Abstract
The present study aimed to describe the expression and purification of cyclophilin-type peptidylprolyl cis-trans isomerase (PPI) from the red alga Pyropia yezoensis. The antioxidant activity of the purified protein was also demonstrated, based on its ability to act against oxidative stress in HepG2 human hepatocellular carcinoma cells. HepG2 cells that were treated with recombinant PPI protein exhibited a reduction in the formation of hydrogen peroxide (H2O2)-mediated reactive oxygen species (ROS). In HepG2 cells, treatment of recombinant PPI protein expression diminished H2O2-mediated oxidative stress and restored both the expression and the activity of certain antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and thioredoxin reductase (TRR). CAT, SOD and TRR activities were upregulated by treatment with the purified protein. CAT mRNA expression was significantly increased in HepG2 cells treated with recombinant PPI protein. These enzymes are the first line of antioxidant defense against ROS generated in times of oxidative stress. Accordingly, data from the present study indicate that the recombinant PPI protein is able to regulate the expression of antioxidant enzymes. Recombinant PPI has antioxidant properties that prevent oxidative stress-induced toxicity, enhance cell viability, decrease ROS production and inhibit oxidative damage and mitochondrial dysfunction in HepG2 cells. Therefore, the present study hypothesizes that the recombinant PPI protein has the potential to protect the liver against oxidative stress-induced cell damage and should be considered as an antioxidant.
Keywords: Pyropia yezoensis, cyclophilin-type peptidylprolyl cis-trans isomerase, antioxidant activity, reactive oxygen species
Introduction
Reactive oxygen species (ROS) that are generated in vivo are a major cause of human aging and disease (1). ROS expression leads to disease through toxic effects on cells and tissues. ROS include free radical species, such as superoxide anion (O2−), hydroxyl radical and singlet oxygen, as well as non-radical species such as hydrogen peroxide (H2O2). ROS are neutralized by antioxidant enzymes, including superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione reductase, or by small molecules, such as ascorbic acid, α-tocopherol and glutathione (GSH), during normal physiological metabolic processes. Oxidizing agents and oxidation inhibitors function to maintain reduction-oxidation (redox) homeostasis in the body. However, perturbations in this balance, either caused by excess production of ROS or decreased or malfunctioning antioxidant defenses, may result in oxidative stress, potentially leading to pathological effects (1–4). ROS may be detrimental to cells by inducing oxidative damage to lipids, proteins and DNA (5). Therefore, it is important to balance ROS levels with endogenous antioxidant enzymes and, when required, antioxidant compounds.
Oxidative stress has been associated with a number of diseases, including inflammatory diseases, acquired immunodeficiency syndrome, gastric ulcers, hypertension, neurological disorder, alcoholism and smoking-related diseases (4). Over 90% of ROS are produced in the mitochondria during cellular respiration. During this process, most oxygen is converted into water (H2O), and a one-electron reduction of molecular oxygen (O2) produces O2−, a primary ROS, which is subsequently transformed into hydrogen peroxide (H2O2) by SOD. H2O2 is an oxygen metabolite of central importance that is produced in the mitochondria, as well as in other sites in the cell. H2O2 itself has weak biological reactivity; however, if present at a cellular concentration of at least 10 µM, it can directly damage cellular components, including inactivating enzymes by oxidizing active site thiol groups (2,3).
ROS levels are tightly controlled in the body by antioxidant enzymes, including SOD, CAT, GPx and thiol-containing small molecules such as GSH. SODs catalyze the dismutation of O2− to H2O2 and O2, and are located in the cytoplasm [copper (Cu)- and zinc (Zn)-dependent SOD] and in the mitochondria [manganese-dependent (Mn)-SOD] (6). CAT is a tetrameric iron-porphyrin protein in peroxisomes that converts H2O2 to H2O and O2 (7). CAT and Cu/Zn-SOD are expressed constitutively, whereas Mn-SOD expression within the mitochondria is induced by oxidative stress. GSH is a sulfhydryl peptide that may directly react with O2− or N2-containing free radicals, or is able to donate electrons in the enzymatic dismutation of H2O2 to H2O and O2 by GPx (8).
