Abstract
Bortezomib, a first-in-class proteasome inhibitor, is a standard method of treatment in multiple myeloma. In the present study, the therapeutic effect of bortezomib was evaluated in an ulcerative colitis model induced by dextran sulfate sodium (DSS) in mice, and the mechanism of action was also investigated. Mice were administered with 3% DSS drinking water for 7 consecutive days and then they were intraperitoneally treated with bortezomib (0.2, 0.6 or 1 mg/kg) for 1, 3 or 7 days. Mice in the control group and the DSS group were provided the same volume of PBS, respectively. Body weight, stool characteristics and hematochezia were observed. Serum levels of tumor necrosis factor-α (TNF-α), C-reactive protein (CRP), albumin (ALB) and colonic activity of superoxide dismutase (SOD) were evaluated using specific kits. The expression of the transcription factor nuclear factor-κB (NF-κB) p65 gene and the DNA-binding activity of NF-κB protein were also evaluated. Administration of bortezomib attenuates colonic inflammation in mice. After 3 or 7 days of treatment, Disease Activity Index (DAI) as well as histological scores and NF-κB p65 protein expression were significantly reduced in mice treated with bortezomib at a dose of 0.6 or 1 mg/kg/day. Furthermore, it was also revealed that bortezomib was able to reduce serum levels of CRP and TNF-α caused by DSS and increase the level of ALB in serum and the activity of SOD in colonic tissues. These results demonstrated that bortezomib exerts a protective effect on DSS-induced colitis, and its underlying mechanisms are associated with the NF-κB gene inhibition that mitigates colon inflammatory responses in intestinal epithelial cells.
Keywords: bortezomib, dextran sulfate sodium, ulcerative colitis, nuclear factor-κB
Introduction
Ulcerative colitis is an inflammatory bowel disease (IBD) of the intestinal tract that features a chronic, relapsing and remitting inflammatory condition of the intestine (1). Common symptoms of ulcerative colitis include abdominal pain, vomiting, diarrhea, weight loss and bloody stools (2). Furthermore, this disease is also associated with increased risk of colorectal cancer (3).
Currently, common agents used to treat ulcerative colitis are sulfasalazine (SASP), 5-aminosalicylic acid (5-ASA), corticosteroids and immunosuppressive agents. However, the majority of these drugs frequently led to multiple adverse events and exhibit limited benefits in long-term disease control (4). SASP is able to cause unwanted effects on male fertility (5) and prolonged or high-dose corticosteroid therapy is associated with hypertension, osteoporosis and immunodeficiency (6). Anti-tumor necrosis factor-α (TNF-α) agents, including infliximab, are approved for ulcerative colitis treatment and although they show high efficacy, they also cause adverse events, including immunogenicity, lymphoma and neuropathy (7,8). Although a number of therapeutic options exist for ulcerative colitis, novel therapies with high efficacy and safety are still required.
Although the pathogenesis and biological molecular mechanism of ulcerative colitis remains to be elucidated, evidence suggests that various inflammatory mediators, particularly pro-inflammatory cytokines, serve a crucial role in the pathogenesis of this disease (9,10). The transcription factor nuclear factor-κB (NF-κB), which has been demonstrated to be constitutively active in ulcerative colitis, serves a role in controlling the activation of various pro-inflammatory cytokine genes involved in ulcerative colitis (11,12). Therefore, inhibition of NF-κB activation has been suggested to be an anti-inflammatory strategy in this disease.
Bortezomib (formerly PS-341), a first-in-class proteasome inhibitor, has become a standard treatment for multiple myeloma. Bortezomib is involved in the ubiquitin-proteasome pathway of cellular protein homeostasis, inhibiting the action of the 26S proteasome (13). The agent is thought to exert its antimyeloma effects partly through the stabilization of NF-κB inhibitor (IκB), resulting in the inhibition of NF-κB (14). However, it remains unclear whether the NF-κB inhibitory activity of bortezomib is effective against ulcerative colitis.
The present study was designed to evaluate the effect of bortezomib on dextran sulfate sodium (DSS)-induced ulcerative colitis in Balb/c mice. Clinical parameters, histological injury and pro-inflammatory cytokine expression were determined. To elucidate the molecular mechanisms, the effect of bortezomib on the NF-κB signaling pathway was examined. Furthermore, the effect of bortezomib dose on its anti-inflammatory properties was examined.
