box 1 protein in rats. Eur J Pharmacol. 2010;638:84–89. doi: 10.1016/j.ejphar.2010.03.059. [DOI] [PubMed] [Google Scholar]
- 5.Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310:1817–1821. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 6.Kataoka M, Wang DZ. Non-coding RNAs including miRNAs and lncRNAs in cardiovascular biology and disease. Cells. 2014;3:883–898. doi: 10.3390/cells3030883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boon RA, Dimmeler S. MicroRNAs in myocardial infarction. Nat Rev Cardiol. 2015;12:135–142. doi: 10.1038/nrcardio.2014.207. [DOI] [PubMed] [Google Scholar]
- 8.Heymans S, Corsten MF, Verhesen W, Carai P, van Leeuwen RE, Custers K, Peters T, Hazebroek M, Stöger L, Wijnands E, et al. Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation. 2013;128:1420–1432. doi: 10.1161/CIRCULATIONAHA.112.001357. [DOI] [PubMed] [Google Scholar]
- 9.Danielson LS, Park DS, Rotllan N, Chamorro-Jorganes A, Guijarro MV, Fernandez-Hernando C, Fishman GI, Phoon CK, Hernando E. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. FASEB J. 2013;27:1460–1467. doi: 10.1096/fj.12-221994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thome JG, Mendoza MR, Cheuiche AV, La Porta VL, Silvello D, Dos Santos KG, Andrades ME, Clausell N, Rohde LE, Biolo A. Circulating microRNAs in obese and lean heart failure patients: A case-control study with computational target prediction analysis. Gene. 2015;574:1–10. doi: 10.1016/j.gene.2015.07.068. [DOI] [PubMed] [Google Scholar]
- 11.Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, Li YR, Li PF. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 12.Ren XP, Wu J, Wang X, Sartor MA, Qian J, Jones K, Nicolaou P, Pritchard TJ, Fan GC. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/CIRCULATIONAHA.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aurora AB, Mahmoud AI, Luo X, Johnson BA, van Rooij E, Matsuzaki S, Humphries KM, Hill JA, Bassel-Duby R, Sadek HA, Olson EN. MicroRNA-214 protects the mouse heart from ischemic injury by controlling Ca2+ overload and cell death. J Clin Invest. 2012;122:1222–1232. doi: 10.1172/JCI59327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Ha T, Zou J, Ren D, Liu L, Zhang X, Kalbfleisch J, Gao X, Williams D, Li C. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc Res. 2014;102:385–395. doi: 10.1093/cvr/cvu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bozok Çetintaş V, Tetik Vardarlı A, Düzgün Z, Tezcanlı Kaymaz B, Açıkgöz E, Aktuğ H, Kosova Can B, Gündüz C, Eroğlu Z. miR-15a enhances the anticancer effects of cisplatin in the resistant non-small cell lung cancer cells. Tumour Biol. 2016;37:1739–1751. doi: 10.1007/s13277-015-3950-9. [DOI] [PubMed] [Google Scholar]
- 16.Renjie W, Haiqian L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett. 2015;356:568–578. doi: 10.1016/j.canlet.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 17.de Groen FL, Timmer LM, Menezes RX, Diosdado B, Hooijberg E, Meijer GA, Steenbergen RD, Carvalho B. Oncogenic role of miR-15a-3p in 13q amplicon-driven colorectal adenoma-to-carcinoma progression. PLoS One. 2015;10:e0132495. doi: 10.1371/journal.pone.0132495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu N, Jiao T, Huang Y, Liu W, Li Z, Ye X. Hepatitis B virus regulates apoptosis and tumorigenesis through the microRNA-15a-Smad7-transforming growth factor beta pathway. J Virol. 2015;89:2739–2749. doi: 10.1128/JVI.02784-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, Dalby CM, Robinson K, Stack C, Latimer PA, et al. Inhibition of miR-15 protects against cardiac ischemic injury. Circ Res. 2012;110:71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamura Y, Hua X, Bergelson S, Lodish HF. Critical role of Smads and AP-1 complex in transforming growth factor-beta-dependent apoptosis. J Biol Chem. 2000;275:36295–36302. doi: 10.1074/jbc.M006023200. [DOI] [PubMed] [Google Scholar]