ROS removal involves the cooperation of 29 associated genes and 15 corresponding gene expression products (3). The regulation of intracellular antioxidant activity is regulated by a complex network of these genes. The expression of antioxidant enzymes is altered by oxidative stress. The antioxidant defense system within cells includes ~50 antioxidant-related genes that are divided into four categories (3–9): i) CAT/SOD family members, which include the classic antioxidant enzymes CAT and SODs; ii) proteins involved in GSH metabolism, including GPx, glutaredoxins, glutathione reductase and glucose-6-phosphate dehydrogenase, which promote the antioxidant activity of thiol-containing small molecules, such as GSH, and GSH recycling; iii) proteins involved in redox balance, including enzymes of thioredoxin (TRX) metabolism, TRX, TRX reductase (TRR) and peroxiredoxin (PRDX); and iv) pentose phosphate cycle proteins, including glucose 6-phosphate dehydrogenase. The present study investigated the antioxidant effects of a purified protein on the mRNA expression the antioxidant enzymes SOD, CAT, GPx, and TRR.
Cyclophilin (Cyp) is a multifunctional protein family, which includes cyclophilin-type peptidylprolyl cis-trans isomerase (PPI), a protein which may be inhibited by cyclosporin A (CsA) (10). CsA generates ROS and lipid peroxidation in cells, which appears to be directly associated with its pathological effects. Perez et al (11) used 2′,7′-dichlorofluorescein diacetate (DCF-DA) and demonstrated that 1–10 µM CsA generated an oxidized DCF signal, interpreted as being H2O2-derived. CsA was demonstrated to increase lipid peroxidation in the rat kidney and liver in vivo (12,13). Cyp is a ubiquitous protein that is present in all subcellular compartments, and therefore may potentially be involved in a variety of processes, including protein trafficking and maturation, receptor complex stabilization, apoptosis, receptor signaling, RNA processing and spliceosome assembly (14–20). It has been proposed that CsA-induced oxidative stress may activate or deactivate transcription factors and thus affect gene transcription. Similarly, ROS generated by CsA may activate or deactivate various signaling molecules and influence their downstream transduction systems. The ROS induced by CsA has been reported to affect mitogen-activated protein kinase and transforming growth factor-β signaling (21). In particular, there is increased expression of TRX 1 via the ERK/MAPK-2 signaling, to overcome the oxidative stress resulting from ROS. In the present study, PPI, known to exhibit antioxidant activity, was cloned from Pyropia yezoensis. In addition, hydrogen peroxide was used to experimentally induce oxidative stress that results from CsA.
In the sea, the exposure of seaweeds to sunlight and oxygen leads to formation of ROS; however, no oxidative damage is evident in their structural and functional components, which suggests that they may have an efficient antioxidant defense system. Hence, several seaweed extracts are attracting scientific interest to identify new and effective antioxidant compounds (22–27). A recent report described antioxidant agents isolated from seaweed (22). Ismail and Tan (23) compared the antioxidant capacity of commercial seaweeds and confirmed high antioxidant activities of Porphyra sp., Laminaria sp., Undaria sp. and Hijikia sp. Other studies have described high antioxidative properties of many compounds isolated from seaweed, including phlorotannin, fucoxanthin, carotenoids and tocopherols (24–27). Previous studies on the antioxidant properties of algae focused primarily on crude extracts (28–30). In the present study, the gene expression and purification of the antioxidant protein PPI from P. yezoensis is described. In addition, the antioxidant activity of recombinant PPI, that is, its protection against ROS, was investigated in a human hepatocellular carcinoma cell line.
Materials and methods
Seaweeds and cDNA synthesis
P. yezoensis was collected directly from Myeongji (Busan, Korea) and stored at −70°C. Frozen samples were lyophilized using a freezing dryer (−80°C, 24 h) and homogenized using a blender into powder, prior to mRNA extraction. mRNA (5 µg) was extracted from 20 mg P. yezoensis with the GeneJET Plant RNA Purification Mini kit (Thermo Scientific, Inc., Waltham, MA, USA), according to the manufacturer's protocol. Quantification of RNA was performed using the NanoDrop 2000 Spectrophotometer (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The ratio of absorbance at 260 nm and 280 nm was used to assess the purity of RNA. A ratio of <2.0 was accepted as pure for RNA. The mRNA was used for first strand cDNA synthesis and double strand cDNA synthesis using a PrimeScript Double strand cDNA synthesis kit (Takara Bio, Inc., Otsu, Japan) following the manufacturer's protocol.
