Abstract
Endothelial dysfunction caused by reactive oxygen species (ROS) has been implicated in numerous cardiovascular diseases. Astragalus polysaccharide (APS), an important bioactive component extracted from the Chinese herb Astragalus membranaceus, has been widely used for the treatment of cardiovascular disease. The present study aimed to investigate the effects of APS on hydrogen peroxide (H2O2)-induced human umbilical vein endothelial cell (HUVEC) injury. Following treatment with 400 µM H2O2 for 24 h, cell viability was decreased and apoptosis was increased. However, pretreatment with APS for 1 h significantly attenuated H2O2-induced injury in HUVECs. In addition, APS decreased intracellular ROS levels, increased the protein expression of endothelial nitric oxide synthase and copper-zinc superoxide dismutase, elevated intracellular cyclic guanosine monophosphate (an activity marker for nitric oxide) levels and restored the mitochondrial membrane potential, compared with cells treated with H2O2 only. In conclusion, the results of the present study suggested that APS may protect HUVECs from injury induced by H2O2 via increasing the cell antioxidant capacity and nitric oxide (NO) bioavailability, which may contribute to the improvement of the imbalance between ROS and NO levels.
Keywords: Astragalus polysaccharide, human umbilical vein endothelial cells, oxidative stress, nitric oxide, apoptosis
Introduction
Vascular endothelial cells are flat cells lining the inner wall of blood vessels, which serve a key role as the mechanical barrier between circulating blood and vascular smooth muscle cells. In addition, the vascular endothelium is an important endocrine organ, which is involved in maintaining vascular wall tension, blood flow, vessel wall inflammation antagonism and angiogenesis through the secretion of numerous vasoactive substances. Due to their barrier function, endothelial cells are more vulnerable to injury by various physical and chemical risk factors. Injury to endothelial cells is a critical event in angiogenesis, atherosclerosis, thrombosis, hypertension and heart failure (1–5). Endothelial dysfunction, particularly endocrine dysfunction, causes the secretion of a variety of active substances, which may lead to dysfunction of the cardiovascular system.
In endothelial cells, oxidative stress is regarded as a critical pathogenic factor for endothelial cell injury, and the accumulation of ROS may result in endothelial cell apoptosis (6–8). Hydrogen peroxide (H2O2) may penetrate the plasma membrane and cause endothelial cell injury. In addition, it has been reported that reactive oxygen species (ROS) are involved in the apoptosis of endothelial cells (9,10) and the primary source of endogenous ROS is H2O2 (11), which has been extensively used to induce oxidative stress in in vitro models (12,13).
Radix astragali is the root of the perennial herb Astragalus membranaceus (14). As a well-established traditional Chinese medicine, it has been used for the treatment of cardiovascular disease and is believed to possess immune stimulatory, antiviral and antioxidative effects. Astragalus polysaccharide (APS) is an important bioactive ingredient obtained from Astragalus membranaceus that has a range of pharmacological effects, including increasing levels of cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP) in plasma and tissues, promotion of immune responses, anti-inflammation (15), protection of vessels, antioxidant effects, anti-insulin resistance and antitumor effects (16–19). Our previous studies reported that APS may inhibit isoprenaline-induced cardiac hypertrophy (20,21). It has been reported that Astragalus membranaceus and its primary components, including APS, may ameliorate endothelial dysfunction induced by homocysteine in which antioxidation is involved. However, the effects of APS on endothelial injury induced by ROS and the underlying mechanism remains to be fully elucidated.
Materials and methods
Materials
APS was purchased from Nanjing Jingzhu Pharmaceutical Technology Co., Ltd. (Nanjing, China). H2O2 was purchased from Tianjin Guangfu Fine Chemical Research Institute (Tianjin, China). Dihydroethidium (DHE) was obtained from Shanghai Haoran Bio-Technology Co., Ltd. (Shanghai, China). MTT and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich; Merck Millipore (Darmstadt, Germany). DAPI, Rhodamine 123 and the Cell Cycle and Apoptosis analysis kit were purchased from Beyotime Institute of Biotechnology (Haimen, China). The cGMP assay kit was supplied by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Rabbit anti-Copper-zinc superoxide dismutase (Cu/Zn-SOD; bs-10216R) and anti-endothelial nitric oxide synthase (eNOS; bs-20609R) antibodies were purchased from Beijing Boosen Biological Technology Co., Ltd (Beijing, China), and anti-β-actin (HRP-600008) was from Wuhan Sanying Biotechnology (Wuhan, China). All other chemicals and reagents used were of analytical grade.
Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd. (Shanghai, China) and were maintained in high-glucose Dulbecco's Modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA), 2 mmol/l L-glutamine, 5 µg/ml endothelial growth factor (Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd.), 100 U/ml penicillin and 100 µg/ml streptomycin. Cells were incubated in a humidified incubator with 5% CO2 at 37°C with media replenishment every 2 days and were passaged at 80–90% confluence. The cells were treated with various concentrations of APS or H2O2 for 24 h incubated in serum-free medium, prior to an MTT assay. Additional groups were pretreated with various concentrations of APS for 1 h prior to H2O2 exposure in fresh medium, following which they were harvested for further analysis. The cells were assigned into the following five groups; A, untreated control group; B, H2O2 (400 µM) model group; C, H2O2+APS (0.1 µg/ml) group; D, H2O2+APS (1 µg/ml) group; E, H2O2+APS (10 µg/ml) group.
