Abstract
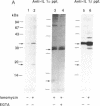
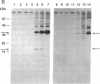
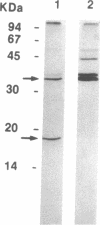
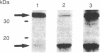
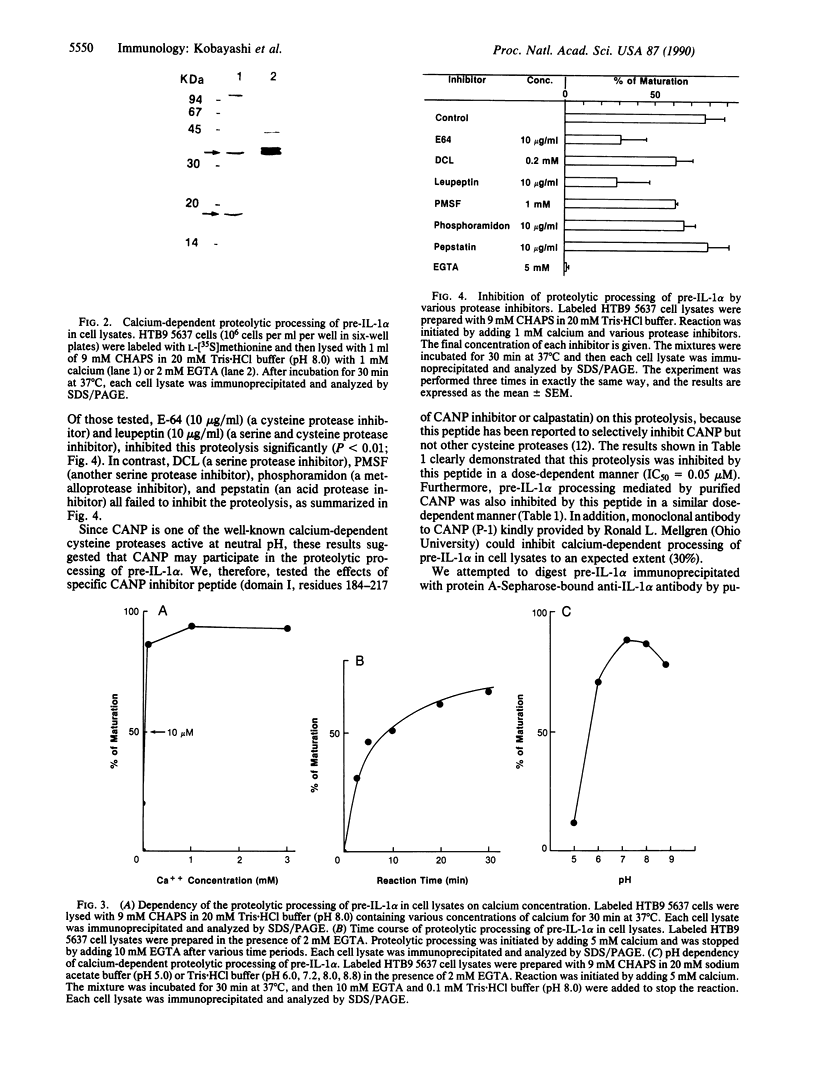
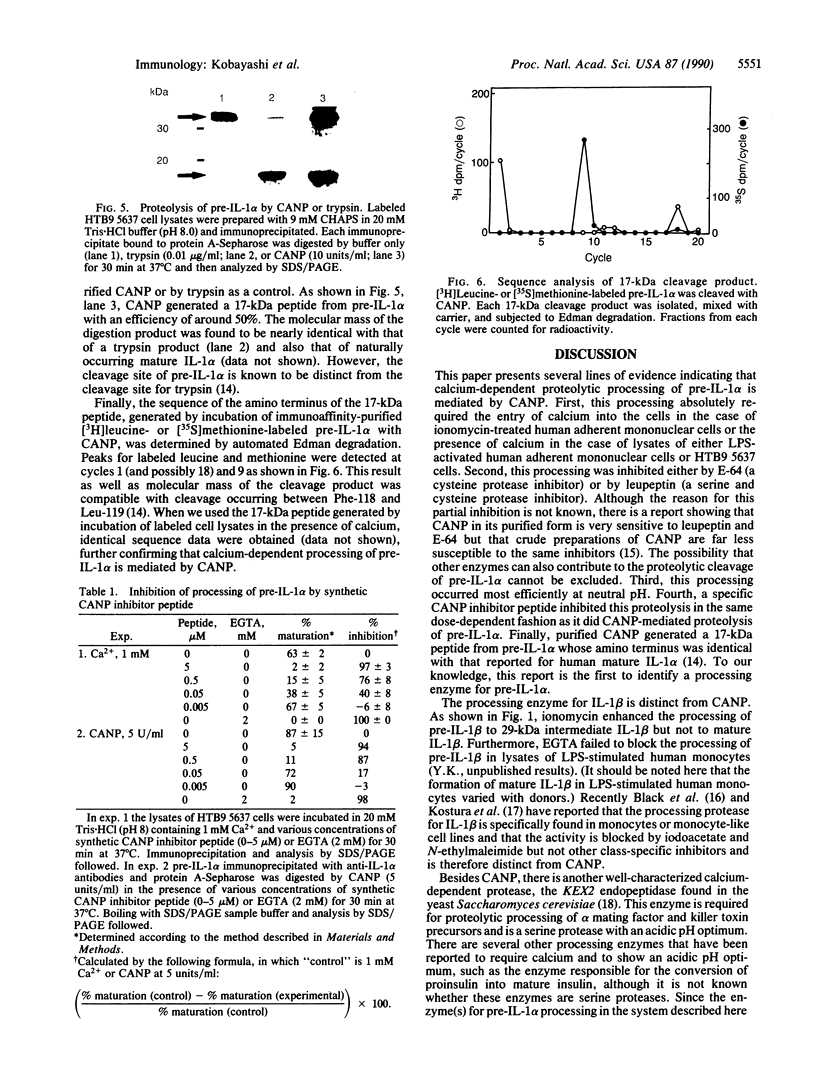
We describe here the involvement of calcium-activated neutral protease (CANP or calpain, EC 3.4.22.17) in calcium-dependent proteolytic processing of the precursor of human interleukin 1 alpha (IL-1 alpha) into mature IL-1 alpha. Calcium ionophore ionomycin enhanced proteolytic processing of pre-IL-1 alpha and the release of mature IL-1 alpha either from lipopolysaccharide (LPS)-activated human adherent mononuclear cells or from a human bladder carcinoma cell line (HTB9 5637) that constitutively produces human IL-1 alpha and -beta. The proteolytic processing of pre-IL-1 alpha was completely inhibited by EGTA. Similar calcium-dependent proteolytic processing of pre-IL-1 alpha was also observed with lysates of either LPS-activated human adherent mononuclear cells or HTB9 5637 cells. Since the optimal pH for processing was between 7 and 8, and E-64 (a cysteine protease inhibitor) and leupeptin (a serine and cysteine protease inhibitor) both inhibited this processing by cell lysates, we hypothesized that a calcium-activated neutral protease, CANP, might be responsible for this processing. This hypothesis was supported by data showing that the specific CANP inhibitor peptide inhibited this proteolysis in cell lysates in a dose-dependent fashion (IC50 = 0.05 microM) and that treatment of pre-IL-1 alpha with purified CANP yielded the 17-kDa mature form of IL-1 alpha, which has an amino terminus identical with that reported for mature human IL-1 alpha. Taken together, these findings indicate that calcium-dependent proteolytic processing of pre-IL-1 alpha is selectively mediated by CANP.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R. A., Kronheim S. R., Sleath P. R. Activation of interleukin-1 beta by a co-induced protease. FEBS Lett. 1989 Apr 24;247(2):386–390. doi: 10.1016/0014-5793(89)81376-6. [DOI] [PubMed] [Google Scholar]