Preparation and identification of recombinant PPI protein
To prepare PPI, a forward primer (5′-GGCCCATATGGGGAACCCGCAGGTGTTCT-3′) containing the Nde1 site (underlined) and initiation codon (italic) and reverse primer (5′-GGCCCTCGAGGAGCTCGCCGCAGTCCGC-3′) containing Xho1 site (underlined), were constructed. Polymerase chain reaction (PCR) amplification was performed using the cDNA of P. yezoensis (one cycle at 95°C for 5 min; 30 cycles at 95°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec; followed by one cycle of 72°C for 7 min) using EXTaq DNA polymerase (Takara Bio Inc., Otsu, Japan). The PCR products (151 bp) and expression vector pET22b+ were digested with two restriction enzymes (NdeI and XhoI). The PCR fragment was subcloned into the digested pET22b+ vector with a DNA ligation kit (Takara Bio Inc.). The resulting plasmid was named pETppi. The pETppi was introduced into Escherichia coli DH5α competent cells, used as the cloning host for propagation of the expression vector, and finally retransformed into expression strain E. coli BL21 (DE3), according to a previously described method (31). The transformation bacteria were selected on LB agar medium containing 100 µg-ml-1 of ampicillin. Cultures of the transformed E. coli BL21 overexpressed a recombinant PPI of the expected molecular mass (~18 kDa), which was purified by affinity chromatography in Ni-NTA purification system (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Bacterial cultures were incubated using LB medium at 37°C until reaching the OD600 of 0.8. For PPI expression, isopropyl-beta-D-thiogalactopyranoside (IPTG) was added to a final concentration of 1 mM. Expression was continued for 4 h. EnterokinaseMax™ enzyme (Thermo Fisher Scientific, Inc.) was used to separate the 6x His-tag from recombinant PPI protein, according to the manufacturer's protocol. Analysis of protein expression and purification were carried out using sodium dodecyl sulfate -poly acrylamide gel electrophoresis (SDS-PAGE). The purified recombinant PPI protein was confirmed using SDS-PAGE and electrospray ionization quadrupole time-of-flight mass spectrometry/mass spectrometry (ESI-Q-TOF MS/MS), according to a previously described method (32). The proteins were identified via an NCBI search using the MASCOT program (http://www.matrixscience.com, Matrixscience, London, UK).
Cell culture and viability
HepG2 human hepatocellular carcinoma cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured at 37°C in a humidified 5% CO2, 95% air equilibrated incubator in minimum essential medium (MEM) supplemented with heat-inactivated 10% fetal bovine serum (HyClone, Logan, UT, USA), penicillin (100 U-ml-1) and streptomycin (100 µg-ml-1). Adherent cells at 70–80% confluence were detached by trypsin-EDTA solution and re-plated.
Cell viability was estimated with the CytoX Cell Viability Assay kit (LPS Solution, Daejeon, Korea). Briefly, cells (1.0×103 cells/well) were seeded in a 96-well plate. Various concentrations (0.001, 0.01, 0.1 and 1 µg-ml−1) of recombinant PPI protein were added to the cells along with 1 mM H2O2 for 1 h at 37°C. Following incubation, Cyto solution was added to each well and incubated for an additional 1 h at 37°C. Absorbance was measured with a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA) at a wavelength of 450 nm. The viability of purified recombinant PPI protein-treated cells was expressed as a percentage of that in negative control cells (−, without H2O2).
Assessment of ROS production
The effects of purified recombinant PPI protein on ROS production was evaluated using the cell-permeable probe DCF-DA. DCF-DA (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved in 10 mg-ml-1 sterile dimethylsulfoxide and was used at a concentration of 50 µg-ml-1. HepG2 cells were seeded onto 96-well plates at 1.0×103 cells/well in MEM, grown until confluence, and pre-incubated with DCF-DA for 20 min at 37°C in the dark. After washing twice with phosphate buffered saline (PBS) to remove the unattached probe, cells were treated with PBS or various concentrations (between 0.001 and 1 µg-ml-1) of purified recombinant PPI protein in the presence or absence of 1 mM H2O2 for 1 h. Fluorescence was measured at 485/20 nm excitation and 535/20 nm emission using a FilterMax F5 Multi-Mode Microplate Reader (Molecular Devices, LLC, Sunnyvale, CA, USA).