Materials and methods
Animals
A total of 160 male Balb/c mice (weight, 20–25 g; age, 8 weeks old) were provided by the Animal Department of The Second Affiliated Hospital, Harbin Medical University (Harbin, China). The animals were maintained in our animal laboratory center under standard conditions (temperature, 24–25°C; humidity, 70–75%; lighting regimen, 12 h day/night cycle). The experimental protocol was approved by the Institutional Animal Care and Use Committee of Harbin Medical University. Prior to conducting experiments, the animals were acclimatized to laboratory conditions for 7 days.
The Balb/c mice were randomly divided into the following 5 groups: Normal control group; model group; and high-dose, medium-dose and low-dose bortezomib groups (n=32 in each group). There was no significant difference in body weight among the groups. P<0.05 was considered to indicate a statistically significant difference.
Induction of colitis and treatment
Normal water intake was administered to the normal control group. The other 4 groups were provided with water containing 3% (w/v) DSS (molecular weight, 36,000–50,000 kDa; MP Biomedicals, Santa Ana, CA, USA) for 7 days. Subsequently, the animals were given the following treatments: normal control [PBS; intraperitoneal (i.p.) injection]; model group (PBS; i.p. injection); high-dose group (bortezomib dose, 1.0 mg/kg; i.p. injection); medium-dose group (bortezomib dose, 0.6 mg/kg; i.p. injection); low-dose group, (bortezomib dose, 0.2 mg/kg; i.p. injection) for 1, 3 or 7 days. Bortezomib was obtained from Ben Venue Laboratories, Inc. (Bedford, OH, USA).
The clinical activity of colitis was evaluated by an independent observer, who was blind to the treatment, using the Disease Activity Index (DAI) as described by Murthy et al (15). This index consisted of three scales: Weight loss; stool consistency; and the presence of rectal bleeding. Weight loss scores were determined as follows: 0, no weight loss; 1, 1–5% weight loss; 2, 6–10% weight loss; 3, 11–15% weight loss; and 4, >15% weight loss. Stool characteristic scores were determined as follows: 0, Normal stools; 2, loose stools; and 4, diarrhea (grades 1 and 3 do not exist in this scale). Bleeding scores were determined as: 0, No bleeding; 1, minimal color change to blue; 2, maximal color change to blue; 3, blood visibly present in the stool and no clotting on the anus and 4, gross bleeding from the anus with clotting present. The sum of the 3 category scores adds up to the total score.
Histopathological evaluation
The mice were euthanized on day 0, 1, 3 and 7 of administration. At the end of the experimental period, the colons were removed, cleaned in physiological saline to remove fecal residues, and subsequently fixed in 4% buffered formaldehyde at 37°C for 24 h and embedded in paraffin. After deparaffinization in xylene and rehydration in a series of graded alcohol at 37°C for 30 min, slices (5 µm) from the paraffin sections were stained with hematoxylin and eosin (H&E) at 37°C for 10 min and observed under a light microscope (Olympus BX51; Olympus Corporation, Tokyo, Japan) and graded by a pathologist who was blind to the experimental protocol. Histological grading criteria are presented in Table I. The rest of the pieces of the colon were collected and frozen in liquid nitrogen.
Table I.
Histological grading criteria.
| Parameters | Score | Histological feature |
|---|---|---|
| Ulcer | 0 | None |
| 1 | Ulcer, <3 mm | |
| 2 | Ulcer, >3 mm | |
| Inflammation | 0 | None |
| 1 | Slight | |
| 2 | Severe | |
| Granuloma | 0 | None |
| 1 | Granuloma | |
| Extent | 0 | None |
| 1 | Mucosa | |
| 2 | Mucosa and submucosa | |
| 3 | Transmural | |
| Fibrosis | 0 | None |
| 1 | Slight | |
| 2 | Severe |
Serum albumin (ALB), C-reactive protein (CRP) and TNF-α evaluation
Animals were anesthetized with i.p. ketamine chloride (90 mg/kg body weight). Blood was collected by cardiac puncture and subsequently centrifuged at 3,000 × g for 15 min at 4°C, to measure the concentrations of albumin, CRP and TNF-α. Serum levels of CRP (mg/l) were determined using a high-sensitivity immunoturbidimetric assay (Denka Seiken Co., Tokyo, Japan). ALB levels (g/l) were determined with the mouse Albumin ELISA kit (Catalogue #CSB-E13878 m, Cusabio, Wuhan, China). The levels of TNF-α (ng/l) were determined using a commercially obtained ELISA kit specific for mouse TNF-α (Wuhan Boster Biological Technology, Ltd., Wuhan, China).
