Abstract
Long non-coding RNA (lncRNA), transcripts of >200 bp in length that do not appear to exhibit any coding capacity, are important in the occurrence and development of cancer, cardiovascular and neurological diseases. However, effects of lncRNAs on photo-aging remain to be elucidated. To explore the potential effects of the lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) on photo-aging in fibroblasts, MALAT1 expression was silenced in fibroblasts using small interference RNA. Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was used to examine MALAT1 expression in normal and silenced fibroblasts following irradiation with 60 mJ/cm2 ultraviolet B (UVB) and an ELISA assay was used to identify matrix metalloproteinase-1 (MMP-1) content in the cellular supernatant. A β-galactosidase kit was applied to measure the number of senescent cells and a western blot assay was used to detect extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 phosphorylation levels. RT-qPCR was additionally used to detect changes in MALAT1 expression following suppression of UVB-induced reactive oxygen species (ROS) generation with N-acetyl-L-cysteine (NAC). Fibroblasts irradiated with 60 mJ/cm2 UVB demonstrated increased MALAT1 expression, MMP-1 secretory volume and number of senescent cells, and greater levels of ERK, p38 and JNK phosphorylation. Following silencing of MALAT1 expression in photo-aged fibroblasts, decreases were observed in MMP-1 secretory volume, number of senescent cells and phosphorylation levels of ERK. NAC reduced ROS content, however, it did not affect MALAT1 expression. Therefore, it was concluded that MALAT1 may participate in UVB-induced photo-aging via regulation of the ERK/mitogen-activated protein kinase signaling pathway and UVB-induced MALAT1 expression is independent of ROS generation.
Keywords: long non-coding RNAs, metastasis-associated lung adenocarcinoma transcript 1, photo-aging, matrix metalloproteinase-1, mitogen-activated protein kinase, reactive oxygen species
Introduction
Photo-aging of skin primarily refers to ultraviolet (UV) irradiation-induced skin aging, which generally manifests as exposed skin laxity, sagging, wrinkles, pigmentation, dryness, roughness, telangiectasia and the appearance of large pores (1). Primary histological features of photo-aged skin include reduction of collagen and accumulation of abnormal elastic fibers (2). UV irradiation may induce abundant secretion of matrix metalloproteinases (MMPs) from fibroblasts and keratinocytes, particularly matrix metalloproteinase-1 (MMP-1), which results in abnormal degradation of the extracellular matrix (1). MMP-1 expression during photo-aging is primarily controlled by the mitogen-activated protein kinase (MAPK) signaling pathway (3). A previous study confirmed that UV-induced reactive oxygen species (ROS) promote MMP-1 expression and induce the occurrence of photo-aging by upregulating phosphorylation levels of the MAPK signaling pathway elements c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK) and p38 (4).
Long non-coding RNA (lncRNA) transcripts that are >200 bp in length, which do not appear to exhibit coding capacity, regulate gene expression at transcriptional, post-transcriptional and translational levels (5,6). Metastasis-associated lung adenocarcinoma transcript 1 (MALAT1), an evolutionarily conserved lncRNA expressed in a wide range of species and tissues, exhibits biological functions including nuclear organization and architecture, gene splicing, and epigenetic modulation of gene expression (7,8). MALAT1 is important in the occurrence and development of cancer, cardiovascular and diabetes-associated microvascular disease (9–11). It has previously been demonstrated that MALAT1 interacts with the MAPK signaling pathway. MALAT1 may activate ERK/MAPK signaling to promote proliferation and metastasis of gallbladder cancer cells (12). Following silencing MALAT1 expression, the p38/MAPK signaling pathway has been demonstrated to be downregulated in endothelial cells (10). Activation of MAPK signaling exerts a key effect on photo-aging, and the present study aimed to investigate the role of MALAT1 in UV-induced photo-aging and any potential interaction with the MAPK signaling pathway elements.