Assessment of antioxidant enzyme activities
HepG2 cells were grown to 70–80% confluence at 37°C. Following 4 h starvation, the cells were treated with PBS or various concentrations (0.001–1 µg-ml-1) of purified recombinant PPI protein in the presence or absence of 1 mM H2O2 for 1 h and harvested in lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM sodium chloride, 0.5% sodium deoxycholate, 0.1% SDS, 1% triton X-100 and 2 mM ethylenediaminetetra-acetic acid with inhibitors (1 mM sodium orthovanadate and 1 mM phenylmethylsulfonyl fluoride). Cell debris was removed by centrifugation at 13,000 × g at 4°C for 10 min and the supernatant was used for further measurements. Protein concentration was determined using the BCA Protein assay (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to the manufacturer's protocol. Each supernatant contained an equal amount of protein (5 µg) and was used for the subsequent enzyme activity assays. The activities of antioxidant enzymes, including CAT, SOD, GPx and TRR were measured using a Catalase Assay kit (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), SOD Assay Kit-WST (Sigma-Aldrich; Merck KGaA), Glutathione Peroxidase Cellular Activity Assay kit (Sigma-Aldrich; Merck KGaA) and Thioredoxin Reductase Assay kit (Sigma-Aldrich; Merck KGaA) according to the manufacturer's protocols. The absorbance was measured using a microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Reverse transcription (RT)-PCR
The effects of recombinant PPI on antioxidant enzyme mRNA expression in H2O2 treated HepG2 cells was evaluated by RT-PCR. Total RNA was extracted from HepG2 cells using TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols. Quantification of RNA was performed as described above. The purity of the RNA was determined at absorbance ratios of 260 and 280 nm (260/280 nm <2.0). cDNA was synthesized using a First-Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. In this experiment, primer sets of SOD2, GPx and CAT were used as reported in Schmidt et al (33) and primer sets of GAPDH and TRR were designed as described by Aguilar-Melero et al (34). PCR amplification was performed using the template cDNA (1 ng) and PCR Amplification Kit (Takara Bio Inc.) Initial denaturation at 95°C for 5 min, followed by 25 cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C for 30 sec, then a final extension of 72°C for 5 min. Amplified products were analyzed by 1% agarose gel electrophoresis and stained with ethidium bromide for detection. The software GeneTools, version 4.03 (SYNGENE, Cambridge, UK) was used for densitometry.
Statistical analysis
Data are expressed as the mean ± standard deviation and were evaluated by one-way analysis of variance using the statistical package for social sciences version 10.0 (SPSS, Ins., Chicago, IL, USA). Values were compared with controls using analysis of variance followed by Duncan's multiple range test. P<0.05 was considered to represent a statistically significant difference.
Results
Expression and purification of recombinant PPI protein
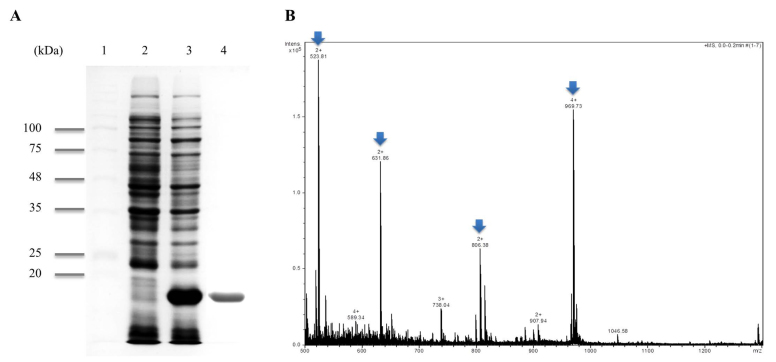
The expression vector pETppi of P. yezoensis was transformed into E. coli BL21 (DE3) and then induced by 1 mM IPTG. SDS-PAGE analysis of the harvested cells from the pETppi transformed E. coli (BL21) exhibited high amounts of a polypeptide with the expected molecular mass of ~18 kDa (Fig. 1A, lane 2) from SDS-PAGE analysis. Recombinant PPI was purified ~18 kDa protein by the Ni-NTA system (Fig. 1A, lane 4). It was highly purified from crude extracts as a His-tagged protein. Recombinant PPI was identified via MALDI-TOF MS/MS. A total of four peaks from MALDI-TOF MS were analyzed by MS/MS and the following sequences were obtained (Fig. 1B, Arrow): i) VFFDMTIGGAPAGR; ii) VITDF MCQGGDFTR; iii) ADENFTLTHTGPGVLSMANAGK; and iv) NGSQFFLTTVK. This was 100% homologous to PPI (accession number KJ728870.1) using the MASCOT program and NCBI database.
Figure 1.
Purification and identification of recombinant PPI protein. (A) SDS-PAGE analysis of PETppi transformed E.coli BL21 (DE3) and purified recombinant PPI. Lane 1, protein marker; lane 2, crude extract of pETppi transformed E. coli BL21 (DE3) prior to induction; lane 3, crude extract of pETppi transformed E. coli BL21 (DE3) after induction with 1 mM IPTG for 4 h; lane 4, purified recombinant PPI protein. (B) ESI-Q-TOF MS of purified recombinant PPI protein. The partial sequences of 4 peaks from the recombinant PPI were identified by MS/MS (Arrow).