- 21.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 22.Ng YY, Hou CC, Wang W, Huang XR, Lan HY. Blockade of NFkappaB activation and renal inflammation by ultrasound-mediated gene transfer of Smad7 in rat remnant kidney. Kidney Int Suppl. 2005:S83–S91. doi: 10.1111/j.1523-1755.2005.09421.x. [DOI] [PubMed] [Google Scholar]
- 23.Zhang B, Zhou M, Li C, Zhou J, Li H, Zhu D, Wang Z, Chen A, Zhao Q. MicroRNA-92a inhibition attenuates hypoxia/reoxygenation-induced myocardiocyte apoptosis by targeting Smad7. PLoS One. 2014;9:e100298. doi: 10.1371/journal.pone.0100298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 26.Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, Dorn GW, II, van Rooij E, Olson EN. MiR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu LF, Liang Z, Lv ZR, Liu XH, Bai J, Chen J, Chen C, Wang Y. MicroRNA-15a/b are up-regulated in response to myocardial ischemia/reperfusion injury. J Geriatr Cardiol. 2012;9:28–32. doi: 10.3724/SP.J.1263.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishi H, Ono K, Iwanaga Y, Horie T, Nagao K, Takemura G, Kinoshita M, Kuwabara Y, Mori RT, Hasegawa K, et al. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J Biol Chem. 2010;285:4920–4930. doi: 10.1074/jbc.M109.082610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu L, Zhang G, Liang Z, Liu X, Li T, Fan J, Bai J, Wang Y. MicroRNA-15b enhances hypoxia/reoxygenation-induced apoptosis of cardiomyocytes via a mitochondrial apoptotic pathway. Apoptosis. 2014;19:19–29. doi: 10.1007/s10495-013-0899-2. [DOI] [PubMed] [Google Scholar]
- 30.Freudlsperger C, Bian Y, Contag Wise S, Burnett J, Coupar J, Yang X, Chen Z, Van Waes C. TGF-β and NF-κB signal pathway cross-talk is mediated through TAK1 and SMAD7 in a subset of head and neck cancers. Oncogene. 2013;32:1549–1559. doi: 10.1038/onc.2012.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valen G. Signal transduction through nuclear factor kappa B in ischemia-reperfusion and heart failure. Basic Res Cardiol. 2004;99:1–7. doi: 10.1007/s00395-003-0442-7. [DOI] [PubMed] [Google Scholar]
- 32.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 33.Hong S, Lee C, Kim SJ. Smad7 sensitizes tumor necrosis factor induced apoptosis through the inhibition of antiapoptotic gene expression by suppressing activation of the nuclear factor-kappaB pathway. Cancer Res. 2007;67:9577–9583. doi: 10.1158/0008-5472.CAN-07-1179. [DOI] [PubMed] [Google Scholar]
- 34.Hamid T, Guo SZ, Kingery JR, Xiang X, Dawn B, Prabhu SD. Cardiomyocyte NF-κB p65 promotes adverse remodelling, apoptosis, and endoplasmic reticulum stress in heart failure. Cardiovasc Res. 2011;89:129–138. doi: 10.1093/cvr/cvq274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ling H, Gray CB, Zambon AC, Grimm M, Gu Y, Dalton N, Purcell NH, Peterson K, Brown JH. Ca2+/Calmodulin-dependent protein kinase II δ mediates myocardial ischemia/reperfusion injury through nuclear factor-κB. Circ Res. 2013;112:935–944. doi: 10.1161/CIRCRESAHA.112.276915. [DOI] [PMC free article] [PubMed] [Google Scholar]