Cell viability assay
The MTT assay was used to evaluate cell viability (22). In brief, HUVECs were cultured in a 96-well plate (1×104 cells/well). A total of 24 h following plating, cells were pretreated with 0.1–100 µg/ml APS for 1 h, following which 400 µM H2O2 was added into the plate and cultured at 37°C for a further 24 h. The viability of cells treated with APS (0.1–100 µg/ml) or H2O2 alone (0–500 µM) was also assessed. MTT was dissolved in PBS, added to each well and incubated at 37°C for 4 h, for a final concentration of 0.5 mg/ml. Subsequently, the culture medium was carefully removed and replaced with 150 µl DMSO. The plates were incubated for 10 min and measured at a wavelength of 490 nm on a microplate reader (DNM-9602G; Beijing Perlong New Technology Co., Ltd., Beijing, China). The viability of HUVECs was expressed as a percentage of the control group.
Apoptosis analysis by flow cytometry
Apoptosis was evaluated using the Cell Cycle and Apoptosis analysis kit. Briefly, following treatment, floating and attached cells were harvested by trypsin and washed with PBS (concentration, 1×106 cells/ml). Cells were fixed with ice-cold 70% alcohol at 4°C overnight, and staining was performed with propidium (PI) according to the manufacturer's protocol. Samples were analyzed on a flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) using CellQuest software v6.1x (BD Biosciences). Cells in the sub-G1 peak were considered apoptotic.
DAPI staining
The morphological characteristics of nuclei were evaluated by DAPI staining as previously described (23). HUVECs were seeded onto a 6-well plate at a density of 1×105 cells/well. Following treatment, cells were fixed with 4% paraformaldehyde for 10 min. Cells were washed once with PBS and stained with 2 µg/ml DAPI for 15 min at 37°C in the dark. The stained cells were visualized under an inverted fluorescence microscope.
Determination of mitochondrial membrane potential
The mitochondrial membrane potential of HUVECs was assessed using the fluorescent dye Rhodamine 123 (24–26). Rhodamine 123, a selective fluorescent dye, permeates cell membranes and is sequestered by active mitochondria, therefore staining the mitochondria of living cells, and is widely used for the detection of mitochondrial membrane potential. Flavovirens fluorescence represents normal cells. HUVECs were cultured in a 6-well plate at a density of 1×105 cells/well. Following treatment, the culture medium was carefully removed and cells were washed twice with PBS. The cells were subsequently stained with 2 µM Rhodamine 123 for 20 min at 37°C in the dark. Cells were analyzed under an inverted fluorescence microscope. The fluorescence intensity was calculated with LAS Software (V4.3) (Leica Microsystems GmbH, Wetzlar, Germany).
Measurement of intracellular cGMP levels
HUVECs were pretreated with 0.1, 1 or 10 µg/ml APS for 1 h, followed by exposure to 400 µM H2O2 for 24 h. The cells were subsequently harvested with a Falcon scraper, centrifuged at 4°C for 5 min at 800 × g and sonicated. The homogenate was prepared in PBS and centrifuged at 1,000 × g for 5 min at 4°C. The cGMP levels in the supernatants were determined with a kit according to the manufacturer's protocol. cGMP levels were expressed as nmol/g protein. Protein concentration was calculated with a Bicinchoninic Acid (BCA) Protein assay kit (Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing, China). cGMP is a second messenger of NO, and its level reflects the level of nitric oxide (NO) (27).
Measurement of intracellular ROS production
The production of ROS was measured using DHE. DHE, an oxidative fluorescent dye, may be oxidized to ethidium bromide and intercalated into DNA in the presence of superoxide anions. HUVECs were seeded on a 6-well plate (1×105 cells/well). Following treatment, cells were washed with PBS and incubated with 2 mM DHE for 30 min at 37°C. Cells were washed with PBS and analyzed under an inverted fluorescence microscope. The fluorescence intensity was calculated using LAS Software V4.3.
Western blot analysis
Following treatment, cells were harvested and lysed with ice-cold radio immunoprecipitation assay buffer (Wuhan Boster Biotechnology Co., Ltd., Wuhan, China). Protein concentration was determined using a BCA Protein assay kit. Protein extracts (30 µg) were electrophoretically separated by 12 or 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Membranes were blocked with 5% nonfat milk powder and incubated overnight at 4°C with the following primary antibodies: Anti-eNOS (1:1,500), anti-Cu/Zn-SOD (1:500) and β-actin (1:500). Following washing with TBS containing Tween-20 (10 mM Tris, 100 mM NaCl, and 0.1% Tween-20), membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (anti-rabbit IgG and anti-mouse IgG; 1706515 and 1706516; 1:1,500; Bio-Rad Laboratories, Inc., Hercules, CA, USA) for 2 h at room temperature. Membranes were washed with TBS, and immunocomplexes were visualized using the Amersham ECL start Western Blotting Detection Reagent enhanced chemiluminescence system (GE Healthcare Life Sciences). β-actin served as an internal control for the experiments. The results were quantified by the Quantity One software v4.62 (Bio-Rad Laboratories, Inc.).