Cell viability
Cell viability was determined with the CytoX assay, which relies on the mitochondrial metabolic capacity of viable cells. The results revealed that recombinant PPI exposure was not cytotoxic to HepG2 cells at any of the concentrations examined, between 0.001 and 1 µg-ml-1 (Fig. 2A). Treatment with H2O2 for 1 h decreased cell viability to 59.9% that of control cells; co-treatment with recombinant PPI did not alter the H2O2-induced decrease in cell viability, which remained constant at 60.4% (at 0.001 µg-ml-1 recombinant PPI), 54.7% (at 0.01 µg-ml-1 recombinant PPI), 55.5% (at 0.1 µg-ml-1 recombinant PPI) and 55.4% (at 1.0 µg-ml-1 recombinant PPI). These results demonstrate that recombinant PPI treatment did not prevent apoptosis from H2O2-induced oxidative stress However, the recombinant PPI was not toxic to HepG2 cells. Therefore, recombinant PPI was then tested further for its antioxidant.
Figure 2.
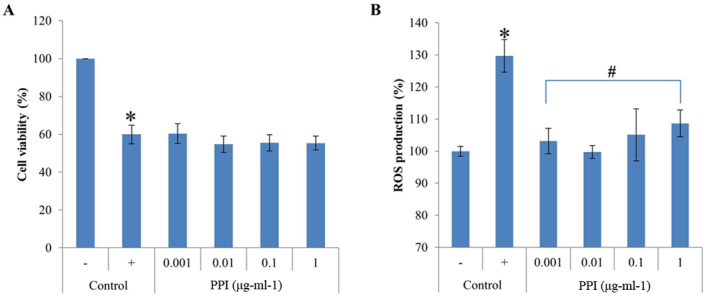
Effect of recombinant PPI in HepG2 cells (A) Recombinant PPI toxicity. (B) Effects of PPI on the formation of H2O2-induced ROS. Cells were pretreated with 2′,7′-dichlorofluorescin diacetate for 20 min and exposed to PPI. Values are expressed as the mean ± standard deviation (n=3). *P<0.05 vs. untreated control; #P<0.05 vs. H2O2 treated control. -, control cells without H2O2; +, control cells treated with 1 mM H2O2; H2O2, hydrogen peroxide; PPI, peptidylprolyl cis-trans isomerase; ROS, reactive oxygen species.
Inhibition of ROS production
DCF-DA staining was used to examine whether recombinant PPI exposure inhibited ROS production in HepG2 cells co-treated with H2O2. HepG2 cells were challenged with H2O2, and the resulting ROS levels were 29.7% greater compared with the unchallenged control. Pretreatment of cells with the various concentrations of recombinant PPI (0.001–1 µg-ml-1) reduced the H2O2-mediated increase in ROS formation (Fig. 2B). Recombinant PPI treatment showed antioxidant effects in the presence of H2O2, implying that PPI directly scavenges ROS or other free radicals.
Induction of antioxidant enzyme activities in HepG2 cells
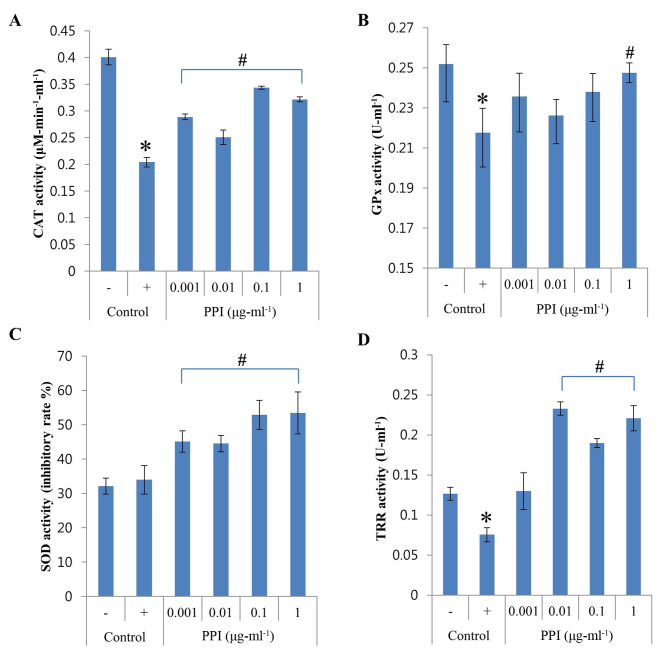
To investigate whether the antioxidant properties of recombinant PPI are related to antioxidant enzyme induction, HepG2 cells were treated with recombinant PPI and the activity of the antioxidant enzymes CAT, GPx, SOD and TRR were measured. As shown in Fig. 3A, CAT activity was dramatically downregulated by H2O2 treatment, whereas the addition of recombinant PPI (0.001–1 µg-ml-1) was able to restore CAT activity. GPx activity was reduced by 14% when cells were exposed to H2O2, but levels recovered in the presence of recombinant PPI at 1 µg-ml-1 (Fig. 3B). The activity of SOD was not altered by H2O2 however, increased with the treatment of recombinant PPI (Fig. 3C). PPI treatment at concentrations between 0.01 and 1 µg-ml-1 was able to increase the H2O2-induced decrease in TRR activity (Fig. 3D). Recombinant PPI treatments increased the activities of CAT, GPx and TRR that were reduced by exposure to H2O2. The results also indicated that recombinant PPI was also able to significantly increase SOD activity. Therefore, recombinant PPI appears to exert its antioxidant effects by modulating the activities of endogenous antioxidant enzymes.