Superoxide dismutase (SOD)
SOD activity in the colon tissue was measured according to the protocol described by Fridovich (16). This protocol uses xanthine and xanthine oxidase to generate superoxide radicals, which are able to react with p-iodonitrotetrazolium violet to form a red formazan dye, which can be measured spectrophotometrically at 505 nm. The assay medium contained 0.01 M phosphate buffer (pH 6.8), 3-cyclohexilamino-1-propanesulfonicacid (CAPS) buffer solution pH 10.2 (50 mM CAPS, 0.94 mM EDTA, saturated NaOH), substrate solution (0.05 mM xanthine, 0.025 mM iodonitrotetrazolium chloride) and 80 µl xanthine oxidase. The results were expressed in SOD U/mg protein in the wet tissues.
Immunohistochemical analysis (NF-κB p65)
Immuno-histochemical (IHC) staining was conducted using the streptavidin-biotin complex (strept-ABC) method (Wuhan Boster Biological Technology, Ltd.). Unstained 4-mm sections were cut from paraffin blocks and incubated at 65°C for 30 min. The slides were deparaffinized in xylene followed by absolute ethanol and subsequent rehydration in graded ethanol. Antigen retrieval was performed by immersing slides in boiling citric acid buffer (pH 6.0) for 15 min. The sections were then pretreated with 3% hydrogen peroxide for 15 min to inactivate endogenous peroxides, followed by incubation with 10% goat serum (Sigma-Aldrich; Merck KGaA) at 37°C for 30 min to block the non-specific binding. Slides were incubated overnight at 4°C with anti-NF-κB p65 antibody (3301; 1:100; Wuhan Boster Biological Technology, Ltd.), subsequently washed with PBS before applying the biotinylated secondary antibody (SE587; Sigma-Aldrich; Merck KGaA) at 37°C for 10 min. Sections were incubated with the strept-ABC complex reagent for 15 min, and subsequently exposed to 3,3′-diaminobenzidine, counterstained with hematoxylin and examined by light microscopy.
The degree of stained sections was scored in duplicate by two independent investigators without any histopathological information. IHC scores were determined by combining the proportion of positively stained cells and intensity of staining in five different high power fields (x400) for each section. Staining intensity was graded as follows: 0, no staining; 1, weak staining (light yellow); 2, moderate staining (yellowish brown); and 3, strong staining (brown). The percentage of positive cells was scored according to the following criteria: 0, no positive cells; 1, ≤25% of cells stained positive; 2, 26–50% of cells stained positive; 3, 51–75% of cells stained positive; and iv) >75% of cells stained positive.
Electrophoretic mobility shift assay (EMSA)
The DNA-binding activity of NF-κB was assessed in nuclear extracts by EMSA. The nuclear extracts were conducted as below: DTT and PIC were added by extraction buffer, incubated on ice for 15 min, centrifuged at 3,000 × g for 10 min at 4°C and prepared for nuclear extraction. Double-stranded oligonucleotides for NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′; Promega Corp., Madison, WI, USA) were labeled on the 3′ end with biotin. EMSA was performed using the Light Shift Chemiluminescent EMSA kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's protocol. Binding reactions containing 10 µg nuclear protein, 10 mM Tris, 50 mM KCl, 1 mM DTT, 2.5% glycerol, 5 mM MgCl2, 0.05% Nonidet P-40 (Sigma-Aldrich; Merck KGaA) and 2 pM oligonucleotide probe were incubated for 15 min at 37°C. Specific binding was confirmed by using a 100-fold excess of unlabeled probe as a specific competitor. Following incubation, samples were separated by electrophoresis on a 6% non-denaturing polyacrylamide gel. Complexes were electroblotted to positively charged nylon membranes and UV-cross-linked in a Stratagene crosslinker. Gel shifts were visualized by exposure to chemiluminescence detection using a luminol-based reagent and X-ray film (Promega Corp.).