Statistical analysis
All data are expressed as the mean ± standard deviation. Each experiment was performed at least three times. Statistical analysis was performed using Student's t-test or one-way analysis of variance with SPSS software version 17.0 (SPSS Inc., Chicago, IL, USA). The post hoc least significant difference test was used to evaluate differences between two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of APS on the viability of HUVECs
Initially, the cytotoxicity of APS on HUVECs was measured using the MTT assay. As presented in Fig. 1A, incubation of HUVECs with 0.1–100 µg/ml APS for 24 h did not significantly affect the cell viability (P>0.05). However, treatment of HUVECs with 100 to 500 µM H2O2 for 24 h resulted in a concentration-dependent decrease in cell viability, when compared with the control group (P<0.05; Fig. 1B). Pretreatment of HUVECs with 0.1, 1 or 10 µg/ml APS for 1 h prior to exposure to H2O2 significantly increased viability in a dose-dependent manner (Fig. 1C). These results indicated that APS may protect HUVECs from oxidative stress-induced injury. Based on these results, further studies employed a H2O2 concentration of 400 µM, and APS concentrations of 0.1, 1.0 and 10 µg/ml.
Figure 1.
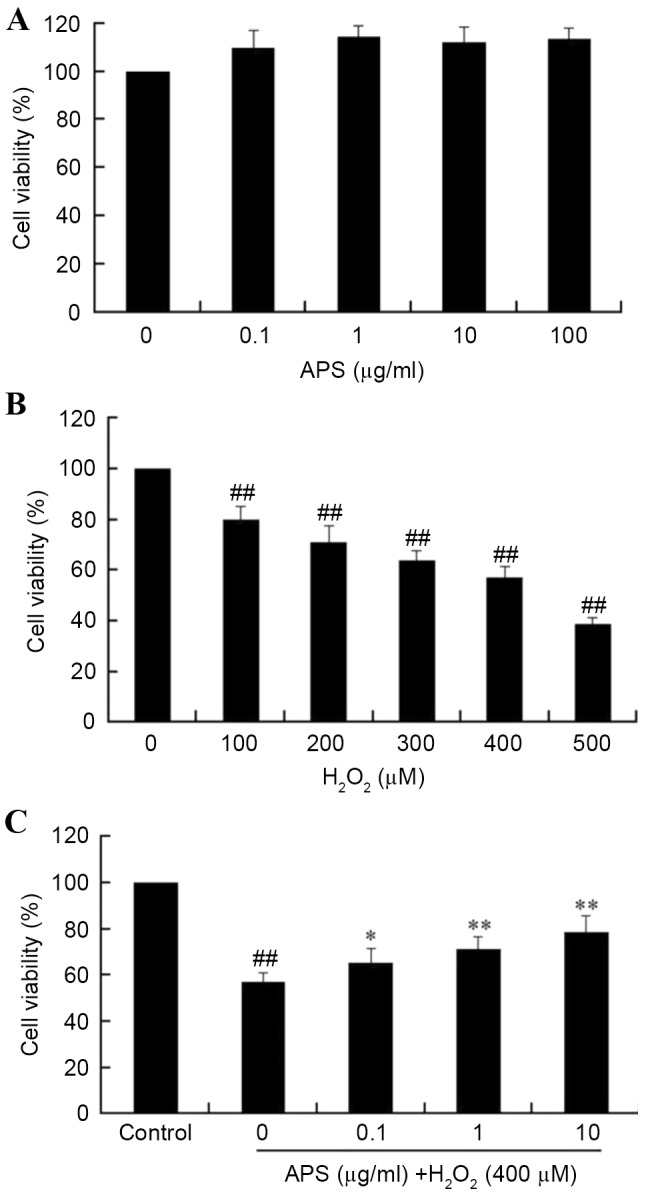
Cell viability, as determined by MTT assay. (A) Effects of APS on the viability of HUVECs. (B) Cells were incubated with of 0–500 µM H2O2 for 24 h. (C) The protective effect of APS pretreatment on H2O2-induced HUVEC damage. Cells were treated with 400 µM H2O2 only or pretreated with 0.1–10 µg/ml APS followed by 400 µM H2O2. Data are expressed as the mean ± standard deviation. ##P<0.01 vs. control; *P<0.05 and **P<0.01 vs. H2O2 only. APS, Astragalus polysaccharide; HUVECs, human umbilical vein endothelial cells.