Figure 3.
Effects of PPI on SOD, GPx, CAT and TRR activities in H2O2-treated HepG2 cells. (A) CAT activity; (B) GPx activity; (C) SOD activity; and (D) TRR activity. Values represent the mean ± standard deviation (n=3). *P<0.05 vs. untreated control; #P<0.05 vs. H2O2-treated control. -, control cells without H2O2; +, control cells treated with 1 mM H2O2; CAT, catalase; GPx, glutathione peroxidase; H2O2, hydrogen peroxide; PPI, peptidylprolyl cis-trans isomerase; ROS, reactive oxygen species; SOD, superoxide dismutase; TRR, thioredoxin reductase.
Induction of antioxidant enzyme expression in HepG2 cells
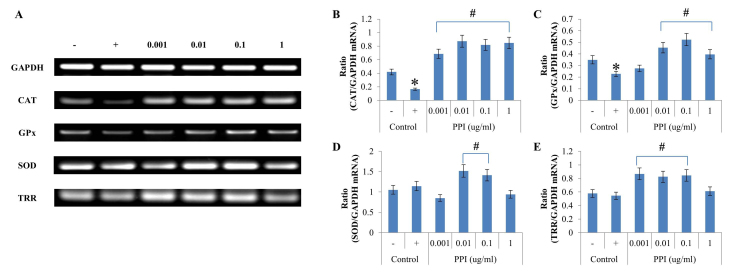
In addition to its effects on antioxidant enzyme activities, the effects of recombinant PPI on mRNA expression were evaluated in HepG2 cells. In cells treated with H2O2, CAT mRNA expression levels of were significantly diminished compared with the H2O2-treated -treated controls and GAPDH mRNA expression (Fig. 4A and B). H2O2 exposure significantly decreased CAT mRNA expression level, which was then reversed five-fold by addition of 0.01 µg-ml-1 recombinant PPI. The level of GPx mRNA expression was highest (ratio 0.52) when cells were pretreated with recombinant PPI at a concentration of 0.1 µg-ml-1 (Fig. 4A,C). The results demonstrated that treatment with H2O2 led to decreased CAT and GPx mRNA expression; however, SOD and TRR mRNA expression levels were not affected by H2O2 treatment (Fig. 4A,D and E). SOD mRNA expression was significantly increased when treated with 0.01 and 0.1 µg-ml-1 recombinant PPI, as compared with the positive control (Fig 4A and D). TRR mRNA levels were highest (ratio 0.87) at a concentration of 0.001 µg-ml-1 recombinant PPI (Fig. 4A and E). Recombinant PPI treatment in HepG2 cells increased the level of mRNA expression of reduced antioxidant enzymes when cells were treated with H2O2. Therefore, PPI increased the expression of the majority of antioxidant enzymes, however this differed depending on the concentration of PPI.
Figure 4.