- Bursten S. L., Locksley R. M., Ryan J. L., Lovett D. H. Acylation of monocyte and glomerular mesangial cell proteins. Myristyl acylation of the interleukin 1 precursors. J Clin Invest. 1988 Nov;82(5):1479–1488. doi: 10.1172/JCI113755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron P. M., Limjuco G. A., Chin J., Silberstein L., Schmidt J. A. Purification to homogeneity and amino acid sequence analysis of two anionic species of human interleukin 1. J Exp Med. 1986 Jul 1;164(1):237–250. doi: 10.1084/jem.164.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui I., Tanaka K., Murachi T. Extracellular appearance of calpain and calpastatin in the synovial fluid of the knee joint. Biochem Biophys Res Commun. 1989 Jul 31;162(2):559–566. doi: 10.1016/0006-291x(89)92347-4. [DOI] [PubMed] [Google Scholar]
- Furutani Y., Notake M., Yamayoshi M., Yamagishi J., Nomura H., Ohue M., Furuta R., Fukui T., Yamada M., Nakamura S. Cloning and characterization of the cDNAs for human and rabbit interleukin-1 precursor. Nucleic Acids Res. 1985 Aug 26;13(16):5869–5882. doi: 10.1093/nar/13.16.5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H., Emori Y., Imajoh-Ohmi S., Minami Y., Suzuki K. Identification and characterization of inhibitory sequences in four repeating domains of the endogenous inhibitor for calcium-dependent protease. J Biochem. 1989 Aug;106(2):274–281. doi: 10.1093/oxfordjournals.jbchem.a122844. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Tabuchi H., Shiota M., Nishizuka Y. Calcium-dependent neural proteases, widespread occurrence of a species of protease active at lower concentrations of calcium. J Biochem. 1981 Sep;90(3):889–892. doi: 10.1093/oxfordjournals.jbchem.a133547. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Appella E., Yamada M., Copeland T. D., Oppenheim J. J., Matsushima K. Phosphorylation of intracellular precursors of human IL-1. J Immunol. 1988 Apr 1;140(7):2279–2287. [PubMed] [Google Scholar]