The present study is the first, to the best of the authors' knowledge, to demonstrate that 60 mJ/cm2 UVB irradiation induces high expression of MALAT1 in fibroblasts. MALAT1 siRNA potentially suppressed UVB-induced MMP-1 secretion and fibroblast senescence by inhibiting activation of the ERK/MAPK signaling pathway.
Materials and methods
Primary culture of fibroblasts
Foreskin tissue was donated (with signed informed consent) by a healthy young male following circumcision. The donor's health status was established through physical examinations and laboratory investigations which revealed no obvious abnormalities. The sample was immersed in an iodine complex for 15 min and washed three times with phosphate buffered saline (PBS). Following removal of subcutaneous tissue, the sample was cut into pieces and digested with trypsin to isolate fibroblasts. Fibroblasts were collected, washed, and then incubated with high-glucose Dulbecco's Modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA). Cells in logarithmic phase from the third to eighth passage were used for experiments.
Ultraviolet B (UVB) irradiation
Cells that had reached ~70% confluence were irradiated with UVB using a SS-07 light therapy device (36 W power; time of irradiation automatically adjusted to dose of irradiation; cat. no. 1,047,469; Shanghai Sigma Hi-Tech Co., Ltd., Shanghai, China). Prior to irradiation, culture medium was removed from cells, which were then washed twice with PBS. Fibroblasts, coated with a thin layer of PBS, were vertically irradiated with UVB immediately following opening the culture dish cover. The distance of irradiation was 20 cm. Complete medium was added to the culture dish immediately following irradiation and the culture dish was then incubated in the original culture environment.
Chemical treatments
MALAT1 siRNA (5′-CACAGGGAAAGCGAGTGGTTGGTAA-3′) and the negative control non-targeting siRNA were designed and synthesized by GeneChem Co., Ltd. (Shanghai, China). When cells reached 30–50% confluence, siRNAs (50 nM) were transfected into cells using Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.,) in serum-free DMEM according to the manufacturer's protocol. Following 6 h of incubation, cells were returned to normal medium in the incubator. When cells reached 70% confluence, they were exposed to irradiation. Cells were pretreated with 10 mM N-acetyl-L-cysteine (NAC; Beyotime Institute of Biotechnology, Haimen, China) 1 h prior to irradiation. Following this, cells were supplemented with 10 mM NAC for 24 h.
Cell proliferation
Cells were seeded into a 96-well plate (104 cells/well). Confluent cells were treated with serum-free medium for 24 h and irradiated with UVB. Following irradiation, cells were incubated with normal medium for 24 h and then treated with 10 µl Cell Counting kit (CCK)-8 (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) for 4 h. Absorbance values were measured at a wavelength of 450 nm using a microplate reader (GEN5 CHS v.2.00, BioTek Instruments, Inc., Winooski, VT, USA).
RT-qPCR
Total RNA was extracted from cells using TRIzol® reagent (Thermo Fisher Scientific, Inc.). Extracted total RNA, following purification and quality assessments, was reverse-transcribed to cDNA. RT-qPCR was performed to detect MALAT1 expression using the SYBR-Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.) with a ABI PRISM 7700 system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling conditions were as follows: Initial denaturation at 95°C for 2 min, then denaturation at 95°C for 15 sec, annealing at 56°C for 15 sec and extension at 68°C for 20 sec, for a total of 40 cycles. β-actin served as an internal reference. The expression level of MALAT1 was calculated using the 2−∆∆Cq method (13) method. The primer sequences used were as follows: Forward, 5′-AAAGCAAGGTCTCCCCACAAG-3′ and reverse, 5′-GGTCTGTGCTAGATCAAAAGGCA-3′ for MALAT1. Experiments were performed in triplicate.
Enzyme-linked immunosorbent assay (ELISA) for MMP-1 expression in supernatants
Following irradiation, cells were incubated with serum-free medium for 24 h. Cell culture medium was collected and centrifuged at 1,000 × g/min for 10 min at room temperature, and then supernatants were harvested. MMP-1 concentration was detected in supernatants using the MMP-1 kit (cat. no. EK0458; Boster Systems, Inc., Pleasanton, CA, USA) according to the manufacturer's protocol.