Effects of PPI on antioxidant enzymes mRNA expressions in H2O2 treated HepG2 cells. (A) Reverse transcription-polymerase chain reaction analysis of SOD, GPx, CAT and TRR. (B-E) Data quantification of antioxidant enzyme/GAPDH mRNA expression for (B) CAT, (C) GPx, (D) SOD, and (E) TRR. Values represent the mean ± standard deviation (n=3). *P<0.05 vs. untreated control; #P<0.05 vs. H2O2-treated control. -, control cells without H2O2; +, control cells treated with 1 mM H2O2; CAT, catalase; GPx, glutathione peroxidase; H2O2, hydrogen peroxide; PPI, peptidylprolyl cis-trans isomerase; ROS, reactive oxygen species; SOD, superoxide dismutase; TRR, thioredoxin reductase.
Discussion
The findings of the present study supported the hypothesis that recombinant PPI has multiple antioxidant properties that enable it to protect cells. First, recombinant PPI treatment made cells more resistant to H2O2-induced oxidative stresses. Second, recombinant PPI treatment increased antioxidant enzyme activities, providing cells with a higher capacity for scavenging ROS. Accordingly, recombinant PPI increased the H2O2-induced decreases in antioxidant enzyme activity and their mRNA expression. These data suggest that recombinant PPI cloned from the red algae P. yezoensis may be able protect cells as an inducer of antioxidant enzymes. PPI treatment increased the activities of antioxidant enzymes, such as CAT, GPx, SOD and TRR.
The mechanism of cell repair against oxidative stress is directly or indirectly regulated by antioxidants. Direct-regulation of antioxidants is possible at low concentrations, however the period of activation time is short. Conversely, the indirect-regulation of antioxidants may occur via the Kelch ECH associating protein 1/nuclear factor erythroid 2-related factor 2/antioxidant response element (Keap1/Nrf2/ARE) pathway, which is relatively long-lasting (35). When oxidative stress was induced by 1 mM H2O2, the activities of CAT and GPx, which primarily act to remove H2O2, were decreased. In addition, the activity of TRR, which helps PRDX directly remove H2O2, decreased. This suggested that the antioxidant enzymes in the cell disappeared following activation to remove the increased H2O2. However, the activity of SOD which is not directly involved in the removal of H2O2 was not different compared with the negative control.
The treatment of 0.001 µg-ml−1 recombinant PPI directly increased the activities of CAT, GPx and TRR to remove H2O2, thereby reducing the intracellular ROS. In addition, as a result of primary antioxidant activity, SOD activity was increased to remove newly generated ROS. The recombinant PPI treatment of 0.001 µg-ml−1 increased the mRNA expressions of CAT, GPx and TRR and did not increase the mRNA expression of SOD. This suggests that the recombinant PPI concentration of 0.001 µg-ml−1 does not affect the expression of SOD, and that the increase in SOD activity may be self-regulated in cells to remove newly generated ROS. In addition, an increase in TRR mRNA expression aided PRDX, which is directly associated with H2O2.
When treated with 1 µg-ml−1 recombinant PPI, the SOD activity was 53.4%, which was increased compared with the positive control. However, expression of mRNA was decreased compared with the positive control. These results suggested that the SOD antioxidant activity was not regulated by its associated signaling pathway, however by the antioxidant activity of the recombinant PPI. It has been reported that the recombinant PPI of the sweet potato roots gene has its own antioxidant activity (36). The recombinant PPI in the present study exhibited 20% antioxidant activity in vitro at 1 µg-ml−1 (data not shown). Therefore, the results of SOD activity and mRNA expression are different due to the fact that the antioxidant activity of SOD is regulated by that of PPI.
The recombinant PPI treatment of 0.01–0.1 µg-ml−1 increased the activities of CAT, GPx, SOD and TRR, and mRNA expression was high. It is predicted that recombinant PPI regulates the Keap1/Nrf2/ARE signal pathway for antioxidant enzyme expression. Therefore, the regulation of antioxidation by recombinant PPI directly promoted the activity of antioxidant enzymes, however was also indirectly controlled. Recently, Lee et al (37) reported that Cyp including PPI, binds to TRR and enhances its antioxidant activity. When the recombinant PPI is treated at a concentration of 0.01 or more, the activity of TRR may be rapidly increased due to the combination of PPI and TRR.
However, the expression of mRNA does not entirely determine the activity of the enzyme (38). Therefore, the present study primarily confirmed the activity and mRNA expression alterations of antioxidant enzymes that regulate induced oxidative stress. Gene regulation and enzyme activity combinations were additionally predicted. In the majority of cases, the expression of mRNA was directly associated with the regulation of the antioxidant enzyme activity. However, when treated with 0.001 µg/ml of PPI, the expression of TRR mRNA rapidly increased, whereas the activity of TRR was decreased compared with other experimental groups. Further experimentation is therefore required on TRR, PRDX and their associated regulatory mechanisms.