Effect of APS on H2O2-induced apoptosis in HUVECs
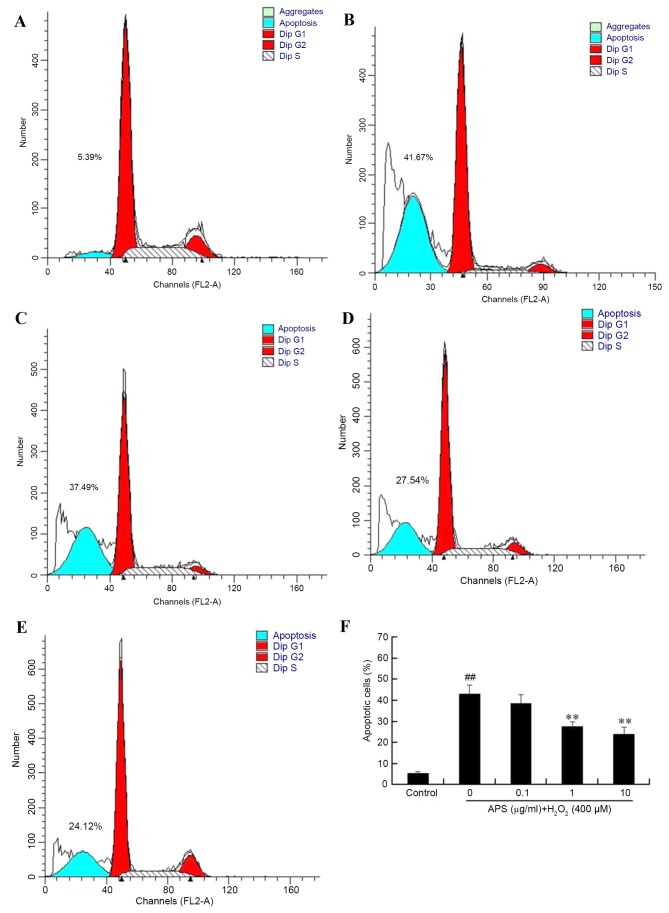
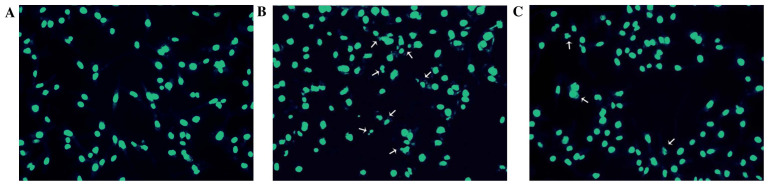
To further evaluate the protective effect of APS against H2O2-induced damage in HUVECs, apoptosis rates were measured by flow cytometry. As presented in Fig. 2, the percentage of apoptotic cells in the control group was 5.44±0.57%. Following exposure to 400 µM H2O2 for 24 h, the percentage of apoptosis increased to 43.14±4.25%. However, pretreatment of the cells with 0.1, 1 and 10 µg/ml APS for 1 h prior to H2O2 exposure reduced the percentage of apoptotic cells to 38.58±4.13, 27.60±2.25 and 24.10±3.26%, respectively. In addition, the morphology of HUVEC nuclei was evaluated by DAPI staining. Compared with the control group (Fig. 3A), nuclei of the H2O2 group (Fig. 3B) were shrunken, irregular and fragmented, typical features of apoptosis. However, APS (1 µg/ml) pretreatment (Fig. 3C) reduced these alterations.
Figure 2.
Apoptosis of human umbilical vein endothelial cells, as determined by flow cytometry. (A) Control, (B) H2O2 only, (C) 0.1 µg/ml APS + H2O2, (D) 1 µg/ml APS + H2O2 and (E) 10 µg/ml APS + H2O2 groups. (F) The effect of APS on the percentage of apoptotic cells induced by 400 µM H2O2. Data are expressed as the mean ± standard deviation. ##P<0.01 vs. control; **P<0.01 vs. H2O2 only. APS, Astragalus polysaccharide.
Figure 3.
Representative images of human umbilical vein endothelial cell nuclear morphology, as evaluated by DAPI staining. (A) Control, (B) 400 µM H2O2 and (C) 1 µg/ml Astragalus polysaccharide + 400 µM H2O2 groups. Magnification, ×200. Arrows point to HUVECS displaying apoptotic nuclei.
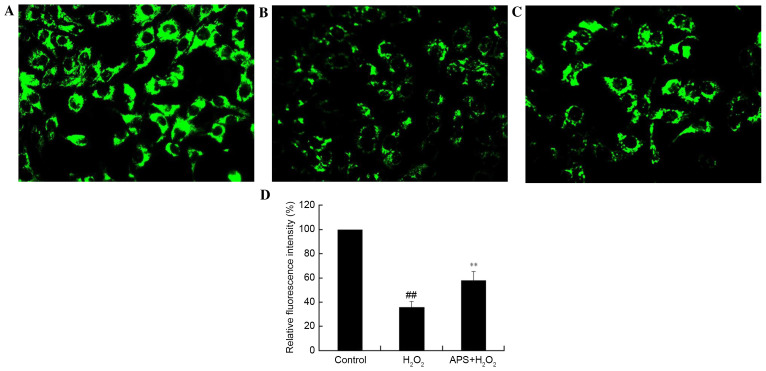
Effect of APS on the mitochondrial membrane potential of HUVECs
The disruption of mitochondria has been reported to be involved in programmed cell death. The maintenance of the mitochondrial membrane potential is essential for mitochondrial integrity and bioenergetic function. To determine whether the anti-apoptotic effect of APS is associated with the inhibition of mitochondrial disruption, mitochondrial membrane potential variation in HUVECs was assessed by Rhodamine 123 staining. Rhodamine 123 is sequestered by active mitochondria of normal HUVECs and revealed yellow-green flavovirens fluorescence. Compared with control cells (Fig. 4A), H2O2-treated cells presented a weak green fluorescence intensity, which reflected the loss of mitochondrial membrane potential (Fig. 4B); this decrease in fluorescence was partially reversed by APS (1 µg/ml) pretreatment (Fig. 4C). Quantification of fluorescence intensity revealed that these alterations were significant (Fig. 4D). This may be a possible underlying mechanism by which APS protects against injury.