Detection of senescent cells using a β-galactosidase (SA-β-Gal) kit
Following 24 h of UVB irradiation, cells were stained using an SA-β-Gal staining kit (Beyotime Institute of Biotechnology) according to the manufacturer's protocol. Senescent cells presented with a blue color around the nuclei and the percentage of senescent cells was equal to number of cells stained blue/total number of cells ×100%.
Detection of ROS content using flow cytometry
Following 24 h of UVB irradiation, cells were treated with serum-free medium containing 10 µM dichloro-dihydro-fluorescein diacetate (DCFH-DA; cat. no. KGT010-1; Nanjing Keygen Biotech Co., Ltd., Nanjing, China) for 30 min at 37°C, according to the manufacturer's protocol, and then washed three times with DMEM. Images were captured using a fluorescence microscope. Cells were collected and the level of green fluorescence was detected to evaluate the levels of intracellular ROS using flow cytometry (FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA) with BD CellQuest Pro software version 6.0 (BD Biosciences) according to the manufacturer's protocol.
Western blot analysis
Following fibroblast irradiation with UVB for 48 h, total protein was collected. Expression levels of ERK, phosphorylated ERK (p-ERK), p38, phosphorylated p38 (p-p38), JNK and phosphorylated JNK (p-JNK) were measured using western blot analysis. The process was conducted as previously described (14). Briefly, the cells were rinsed with PBS and lysed in radioimmunoprecipitation lysis buffer (Beyotime Institute of Biotechnology) with a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific, Inc.) on ice for 30 min. Cell lysates were centrifuged at 13,000 × g at 4°C for 20 min. The supernatants were collected and the proteins were quantified using a bicinchoninic acid protein assay kit (Beyotime Institute of Biotechnology). Protein samples (20 µg) were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Gels were transferred to nitrocellulose membranes, blocked with 5% non-fat-milk and then probed with anti-ERK (dilution, 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA; cat. no. 9926), anti-p-ERK (dilution, 1:1,000; Cell Signaling Technology, Inc.; cat. no. 9910), anti-p38 (dilution, 1:1,000; Cell Signaling Technology, Inc.; cat. no. 9926), anti-p-p38 (dilution, 1:1000; Cell Signaling Technology, Inc.; cat. no. 9910), anti-JNK (dilution, 1:1,000; Cell Signaling Technology, Inc.; cat. no. 9926), or anti-p-JNK (dilution, 1:1,000; Cell Signaling Technology, Inc.; cat. no. 9910) antibody at 4°C overnight. Following incubation with a rabbit horseradish peroxidase-conjugated secondary antibody (dilution, 1:2,000; Abcam, Cambridge, UK; cat. no. ab6721) at room temperature for 2 h, immunoreactive bands were visualized using a electrochemiluminescence detection reagent (Yeasen; Shanghai Yi Sheng Biological Technology Co., Ltd., Shanghai, China; cat. no. 36208ES60) according to the manufacturer's instructions. Integrated optical density (IOD) was calculated using ImageJ version 1.46 software (http://rsb.info.nih.gov/ij). The relative intensity of the level of the protein of interest was calculated using the following formula: IODphosphorylated protein band of interest/IODcorresponding total protein band. Representative blots of at least three independent experiments are presented.
Statistical analysis
Data were analyzed using SPSS software, version 22.0 (IBM SPSS, Armonk, NY, USA) and expressed as the mean ± standard deviation. All experiments were performed at least in triplicate. Significance tests were conducted on the data groups using analysis of variance followed by a comparison between the specific groups using a Student-Newman-Keuls test and analysis of differences between only two groups was performed using an unpaired Student's t-test. P<0.05 was considered to indicate a statistically significant difference.