Statistical analyses
All data are expressed as the mean ± standard deviation. Differences among groups were tested for statistical significance using analysis of variance and the Student-Newman-Keuls test. Statistical analysis was performed using SPSS software (version 17.0; SSPS, Inc., Chicago, IL, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of treatment with bortezomib on DSS-induced ulcerative colitis
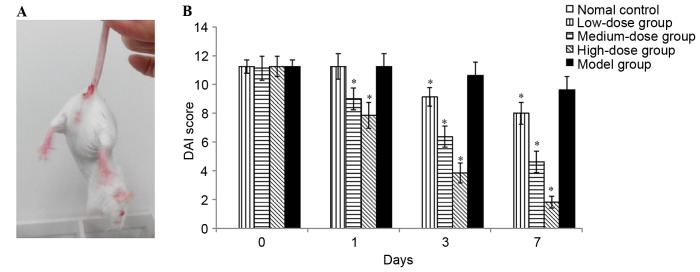
The clinical symptoms of body weight loss, rectal bleeding and loose stools were observed on day 5 in the mice treated with DSS (Fig. 1A). As presented in Fig. 1B, DAI was 0 in the normal control group from day 0 to 7 of administration, indicating no disease symptoms (diarrhea and blood). By contrast, the DAI scores were increased in all DSS-treated groups. There were significantly decreased DAI scores in the high-dose and medium-dose bortezomib groups when compared with the PBS-treated model group. However, no significant improvements were observed for 1-day bortezomib treatment at a dose of 0.2 mg/kg. The results demonstrated that bortezomib exerted protective effects in a time- and dose-dependent manner. High-dose (1 mg/kg) bortezomib treatment exhibited a significantly improved therapeutic effect compared with medium-(0.6 mg/kg) and low-dose group (0.2 mg/kg). Following a 7-day treatment with high-dose bortezomib, the clinical symptoms of colitis improved significantly with an average DAI score of 1.83 (Fig. 1B) However, four mice died in the high-dose (1 mg/kg) group, one died in 1 day, one in 3 days and two in 7 days of administration.
Figure 1.
Effect of bortezomib on DSS-induced ulcerative colitis in mice. (A) Severe clinical symptoms, including rectal bleeding, were observed on day 5 in mice treated with DSS. (B) DAI was evaluated as the average score of body weight changes, rectal bleeding and stool consistency. Each value represents the mean ± standard deviation of the mean. *P<0.05 vs. model group. DSS, dextran sulfate sodium; DAI, disease activity index.
Histological evaluation
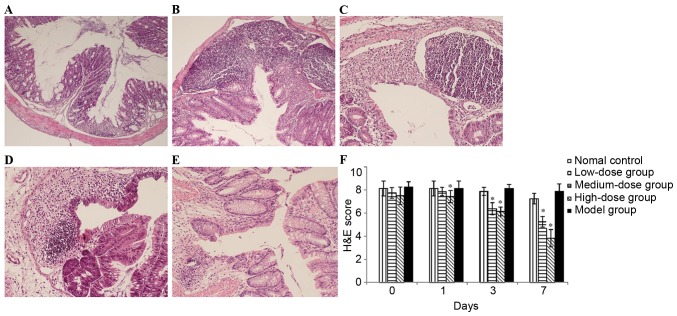
To further evaluate disease severity, histopathological evaluation of H&E-stained sections was performed. Histological sections of colonic tissue from the control group demonstrated a normal structure without histological changes (Fig. 2A). By contrast, the administration of DSS caused injuries that affected the majority of the mucosa, with the loss of histological structure, strong epithelial disintegration, inflammatory cell infiltration, edema and crypt abscesses (Fig. 2B). Following treatment with medium-dose or high-dose bortezomib for 3 or 7 days, inflammation and crypt damages were significantly reduced and the histological scores were also significantly decreased (Fig. 2C-F), which was consistent with clinical findings. In addition, low-dose bortezomib treatment did not induce a significant decrease in the histological scores. Therefore, treatment with bortezomib (1 and 0.6 mg/kg) was able to reduce DSS-induced colitis clinically and histopathologically.