Figure 4.
Analysis of the mitochondrial membrane potential, as assessed by Rhodamine 123 staining. Representative images from the (A), control, (B) 400 µM H2O2 only and (C) 1 µg/ml APS + 400 µM H2O2 groups. Magnification, ×200. (D) Relative fluorescence intensity, expressed as a percentage of the control group. Data are expressed as the mean ± standard deviation. ##P<0.01 vs. control; **P<0.01 vs. H2O2 only. APS, Astragalus polysaccharide.
Effect of APS on cGMP levels in HUVECs
To further investigate the protective effects of APS, the influence of H2O2 on cGMP accumulation was measured. As presented in Fig. 5, basal production of cGMP decreased from 9.8±0.8 nmol/g protein in control cells to 4.2±0.4 nmol/g protein in H2O2-treated cells. However, pretreatment of cells with 0.1–10 µg/ml APS for 1 h prior to H2O2 exposure increased the levels of cGMP compared with H2O2 treatment alone, in a dose-dependent manner.
Figure 5.
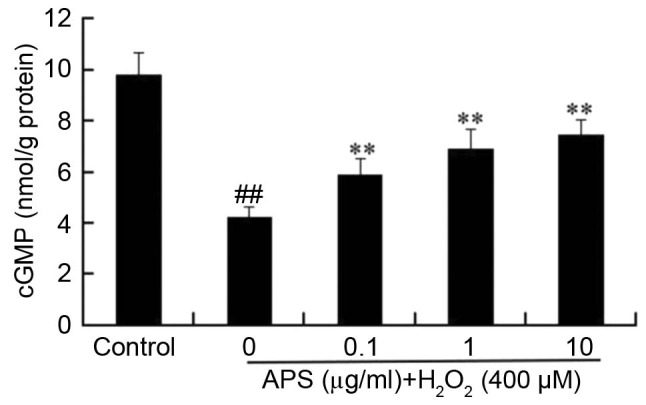
APS partially restored H2O2-induced decrease of cGMP levels in human umbilical vein endothelial cells. Cells were pretreated 0, 0.1, 1 or 10 µg/ml APS for 1 h prior to incubation with 400 µM H2O2. Data are expressed as the mean ± standard deviation. ##P<0.01 vs. control; **P<0.01 vs. H2O2 only. APS, Astragalus polysaccharide; cGMP, cyclic guanosine monophosphate.
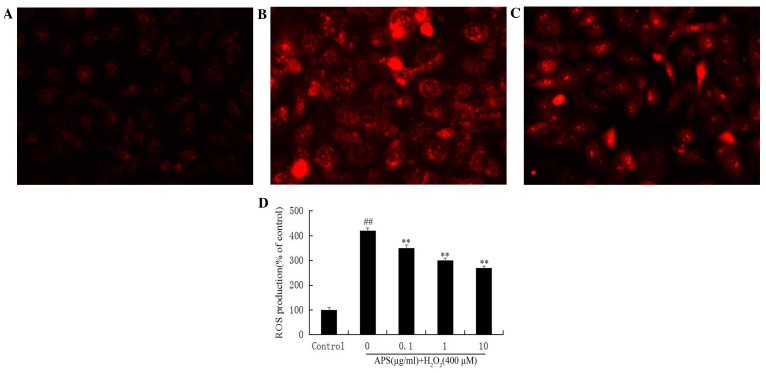
Effect of APS on ROS formation in HUVECs
The secondary generation of ROS is another potential mechanism underlying cell damage induced by H2O2. Therefore, intracellular ROS levels were measured by DHE staining. Compared with the control group (Fig. 6A), DHE fluorescence in HUVECs exposed to H2O2 was increased (Fig. 6B). This increase was attenuated by pretreatment with APS (Fig. 6C). Quantification of fluorescence revealed significantly increased ROS production in H2O2-treated cells (P<0.01), and a significant and dose-dependent effect of APS (P<0.01; Fig. 6D).
Figure 6.
Effect of APS on H2O2-induced ROS formation in human umbilical vein endothelial cells. Representative images from the (A) control, (B) 400 µM H2O2 only and (C) 1 µg/ml APS + 400 µM H2O2 groups. Magnification, ×400. (D) Quantification of the effect of APS on the ROS formation induced by 400 µM H2O2, expressed as a percentage of the control group. Data are expressed as the mean ± standard deviation. ##P<0.01 vs. control; **P<0.01 vs. H2O2 only. APS, Astragalus polysaccharide; ROS, reactive oxygen species.
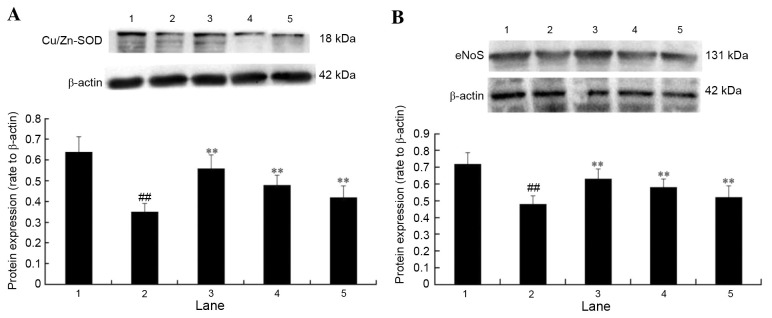
Effect of APS on the protein expression levels of eNOS and Cu/Zn-SOD in H2O2-treated cells
The protein expression levels of eNOS and Cu/Zn-SOD were examined to investigate the potential underlying molecular mechanisms contributing to APS cytoprotection. Protein expression levels of Cu/Zn-SOD (Fig. 7A) and eNOS (Fig. 7B) in HUVECs exposed to H2O2 were significantly decreased (P<0.01). However, pretreatment with 10, 1 or 0.1 µg/ml APS significantly attenuated the H2O2-mediated decrease of eNOS and Cu/Zn-SOD expression (P<0.01), which may therefore contribute to the APS-mediated protective effects of HUVECs.