Results
MALAT1 expression increases following UVB irradiation
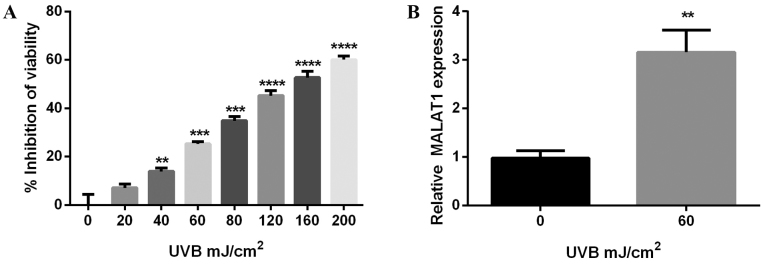
To investigate the phototoxicity of UVB on fibroblasts, cell viability was detected using the CCK-8 method, on cells irradiated with various doses of UVB for 24 h. The results demonstrated UVB suppressed melanocyte viability, an inhibitory effect enhanced by increasing doses of UVB. The inhibitory rate of 60 mJ/cm2 UVB was ~25% (P<0.001; Fig. 1A) and results further demonstrated that 60 mJ/cm2 UVB increased MALAT1 expression (P<0.01; Fig. 1B).
Figure 1.
A total of 60 mJ/cm2 UVB upregulates MALAT1 expression in fibroblasts. (A) Effect of UVB irradiation on cell viability, detected via Cell Counting kit-8. The inhibitory rate on cell viability increased in a dose-dependent manner. The inhibitory rate was approximately 25% in the 60 mJ/cm2 group. (B) Reverse transcription-quantitative polymerase chain reaction demonstrated an increase in MALAT1 expression with 60 mJ/cm2 UVB. ****P<0.0001, ***P<0.001 and **P<0.01 vs. control. UVB, ultraviolet B; MALAT1, metastasis-associated lung adenocarcinoma transcript 1.
MALAT1 siRNA inhibits UVB-induced MMP-1 secretion
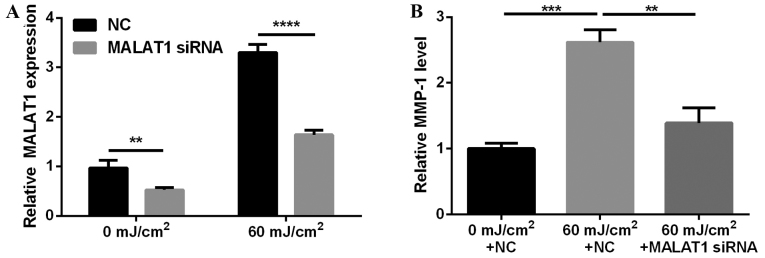
To investigate the effects of MALAT1 on photo-aging, MALAT1 expression in fibroblasts was silenced and effects on MMP-1 secretion observed. The results demonstrated that MALAT1 siRNA suppressed MALAT1 expression (P<0.01 and P<0.0001; Fig. 2A) and inhibited 60 mJ/cm2 UVB-induced MMP-1 secretion (P<0.01 and P<0.001; Fig. 2B).
Figure 2.
MALAT1 siRNA inhibits UVB-induced MMP-1 secretion. (A) Effect of MALAT1 siRNA on MALAT1 expression in fibroblasts, detected by reverse transcription-quantitative polymerase chain reaction. (B) Effect on MMP-1 expression levels following fibroblast irradiation with 60 mJ/cm2 UVB and MALAT1 siRNA, detected by enzyme-linked immunosorbent assay. ****P<0.0001 and **P<0.01 vs. NC. ***P<0.001 vs. 0 mJ/km2 + NC. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; siRNA, small interfering; UVB, ultraviolet B; MMP-1, matrix metalloproteinase-1; NC, negative control.
MALAT1 siRNA inhibits UVB-induced fibroblast senescence
The ratio of senescent cells was markedly greater following UVB irradiation (74.4%) compared with cells that had not been exposed to irradiation (15.7%). In addition, the ratio of senescent cells was significantly lower following intervention with MALAT1 siRNA (52.1%) compared with the UVB irradiation group (P<0.01 and P<0.0001; Fig. 3).
Figure 3.