- Kostura M. J., Tocci M. J., Limjuco G., Chin J., Cameron P., Hillman A. G., Chartrain N. A., Schmidt J. A. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5227–5231. doi: 10.1073/pnas.86.14.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Oppenheim J. J. Calcium ionophore (A23187) increases interleukin 1 (IL-1) production by human peripheral blood monocytes and interacts synergistically with IL-1 to augment concanavalin A stimulated thymocyte proliferation. Cell Immunol. 1985 Jan;90(1):226–233. doi: 10.1016/0008-8749(85)90184-4. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Taguchi M., Kovacs E. J., Young H. A., Oppenheim J. J. Intracellular localization of human monocyte associated interleukin 1 (IL 1) activity and release of biologically active IL 1 from monocytes by trypsin and plasmin. J Immunol. 1986 Apr 15;136(8):2883–2891. [PubMed] [Google Scholar]
- Mizuno K., Nakamura T., Ohshima T., Tanaka S., Matsuo H. Characterization of KEX2-encoded endopeptidase from yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1989 Feb 28;159(1):305–311. doi: 10.1016/0006-291x(89)92438-8. [DOI] [PubMed] [Google Scholar]
- Mochizuki D. Y., Eisenman J. R., Conlon P. J., Larsen A. D., Tushinski R. J. Interleukin 1 regulates hematopoietic activity, a role previously ascribed to hemopoietin 1. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5267–5271. doi: 10.1073/pnas.84.15.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Salamino F., Sparatore B., Michetti M., Sacco O., Melloni E. Following association to the membrane, human erythrocyte procalpain is converted and released as fully active calpain. Biochim Biophys Acta. 1985 Oct 18;831(3):335–339. doi: 10.1016/0167-4838(85)90116-5. [DOI] [PubMed] [Google Scholar]
- Suttles J., Giri J. G., Mizel S. B. IL-1 secretion by macrophages. Enhancement of IL-1 secretion and processing by calcium ionophores. J Immunol. 1990 Jan 1;144(1):175–182. [PubMed] [Google Scholar]
- Suzuki K., Imajoh S., Emori Y., Kawasaki H., Minami Y., Ohno S. Calcium-activated neutral protease and its endogenous inhibitor. Activation at the cell membrane and biological function. FEBS Lett. 1987 Aug 17;220(2):271–277. doi: 10.1016/0014-5793(87)80828-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Kosaki G., Suzuki K., Tanoue K., Yamazaki H. Cleavage site of calcium-dependent protease in human platelet membrane glycoprotein Ib. Thromb Res. 1986 Jul 1;43(1):41–55. doi: 10.1016/0049-3848(86)90043-5. [DOI] [PubMed] [Google Scholar]