Figure 7.
Effect of APS on the expression of and (A) Cu/Zn-SOD (B) eNOS in H2O2-treated cells. Lane 1, control group; lane 2, 400 µM H2O2 only group; lane 3, 10 µg/ml APS + 400 µM H2O2 group; lane 4, 1 µg/ml APS + 400 µM H2O2 group; lane 5, 0.1 µg/ml APS + 400 µM H2O2 group. Data are expressed as the mean ± standard deviation. ##P<0.01 vs. control; **P<0.01 vs. H2O2 only. APS, Astragalus polysaccharide; Cu/Zn-SOD, copper-zinc superoxide dismutase; eNOS, endothelial nitric oxide synthase.
Discussion
Vascular endothelial cells form a crucial functional barrier between tissues and the circulation, which acts as a receptor that senses physical or chemical stimuli occurring inside the vessel. In addition, this barrier may secrete a variety of substances that contribute to the response of the blood vessel to different physiologic and pathologic stimuli, including regulation of coagulation and participation in the immune response. Abnormalities in endothelial cell structure and function potentially lead to numerous cardiovascular diseases. Therefore, endothelial function has been identified as a biomarker/mediator of cardiovascular risk factors (28–30) that may act as an independent predictor of cardiovascular disease (3,31). APS, isolated from Astragalus membranaceus, has a variety of pharmacological effects, including anti-inflammatory, antiviral and antioxidant effects, and it increases levels of cGMP and cAMP in plasma and tissues (32). It has been reported that APS has cytoprotective effects on the erythroid lineage K562 cells (33). In addition, APS may improve the response of C2C12 skeletal muscle myotubes and myoblasts to peroxide-induced injury in vitro by inhibiting apoptosis (34). However, the effect and underlying mechanisms of APS on H2O2-induced injury in HUVECs remain to be elucidated.
In the present study, the effects of APS on H2O2-induced injury in HUVECs in vitro and the possible underlying mechanisms were investigated. The results indicated that APS protected HUVECs from H2O2-induced apoptosis. Furthermore, APS markedly decreased intracellular ROS levels, increased protein expression levels of eNOS and Cu/Zn-SOD, partially restored the mitochondrial membrane potential and increased levels of cGMP, compared with cells treated with H2O2 alone. The underlying mechanisms of APS against HUVEC apoptosis may involve an increase in antioxidant defense systems and an increase in the production and bioavailability of NO.
NO, an endothelium-dependent vasodilator, is an important mediator in the regulation of endothelial cell function. In addition, it has numerous biochemical activities, including directly scavenging superoxide, maintaining endothelial integrity, attenuating leukocyte adhesion and inhibiting platelet aggregation (5). Endothelial NO produced by eNOS may inhibit apoptosis, and is regarded as a survival factor for endothelial cells (35). It has been reported that the reduced formation of NO or impairment of NO effects may be associated with aortic sclerosis (36). Additional studies have indicated that reduced NO bioavailability enhances atherogenesis in animal models (8), and contributes to endothelial dysfunction (37). In the current study, the intracellular cGMP levels were detected to evaluate the bioavailability of NO, as it is the second messenger of NO. The results suggested that APS pretreatment may increase the levels of cGMP and the protein expression levels of eNOS, compared with cells treated with H2O2 alone. This indicated that APS may protect HUVECs from injury induced by H2O2 via increasing the production and bioavailability of NO.
ROS, the free radicals present in all vascular cells, have been demonstrated to serve an important role in endothelial injury (38). Numerous studies have suggested that ROS are involved in vascular remodeling and endothelial dysfunction (39). Endothelial cells express a variety of enzymes from which ROS may be generated. Therefore, endothelial cells are regarded as an important source of vascular ROS production (40). In addition, mitochondria are important physiological sources of ROS. Furthermore, it has been reported that H2O2 may cause endothelial cell injury by inducing mitochondrial dysfunction, including loss of mitochondrial membrane potential (41).
Reduced bioavailability of NO contributes to endothelial dysfunction. The overproduction of ROS may inactivate NO by reacting with it to decrease NO synthesis and reduce NO bioavailability. Previous studies have suggested that the imbalance between ROS and NO levels, rather than the levels of each, may be a primary cause of endothelial dysfunction in numerous cardiovascular diseases. The results of the present study suggested that APS may protect endothelial cells against injury induced by H2O2 via increasing the protein expression levels of Cu/Zn-SOD. This may lead to superoxide scavenging, restoring mitochondrial membrane potential and decreasing the intracellular ROS levels detected by DHE staining.