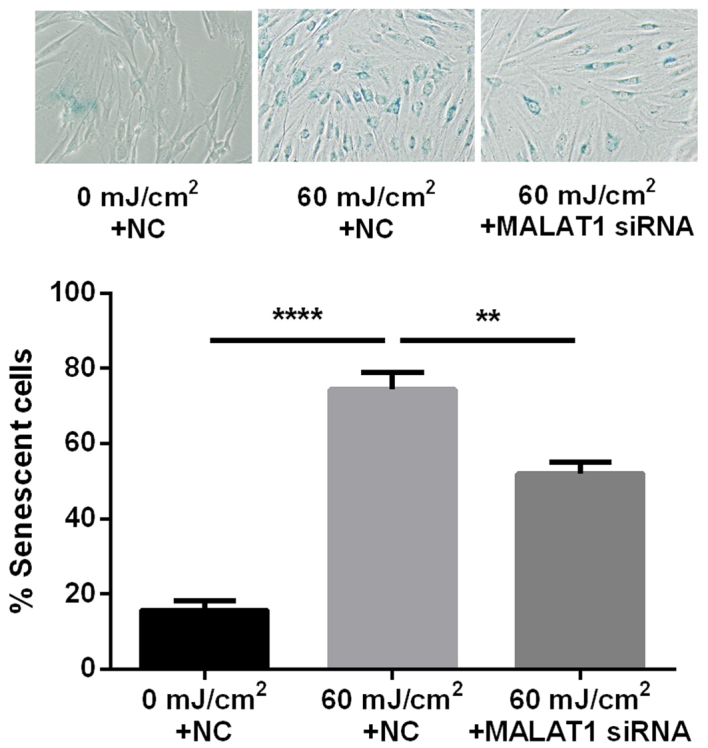
MALAT1 siRNA suppresses UVB-induced fibroblast senescence. Ratio of senescent cells detected via a β-galactosidase SA-β-Gal kit, following 60 mJ/cm2 UVB irradiation and then intervention with MALAT1 siRNA. Magnification, ×40. ****P<0.0001 vs. 0 mJ/km2 + NC; **P<0.01 vs. 60 mJ/km2 + NC. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; siRNA, small interfering RNA; UVB, ultraviolet B; NC, negative control.
MALAT1 siRNA inhibits UVB-induced ERK phosphorylation in fibroblasts
Activation of MAPK signaling is important in photo-aging. The present study investigated the effect of the silencing of MALAT1 on activation of MAPK signaling pathway elements, induced by UVB. The results demonstrated that 60 mJ/cm2 UVB upregulated ERK, JNK and p38 phosphorylation levels, however MALAT1 siRNA inhibited UVB-induced ERK phosphorylation (P<0.01), with no significant influence on JNK and p38 phosphorylation levels (P>0.05; Fig. 4).
Figure 4.
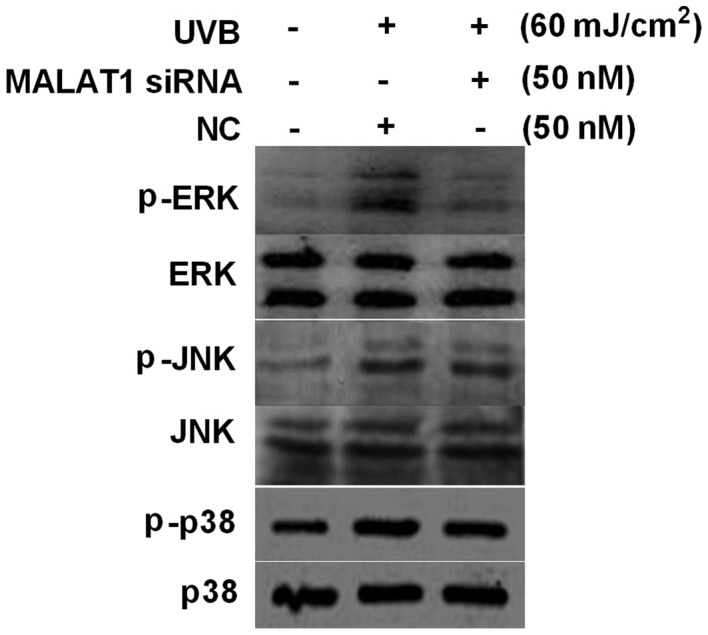
MALAT1 siRNA suppresses UVB-induced p-ERK activation in fibroblasts. Effect of 60 mJ/cm2 UVB irradiation and MALAT1 siRNA on fibroblast p-ERK, p-p38 and p-JNK expression levels, detected by western blot analysis. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; siRNA, small interfering; UVB, ultraviolet B; NC, negative control; p, phosphorylated; ERK, extracellular signal-regulated kinase, JNK, c-Jun N-terminal kinase.