Figure 2.
Examination and scoring of histological sections of colonic sections following H&E staining. (A) mice in normal control group; (B) mice in model group; (C) mice treated with 1 day bortezomib at dose of 1.0 mg/kg; (D) mice treated with 3 days bortezomib at dose of 1.0 mg/kg; (E) mice treated with 7 days bortezomib at dose of 1.0 mg/kg; (F) histological analysis using a colitis score. Each value is presented as the mean ± standard deviation of the mean. *P<0.05 vs. the model group. H&E, hematoxylin and eosin.
Effects of treatment with bortezomib on serum ALB, CRP and TNF-α levels
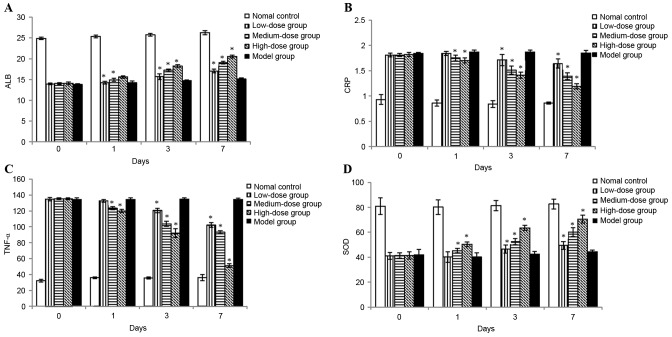
To assess the effect of bortezomib on anti-inflammatory activity, serum levels of ALB, CRP and TNF-α were measured following drug administration. Compared with the normal control group, the levels of CRP and TNF-α were significantly increased in the model group. However, the increase of these cytokines was suppressed by treatment with bortezomib in a time- and dose-dependent manner (Fig. 3). Serum ALB levels, which were negatively associated with inflammatory activity, were significantly decreased with DSS-induction. Following treatment with bortezomib, ALB levels were markedly increased (Fig. 3).
Figure 3.
Effects of bortezomib on inflammation-associated substances following colitis induction. (A) ALB (g/l) in serum; (B) CRP (mg/l) in serum; (C) TNF-α (ng/l) in serum; and (D) SOD (U/mg) activity in the colon. Each value is presented as the mean ± standard deviation of the mean. *P<0.05 vs. the model group. ALB, albumin; CRP, C-reactive protein; TNF-α, tumor necrosis factor-α; SOD, superoxide dismutase.
SOD in colon tissue increases after bortezomib treatment
To assess the effect of bortezomib on antioxidative functions, SOD activity in colon tissue was evaluated. Colonic injury induced by DSS administration was accompanied by markedly decreased SOD activity as compared with normal controls. Treatment with bortezomib significantly increased the SOD activity and restored the levels towards normal control values. In addition, the increase in SOD level was markedly increased with high-dose bortezomib when compared with low-dose and medium-dose treatment with bortezomib (Fig. 3).
Effects of treatment with bortezomib on NF-κB expression in DSS-induced ulcerative colitis
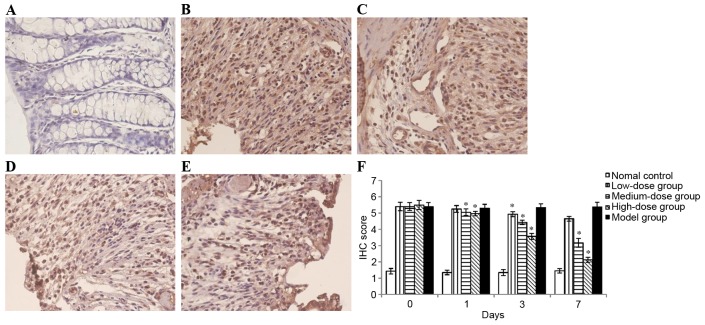
NF-κB is an essential transcription factor that serves a central role in the activation of various inflammatory mediators. According to the results obtained, nuclear protein extracts of colon tissues demonstrated that the nuclear translocation levels of p65 protein were significantly increased in mice treated with DSS as compared with normal control (Fig. 4). However, treatment with bortezomib decreased the DSS-induced nuclear translocation level of p65 in colonic mucosa (Fig. 4). In addition, the administration of DSS enhanced NF-κB DNA binding activity in colonic tissue, which was suppressed by bortezomib administration in time- and dose-dependent manner (Fig. 5).