In conclusion, the present study demonstrated that APS had a protective effect against H2O2-induced injury and apoptosis in HUVECs. The underlying mechanisms of the protective effects of APS may involve restoration of the balance between ROS and NO levels via increasing the cell antioxidant capacity and NO bioavailability.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (grant no. 81374008).
Glossary
Abbreviations
- APS
Astragalus polysaccharide
- ROS
reactive oxygen species
- HUVECs
human umbilical vein endothelial cells
References
- 1.Karsan A, Harlan JM. Modulation of endothelial cell apoptosis: Mechanisms and pathophysiological roles. J Atheroscler Thromb. 1996;3:75–80. doi: 10.5551/jat1994.3.75. [DOI] [PubMed] [Google Scholar]
- 2.Harrison D, Griendling KK, Landmesser U, Hornig B, Drexler H. Role of oxidative stress in atherosclerosis. Am J Cardiol. 2003;91:7A–11A. doi: 10.1016/S0002-9149(02)03144-2. [DOI] [PubMed] [Google Scholar]
- 3.Szymanski MK, Buikema JH, Van Veldhuisen DJ, Koster J, van der Velden J, Hamdani N, Hillege JL, Schoemaker RG. Increased cardiovascular risk in rats with primary renal dysfunction; mediating role for vascular endothelial function. Basic Res Cardiol. 2012;107:242. doi: 10.1007/s00395-011-0242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li JM, Shah AM. Endothelial cell superoxide generation: Regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 5.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 6.Birukov KG. Cyclic stretch, reactive oxygen species, and vascular remodeling. Antioxid Redox Signal. 2009;11:1651–1667. doi: 10.1089/ars.2008.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urso C, Caimi G. Oxidative stress and endothelial dysfunction. Minerva Med. 2011;102:59–77. [PubMed] [Google Scholar]
- 8.Lu X, Dang CQ, Guo X, Molloi S, Wassall CD, Kemple MD, Kassab GS. Elevated oxidative stress and endothelial dysfunction in right coronary artery of right ventricular hypertrophy. J Appl Physiol (1985) 2011;110:1674–1681. doi: 10.1152/japplphysiol.00744.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee YJ, Kang IJ, Bünger R, Kang YH. Mechanisms of pyruvate inhibition of oxidant-induced apoptosis in human endothelial cells. Microvasc Res. 2003;66:91–101. doi: 10.1016/S0026-2862(03)00052-9. [DOI] [PubMed] [Google Scholar]
- 10.Maio R, Perticone M, Sciacqua A, Tassone EJ, Naccarato P, Bagnato C, Iannopollo G, Sesti G, Perticone F. Oxidative stress impairs endothelial function in nondipper hypertensive patients. Cardiovasc Ther. 2012;30:85–92. doi: 10.1111/j.1755-5922.2010.00183.x. [DOI] [PubMed] [Google Scholar]
- 11.Nohl H, Kozlov AV, Gille L, Staniek K. Cell respiration and formation of reactive oxygen species: Facts and artefacts. Biochem Soc Trans. 2003;31:1308–1311. doi: 10.1042/bst0311308. [DOI] [PubMed] [Google Scholar]
- 12.Yang B, Oo TN, Rizzo V. Lipid rafts mediate H2O2 prosurvival effects in cultured endothelial cells. FASEB J. 2006;20:1501–1503. doi: 10.1096/fj.05-5359fje. [DOI] [PubMed] [Google Scholar]
- 13.Wang YK, Hong YJ, Wei M, Wu Y, Huang ZQ, Chen RZ, Chen HZ. Curculigoside attenuates human umbilical vein endothelial cell injury induced by H2O2. J Ethnopharmacol. 2010;132:233–239. doi: 10.1016/j.jep.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Chen HB, Guo BL, Zhao ZZ, Liang ZT, Yi T. Study of the relationship between genetics and geography in determining the quality of Astragali Radix. Biol Pharm Bull. 2011;34:1404–1412. doi: 10.1248/bpb.34.1404. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Wang HX, Zhang YJ, Lu ML, Zhang J, Li ST, Zhang SP, Li G. Astragaloside IV attenuates inflammatory cytokines by inhibiting TLR4/NF-κB signaling pathway in isoproterenol-induced myocardial hypertrophy. J Ethnopharmacol. 2013;150:1062–1070. doi: 10.1016/j.jep.2013.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Hu Y, Sun J, Kong X, Zhang B, Liu J. Comparative study on adjuvanticity of compound Chinese herbal medicinal ingredients. Vaccine. 2005;23:3704–3708. doi: 10.1016/j.vaccine.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Huang WM, Liang YQ, Tang LJ, Ding Y, Wang XH. Antioxidant and anti-inflammatory effects of Astragalus polysaccharide on EA.hy926 cells. Exp Ther Med. 2013;6:199–203. doi: 10.3892/etm.2013.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu J, Chen X, Zhang Y, Xu J, Zhang L, Li Z, Liu W, Ouyang J, Han S, He X. Astragalus polysaccharide induces anti-inflammatory effects dependent on AMPK activity in palmitate-treated RAW264.7 cells. Int J Mol Med. 2013;31:1463–1470. doi: 10.3892/ijmm.2013.1335. [DOI] [PubMed] [Google Scholar]