UVB-induced MALAT1 expression is independent of ROS generation
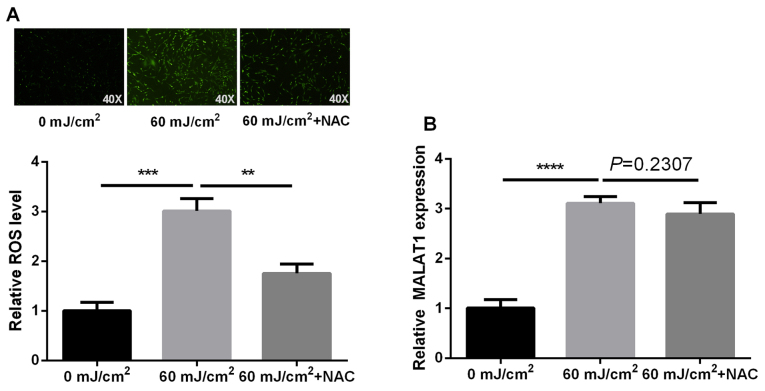
ROS alters the expression levels of numerous molecules. The present study examined whether ROS underlies UVB-induced MALAT1 alteration using NAC, which is a ROS scavenger. The effects on MALAT1 expression in NAC-pretreated cells were investigated. The results indicated an increase in ROS content following fibroblast irradiation with UVB, which was then inhibited by NAC. However, NAC did not have a significant effect on MALAT1 expression (P=0.2307). These findings indicated that UVB-induced MALAT1 expression is not dependent on ROS generation (P<0.01, P<0.001 and P<0.0001 respectively; Fig. 5).
Figure 5.
UVB-induced MALAT1 is independent of ROS generation. Effect on (A) ROS generation (magnification, ×40) and (B) MALAT1 expression following fibroblast irradiation with 60-mJ/cm2 UVB, and then addition of NAC, detected by flow cytometry and reverse transcription-quantitative polymerase chain reaction respectively. ****P<0.0001 and ***P<0.001 vs. 0 mJ/km2; **P<0.01 vs. 60 mJ/km2 + NAC. MALAT1, metastasis-associated lung adenocarcinoma transcript 1; UVB, ultraviolet; ROS, reactive oxygen species; NAC, N-acetyl-L-cysteine.
Discussion
It has recently been demonstrated that microRNA expression profiles within non-coding RNA alter in fibroblasts following administration of UV irradiation (15). An additional study verified that two microRNAs, miR-146a and miR-155, potentially regulate photo-aging via targeting Smad4 and c-Jun regulation (16,17). The role of lncRNAs in UV-induced photo-aging of skin remains to be elucidated.
The potential of UV radiation to alter lncRNA expression profiles within skin cells has implications on numerous important biological functions associated with lncRNAs. Hall et al (18) demonstrated that UVB-induced lincRNA-p21 is important in keratinocyte apoptosis. The authors previously demonstrated that >two-fold alterations in the expression of 807 lncRNAs occurred, following melanocyte irradiation with UVB (14), including a markedly increased expression of MALAT1 (data not shown). MALAT1, a well-known cancer-promoting gene within lncRNAs, regulates numerous biological activities including tumor proliferation, metastasis and epithelial-mesenchymal transitioning (19). The current study revealed increases in MALAT1 expression following UVB irradiation of fibroblasts, indicating a potentially important role of MALAT1 in the photo-aging of fibroblasts.