Figure 4.
IHC staining of NF-κB p65 in colon sections. (A) NF-κB p65 expression in normal colon; (B) NF-κB p65 expression in the colon of mice in the model group; NF-κB p65 expression in the colon of mice treated with bortezomib for 3 days at a dose of (C) 0.2, (D) 0.6 or (E) 1 mg/kg; (F) the degree of stained sections was scored. Each value is presented as the mean ± standard deviation of the mean. *P<0.05 vs. the model group. IHC, immunohistochemical; NF-κB, nuclear factor-κB.
Figure 5.
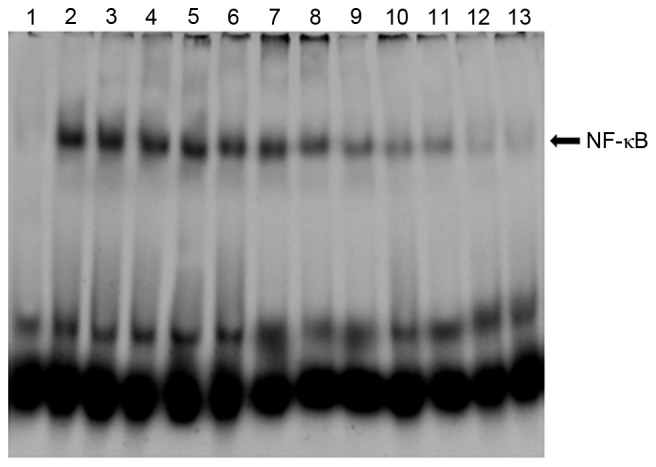
DNA binding activities of transcription factor NF-κB in colon tissues evaluated by the electrophoretic mobility shift assay. Lane 1, mice in normal control group; lane 2, mice in model group treated with PBS for 1 day; lane 3, mice in model group treated with PBS for 3 days; lane 4, mice in model group treated with PBS for 7 days; lane 5, mice in low-dose group treated with bortezomib for 1 day; lane 6, mice in low-dose group treated with bortezomib for 3 days; lane 7, mice in low-dose group treated with bortezomib for 7 days; lane 8, mice in medium-dose group treated with bortezomib for 1 day; lane 9, mice in medium-dose group treated with bortezomib for 3 days; lane 10, mice in medium-dose group treated with bortezomib for 7 days; lane 11, mice in high-dose group treated with bortezomib for 1 day; lane 12, mice in high-dose group treated with bortezomib for 3 days; lane 13, mice in high-dose group treated with bortezomib for 7 days. NF-κB, nuclear factor-κB.
Discussion
The present study focused on studying the effects of bortezomib on DSS-induced ulcerative colitis in mice. It was demonstrated that administration of bortezomib effectively attenuates colonic inflammation in mice. After 3- or 7-days of treatment, the DAI score, histological score and NF-κB expression were significantly reduced in mice treated with DSS at a dose of 0.6 or 1 mg/kg/day. Furthermore, it was revealed that bortezomib was able to reduce serum levels of CRP and TNF-α caused by DSS, increase serum levels of ALB, and colonic activity of SOD.
Various chemicals are used to induce ulcerative colitis in animal models, including 2,4,6-trinitrobenzenesulfonic acid, DSS, oxazolone, acetic acid and nonsteroidal anti-inflammatory drugs (17–21). The DSS-induced ulcerative colitis model is employed as a model of experimental colitis as it causes acute inflammatory reactions and ulceration in the colon, similar to that observed in patients. It is characterized by bloody diarrhea, body weight loss, inflammatory cell infiltration and surface epithelial cell loss (22,23). In the present study, DSS administration induced severe injury to the colon, represented by the elevated DAI score and histological damage score; however, it was markedly suppressed by bortezomib at a dose of 0.6 or 1 mg/kg/day. These results indicated that bortezomib is able to suppress DSS-induced ulcerative colitis clinically and histopathologically.