- 19.Shao BM, Xu W, Dai H, Tu P, Li Z, Gao XM. A study on the immune receptors for polysaccharides from the roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem Biophys Res Commun. 2004;320:1103–1111. doi: 10.1016/j.bbrc.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 20.Luan A, Tang F, Yang Y, Lu M, Wang H, Zhang Y. Astragalus polysaccharide attenuates isoproterenol-induced cardiac hypertrophy by regulating TNF-α/PGC-1α signaling mediated energy biosynthesis. Environ Toxicol Pharmacol. 2015;39:1081–1090. doi: 10.1016/j.etap.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Dai H, Jia G, Liu X, Liu Z, Wang H. Astragalus polysaccharide inhibits isoprenaline-induced cardiac hypertrophy via suppressing Ca2+-mediated calcineurin/NFATc3 and CaMKII signaling cascades. Environ Toxicol Pharmacol. 2014;38:263–271. doi: 10.1016/j.etap.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Li ZL, Liu JC, Hu J, Li XQ, Wang SW, Yi DH, Zhao MG. Protective effects of hyperoside against human umbilical vein endothelial cell damage induced by hydrogen peroxide. J Ethnopharmacol. 2012;139:388–394. doi: 10.1016/j.jep.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 23.Gong G, Qin Y, Huang W, Zhou S, Yang X, Li D. Rutin inhibits hydrogen peroxide-induced apoptosis through regulating reactive oxygen species mediated mitochondrial dysfunction pathway in human umbilical vein endothelial cells. Eur J Pharmacol. 2010;628:27–35. doi: 10.1016/j.ejphar.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 24.Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, Nakada K, Honma Y, Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- 25.Gonda K, Tsuchiya H, Sakabe T, Akechi Y, Ikeda R, Nishio R, Terabayashi K, Ishii K, Matsumi Y, Ashla AA, et al. Synthetic retinoid CD437 induces mitochondria-mediated apoptosis in hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2008;370:629–633. doi: 10.1016/j.bbrc.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 26.Han YS, Lee CS. Antidepressants reveal differential effect against 1-methyl-4-phenylpyridinium toxicity in differentiated PC12 cells. Eur J Pharmacol. 2009;604:36–44. doi: 10.1016/j.ejphar.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Calderone A, Thaik CM, Takahashi N, Chang DL, Colucci WS. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest. 1998;101:812–818. doi: 10.1172/JCI119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bleske BE, Hwang HS, Zineh I, Ghannam MG, Boluyt MO. Evaluation of immunomodulatory biomarkers in a pressure overload model of heart failure. Pharmacotherapy. 2007;27:504–509. doi: 10.1592/phco.27.4.504. [DOI] [PubMed] [Google Scholar]
- 29.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: The role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.RES.87.10.840. [DOI] [PubMed] [Google Scholar]
- 30.Vita JA, Keaney JF., Jr Endothelial function: A barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.CIR.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 31.Poulikakos D, Ross L, Recio-Mayoral A, Cole D, Andoh J, Chitalia N, Sharma R, Kaski J Carlos, Banerjee D. Left ventricular hypertrophy and endothelial dysfunction in chronic kidney disease. Eur Heart J Cardiovasc Imaging. 2014;15:56–61. doi: 10.1093/ehjci/jet120. [DOI] [PubMed] [Google Scholar]
- 32.Zhang BQ, Hu SJ, Qiu LH, Zhu JH, Xie XJ, Sun J, Zhu ZH, Xia Q, Bian K. Effects of Astragalus membranaceus and its main components on the acute phase endothelial dysfunction induced by homocysteine. Vascul Pharmacol. 2007;46:278–285. doi: 10.1016/j.vph.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Yang M, Qian XH, Zhao DH, Fu SZ. Effects of Astragalus polysaccharide on the erythroid lineage and microarray analysis in K562 cells. J Ethnopharmacol. 2010;127:242–250. doi: 10.1016/j.jep.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Lu L, Wang DT, Shi Y, Yin Y, Wei LB, Zou YC, Huang B, Zhao Y, Wang M, Wan H, et al. Astragalus polysaccharide improves muscle atrophy from dexamethasone- and peroxide-induced injury in vitro. Int J Biol Macromol. 2013;61:7–16. doi: 10.1016/j.ijbiomac.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Dimmeler S, Zeiher AM. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 1999;6:964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- 36.Nightingale AK, Sverdlov AL, Rajendran S, Mishra K, Heresztyn T, Ngo DT, Horowitz JD. Lack of association between aortic sclerosis and left ventricular hypertrophy in elderly subjects. Int J Cardiol. 2011;150:33–38. doi: 10.1016/j.ijcard.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 37.Clapp BR, Hingorani AD, Kharbanda RK, Mohamed-Ali V, Stephens JW, Vallance P, MacAllister RJ. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc Res. 2004;64:172–178. doi: 10.1016/j.cardiores.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 38.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: A new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: Implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- 40.Cai H. Hydrogen peroxide regulation of endothelial function: Origins, mechanisms and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 41.Liu YM, Jiang B, Bao YM, An LJ. Protocatechuic acid inhibits apoptosis by mitochondrial dysfunction in rotenone-induced PC12 cells. Toxicol In Vitro. 2008;22:430–437. doi: 10.1016/j.tiv.2007.10.012. [DOI] [PubMed] [Google Scholar]