MMP-1 is an important indicator of photo-aging within fibroblasts. In the present study, MMP-1 content increased within fibroblasts following 60 mJ/cm2 UVB irradiation, suggesting aging of fibroblasts. MMP-1 secretory volume was markedly reduced in photo-aged fibroblasts following silencing of MALAT1 expression, indicating MALAT1 l promoted photo-aging of fibroblasts. Cell senescence staining experiments confirmed the ability of MALAT1 siRNA to suppress UVB-induced photo-aging of fibroblasts.
The MAPK signaling pathway is activated during photo-aging of fibroblasts (4). Furthermore, recent studies have demonstrated an interaction between MALAT1 and the MAPK signaling pathway (10,12). Therefore, the present study explored the effects of MALAT1 on MAPK signaling in fibroblasts. The results demonstrated MALAT1 siRNA inhibited UVB-induced p-ERK activation, indicating MALAT1 may participate in the photo-aging of fibroblasts via activation of the ERK/MAPK signaling pathway elements.
The authors previously demonstrated that UVB-induced lncRNAs (including lnc-CD1D-2:1) depend on ROS generation (14), and therefore, further proceeded to investigate whether UVB-induced MALAT1 upregulation is ROS-dependent. The results demonstrated no inhibition of MALAT1 expression by NAC (a ROS scavenger), indicating UVB-induced MALAT1 upregulation is independent of ROS. In conclusion, MALAT1 may participate in UVB-induced photo-aging via regulation of the ERK/MAPK signaling pathway elements and UVB-induced MALAT1 upregulation does not depend on ROS generation.
Acknowledgements
The present study was supported by the Science and Technology Plan of Hunan province (grant no. 2013SK3056) and the New Xiangya Talent Projects of the Third Xiangya Hospital of Central South University (grant no. JY201623).
Glossary
Abbreviations
- DCFH-DA
dichloro-dihydro-fluorescein diacetate
- DMEM
Dulbecco's modified Eagle's medium
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun N-terminal kinase
- lncRNA
long non-coding RNA
- MALAT1
metastasis-associated lung adenocarcinoma transcript 1
- MMP-1
metalloproteinase-1
- MAPK
mitogen-activated protein kinase
- NAC
N-acetyl-L-cysteine
- PBS
phosphate-buffered saline
- P-ERK
phosphorylated ERK
- P-JNK
phosphorylated JNK
- P-p38
phosphorylated p38
- ROS
reactive oxygen species
- siRNA
small interference RNA
- UVB
ultraviolet B
References
- 1.Ham SA, Kang ES, Lee H, Hwang JS, Yoo T, Paek KS, Park C, Kim JH, Lim DS, Seo HG. PPARδ inhibits UVB-induced secretion of MMP-1 through MKP-7-mediated suppression of JNK signaling. J Invest Dermatol. 2013;133:2593–2600. doi: 10.1038/jid.2013.202. [DOI] [PubMed] [Google Scholar]
- 2.Stoebner PE, Meunier L. Photoaging of face. Ann Dermatol Venereol. 2008;135:1S21–1S26. doi: 10.1016/S0151-9638(08)70207-2. (In French) [DOI] [PubMed] [Google Scholar]
- 3.Park G, Baek S, Kim JE, Lim TG, Lee CC, Yang H, Kang YG, Park JS, Augustin M, Mrosek M, et al. Flt3 is a target of coumestrol in protecting against UVB-induced skin photoaging. Biochem Pharmacol. 2015;98:473–483. doi: 10.1016/j.bcp.2015.08.104. [DOI] [PubMed] [Google Scholar]
- 4.Kim MJ, Woo SW, Kim MS, Park JE, Hwang JK. Anti-photoaging effect of aaptamine in UVB-irradiated human dermal fibroblasts and epidermal keratinocytes. J Asian Nat Prod Res. 2014;16:1139–1147. doi: 10.1080/10286020.2014.983092. [DOI] [PubMed] [Google Scholar]