Although the exact etiology of ulcerative colitis remains to be elucidated, data from many studies in humans and animal models suggest that it is associated with the dysfunctional immunoregulation of the gastrointestinal tract. Among immunoregulatory factors, the abnormal presence of inflammatory cells is responsible for disrupting epithelial integrity, causing colon injury and leading to increased levels of pro-inflammatory cytokines, including TNF-α and CRP (24,25). TNF-α, which is a pleiotropic cytokine, serves an integral role in inflammatory conditions, including ulcerative colitis, by triggering leukocyte activation and tissue accumulation (25). CRP is one of the most important molecules in the host innate immune system, involved in protection against auto immunity and it is an acute phase protein stimulated by infection, inflammatory diseases, tissue infarction and neoplasia (26). In the present study, it was observed that the levels of TNF-α and CRP were elevated in mice serum with DSS-induced colitis. The administration of bortezomib significantly reduced the levels of serum of TNF-α and CRP. These results indicated that bortezomib ameliorates DSS-induced colitis by suppressing pro-inflammatory mediators, including TNF-α and CRP.
Oxidative stress also serves an important role in the pathogenesis of ulcerative colitis (25). SOD is an important element of cellular defense against oxidative damage (26). It catalyzes the dismutation of superoxide free radical to hydrogen peroxide and molecular oxygen (27). The results of the present study demonstrated that the colonic level of SOD activity decreased in DSS-induced colitis, whereas bortezomib significantly improved SOD activity after 3 or 7 days of treatment, therefore suggesting the anti-oxidative ability of bortezomib. These results suggested that bortezomib suppressed DSS-induced colitis through the enhancement of antioxidative functions in the colon.
It has been demonstrated that NF-κB serves an essential role in the activation of various inflammatory cytokines. Evidence indicated that NF-κB is a key regulator in ulcerative colitis as the expression and activation of NF-κB is markedly enhanced in the inflamed gut among patients with ulcerative colitis (28). In addition, the amount of activated NF-κB correlated significantly with the severity of intestinal inflammation (29). The results of the present study revealed that treatment with bortezomib decreased the DSS-induced nuclear translocation level of p65 and inhibited NF-κB DNA binding activity. The results suggested that administration of bortezomib effectively suppresses mucosal inflammation in the colon through the inhibition of NF-κB signal transduction pathways.
In the present study, it was revealed that bortezomib exerted a protective effect in a time- and dose-dependent manner. High-dose (1 mg/kg) bortezomib treatment had a significantly better therapeutic effect than medium- and low-dose treatment. However, four deaths were observed in the high-dose group, which implied the toxicity of high-dose bortezomib. Further studies are required in order to investigate which dose of bortezomib is more effective and safe in the treatment of ulcerative colitis.
In conclusion, the results of the present study demonstrated that treatment with bortezomib were able to significantly attenuate DSS-induced ulcerative colitis in mice. The mechanism underlying the protective effects involves NF-κB inhibition, which decreases the expressions of TNF-α and CRP. It is proposed that bortezomib may be a novel therapeutic drug in the treatment of ulcerative colitis.
Acknowledgements
The present study was supported by grants from the research project of Health and family planning commission of Heilongjiang province (grant no. 2014-332).
References
- 1.Poxton IR, Brown R, Sawyerr A, Ferguson A. Mucosa-associated bacterial flora of the human colon. J Med Microbiol. 1997;46:85–91. doi: 10.1099/00222615-46-1-85. [DOI] [PubMed] [Google Scholar]
- 2.Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J Int Med. 2008;263:597–606. doi: 10.1111/j.1365-2796.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 3.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lissner D, Siegmund B. Ulcerative colitis: Current and future treatment strategies. Dig Dis. 2013;31:91–94. doi: 10.1159/000347194. [DOI] [PubMed] [Google Scholar]
- 5.Alonso V, Linares V, Bellés M, Albina ML, Sirvent JJ, Domingo JL, Sánchez DJ. Sulfasalazine induced oxidative stress: A possible mechanism of male infertility. Reprod Toxicol. 2009;27:35–40. doi: 10.1016/j.reprotox.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Wang F, Zhang HJ, Sheng JQ, Yan WF, Ma MX, Fan RY, Gu F, Li CF, Chen DF, et al. Corticosteroid therapy in ulcerative colitis: Clinical response and predictors. World J Gastroenterol. 2015;21:3005–3015. doi: 10.3748/wjg.v21.i10.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrante M, Vermeire S, Fidder H, Schnitzler F, Noman M, Van Assche G, De Hertogh G, Hoffman I, D'Hoore A, Van Steen K, et al. Long-term outcome after infliximab for refractory ulcerative colitis. J Crohns Colitis. 2008;2:219–225. doi: 10.1016/j.crohns.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Khanna R, Feagan BG. Safety of infliximab for the treatment of inflammatory bowel disease: Current understanding of the potential for serious adverse events. Expert Opin Drug Saf. 2015;14:987–997. doi: 10.1517/14740338.2015.1029915. [DOI] [PubMed] [Google Scholar]
- 9.Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009;361:2066–2078. doi: 10.1056/NEJMra0804647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braus NA, Elliott DE. Advances in the pathogenesis and treatment of IBD. Clin Immunol. 2009;132:1–9. doi: 10.1016/j.clim.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto Y, Gaynor RB. Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest. 2001;107:135–142. doi: 10.1172/JCI11914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Setia S, Nehru B, Sanyal SN. Activation of NF-κB: Bridging the gap between inflammation and cancer in colitis-mediated colon carcinogenesis. Biomed Pharmacother. 2014;68:119–128. doi: 10.1016/j.biopha.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Richardson PG, Hideshima T, Anderson KC. Bortezomib (PS-341): A novel, first-in-class proteasome inhibitor for the treatment of multiple myeloma and other cancers. Cancer Control. 2003;10:361–369. doi: 10.1177/107327480301000502. [DOI] [PubMed] [Google Scholar]
- 14.Adams J. Proteasome inhibition in cancer: Development of PS-341. Semin Oncol. 2001;28:613–619. doi: 10.1053/sonc.2001.28609. [DOI] [PubMed] [Google Scholar]
- 15.Murthy SN, Cooper HS, Shim H, Shah RS, Ibrahim SA, Sedergran DJ. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–1734. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 16.Fridovich I. Superoxide radical: An endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983;23:239–257. doi: 10.1146/annurev.pa.23.040183.001323. [DOI] [PubMed] [Google Scholar]
- 17.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. doi: 10.1016/0016-5085(89)90904-9. [DOI] [PubMed] [Google Scholar]
- 18.Dharmani P, Leung P, Chadee K. Tumor necrosis factor-α and Muc2 mucin play major roles in disease onset and progression in dextran sodium sulphate-induced colitis. PLoS One. 2011;6:e25058. doi: 10.1371/journal.pone.0025058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Zhao J, Nakaguchi T, Gregersen H. Biomechanical changes in oxazolone-induced colitis in BALB/C mice. J Biomech. 2009;42:811–817. doi: 10.1016/j.jbiomech.2009.01.028. [DOI] [PubMed] [Google Scholar]
- 20.Hagar HH, El-Medany A, El-Eter E, Arafa M. Ameliorative effect of pyrrolidinedithiocarbamate on acetic acid-induced colitis in rats. Eur J Pharmacol. 2007;554:69–77. doi: 10.1016/j.ejphar.2006.09.066. [DOI] [PubMed] [Google Scholar]
- 21.Narushima S, Spitz DR, Oberley LW, Toyokuni S, Miyata T, Gunnett CA, Buettner GR, Zhang J, Ismail H, Lynch RG, Berg DJ. Evidence for oxidative stress in NSAID-induced colitis in IL10-/- mice. Free Radic Biol Med. 2003;34:1153–1166. doi: 10.1016/S0891-5849(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 22.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis. 2003;24:353–362. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 23.Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sandborn WJ, Targan SR. Biologic therapy of inflammatory bowel disease. Gastroenterology. 2002;122:1592–1608. doi: 10.1053/gast.2002.33426. [DOI] [PubMed] [Google Scholar]
- 25.Vermeire S, Assche G, Rutgeerts P. C-reactive protein as a marker for inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:661–665. doi: 10.1097/00054725-200409000-00026. [DOI] [PubMed] [Google Scholar]
- 26.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 27.Piechota-Polanczyk A, Fichna J. Review article: The role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:605–620. doi: 10.1007/s00210-014-0985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neurath MF, Pettersson S, zum Buschenfelde KH Meyer, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 29.Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, Knuechel R, Baeuerle PA, Schölmerich J, Gross V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/S0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]