- 5.He X, Bao W, Li X, Chen Z, Che Q, Wang H, Wan XP. The long non-coding RNA HOTAIR is upregulated in endometrial carcinoma and correlates with poor prognosis. Int J Mol Med. 2014;33:325–332. doi: 10.3892/ijmm.2013.1570. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Wang X. Role of long noncoding RNAs in malignant disease (Review) Mol Med Rep. 2016;13:1463–1469. doi: 10.3892/mmr.2015.4711. [DOI] [PubMed] [Google Scholar]
- 7.Xu S, Sui S, Zhang J, Bai N, Shi Q, Zhang G, Gao S, You Z, Zhan C, Liu F, Pang D. Downregulation of long noncoding RNA MALAT1 induces epithelial-to-mesenchymal transition via the PI3K-AKT pathway in breast cancer. Int J Clin Exp Pathol. 2015;8:4881–4891. [PMC free article] [PubMed] [Google Scholar]
- 8.Dey BK, Mueller AC, Dutta A. Long non-coding RNAs as emerging regulators of differentiation, development, and disease. Transcription. 2014;5:e944014. doi: 10.4161/21541272.2014.944014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida S, Dimmeler S. Long noncoding RNAs in cardiovascular diseases. Circ Res. 2015;116:737–750. doi: 10.1161/CIRCRESAHA.116.302521. [DOI] [PubMed] [Google Scholar]
- 10.Liu JY, Yao J, Li XM, Song YC, Wang XQ, Li YJ, Yan B, Jiang Q. Pathogenic role of lncRNA-MALAT1 in endothelial cell dysfunction in diabetes mellitus. Cell Death Dis. 2014;5:e1506. doi: 10.1038/cddis.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei Y, Niu B. Role of MALAT1 as a prognostic factor for survival in various cancers: A systematic review of the literature with meta-analysis. Dis Markers. 2015;2015:164635. doi: 10.1155/2015/164635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu XS, Wang XA, Wu WG, Hu YP, Li ML, Ding Q, Weng H, Shu YJ, Liu TY, Jiang L, et al. MALAT1 promotes the proliferation and metastasis of gallbladder cancer cells by activating the ERK/MAPK pathway. Cancer Biol Ther. 2014;15:806–814. doi: 10.4161/cbt.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 14.Zeng Q, Wang Q, Chen X, Xia K, Tang J, Zhou X, Cheng Y, Chen Y, Huang L, Xiang H, et al. Analysis of lncRNAs expression in UVB-induced stress responses of melanocytes. J Dermatol Sci. 2016;81:53–60. doi: 10.1016/j.jdermsci.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 15.Syed DN, Khan MI, Shabbir M, Mukhtar H. MicroRNAs in skin response to UV radiation. Curr Drug Targets. 2013;14:1128–1134. doi: 10.2174/13894501113149990184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li W, Zhou BR, Hua LJ, Guo Z, Luo D. Differential miRNA profile on photoaged primary human fibroblasts irradiated with ultraviolet A. Tumour Biol. 2013;34:3491–3500. doi: 10.1007/s13277-013-0927-4. [DOI] [PubMed] [Google Scholar]
- 17.Song J, Liu P, Yang Z, Li L, Su H, Lu N, Peng Z. MiR-155 negatively regulates c-Jun expression at the post-transcriptional level in human dermal fibroblasts in vitro: Implications in UVA irradiation-induced photoaging. Cell Physiol Biochem. 2012;29:331–340. doi: 10.1159/000338488. [DOI] [PubMed] [Google Scholar]
- 18.Hall JR, Messenger ZJ, Tam HW, Phillips SL, Recio L, Smart RC. Long noncoding RNA lincRNA-p21 is the major mediator of UVB-induced and p53-dependent apoptosis in keratinocytes. Cell Death Dis. 2015;6:e1700. doi: 10.1038/cddis.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutschner T, Hammerle M, Diederichs S. MALAT1-a paradigm for long noncoding RNA function in cancer. J Mol Med (Berl) 2013;91:791–801. doi: 10.1007/s00109-013-1028-y. [DOI] [PubMed] [Google Scholar]