The present study revealed for the first time, to the best of our knowledge, the antioxidant properties of P. yezoensis recombinant PPI. The results of the present study indicated that recombinant PPI treatment controlled the expression of antioxidant enzymes and maintained the antioxidant capacity of the cell. Additional studies into the interaction between recombinant PPI and antioxidant enzymes are necessary to elucidate the antioxidant mechanisms including the Keap1/Nrf2/ARE signal pathway of recombinant PPI in the cell.
The present study also reported for the first time, and to the best of our knowledge, the isolation of PPI mRNA from P. yezoensis. The biochemical and physiological effects of recombinant PPI protein during oxidative stress requires further study. However, results from the present study suggest that recombinant PPI is an excellent candidate for the development of therapeutically useful antioxidant agents.
Acknowledgements
The present study was supported by The Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education (grant no. 2012R1A6A1028677).
References
- 1.Harman D. Role of free radicals in aging and disease. Ann N Y Acad Sci. 1992;673:126–141. doi: 10.1111/j.1749-6632.1992.tb27444.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bentley AR, Emrani P, Cassano PA. Genetic variation and gene expression in antioxidant related enzymes and risk of COPD: A systematic review. Thorax. 2008;63:956–961. doi: 10.1136/thx.2007.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young IS, Woodside JV. Antioxidants in health and disease. J Clin Pathol. 2001;54:176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apel K, Hirt H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. doi: 10.1146/annurev.arplant.55.031903.141701. [DOI] [PubMed] [Google Scholar]
- 7.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/S0891-5849(02)00905-X. [DOI] [PubMed] [Google Scholar]
- 8.Flora SJ. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev. 2009;2:191–206. doi: 10.4161/oxim.2.4.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de M, Bandeira S, da Fonseca LJ, da S Guedes G, Rabelo LA, Goulart MO, Vasconcelos SM. Oxidative stress as an underlying contributor in the development of chronic complications in diabetes mellitus. Int J Mol Sci. 2013;14:3265–3284. doi: 10.3390/ijms14023265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahashi N, Hayano T, Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989;337:473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- 11.Pérez de Lema G, Arribas-Gómez I, Ruiz-Ginés JA, de Arriba G, Prieto A, Rodriguez-Puyol D, Rodriguez-Puyol M. Reactive oxygen species mediate the effects of cyclosporine A on human cultured mesangial cells. Transplant Proc. 1997;29:1241–1243. doi: 10.1016/S0041-1345(96)00482-4. [DOI] [PubMed] [Google Scholar]
- 12.McGrath LT, Treacy R, McClean E, Brown JH. Oxidative stress in cyclosporin and azathioprine treated renal transplant patients. Clin Chim Acta. 1997;264:1–12. doi: 10.1016/S0009-8981(97)00088-0. [DOI] [PubMed] [Google Scholar]
- 13.Zhong Z, Arteel GE, Connor HD, Yin M, Frankenberg MV, Stachlewitz RF, Raleigh JA, Mason RP, Thurman RG. Cyclosporin A increases hypoxia and free radical production in rat kidneys: Prevention by dietary glycine. Am J Physiol. 1998;275:F595–F604. doi: 10.1152/ajprenal.1998.275.4.F595. [DOI] [PubMed] [Google Scholar]
- 14.Brazin KN, Mallis RJ, Fulton DB, Andreotti AH. Regulation of the tyrosine kinase Itk by the peptidyl-prolyl isomerase cyclophilin A; Proc Natl Acad Sci USA; 2002; pp. 1899–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreira PA, Nakayama TA, Pak WL, Travis GH. Cyclophilin-related protein RanBP2 acts as chaperone for red/green opsin. Nature. 1996;383:637–640. doi: 10.1038/383637a0. [DOI] [PubMed] [Google Scholar]
- 16.Galat A. Variations of sequences and amino acid compositions of proteins that sustain their biological functions: An analysis of the cyclophilin family of proteins. Arch Biochem Biophys. 1999;371:149–162. doi: 10.1006/abbi.1999.1434. [DOI] [PubMed] [Google Scholar]
- 17.Horowitz DS, Lee EJ, Mabon SA, Misteli T. A cyclophilin functions in pre-mRNA splicing. EMBO J. 2002;21:470–480. doi: 10.1093/emboj/21.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krzywicka A, Beisson J, Keller AM, Cohen J, Jerka-Dziadosz M, Klotz C. KIN241: A gene involved in cell morphogenesis in Paramecium tetraurelia reveals a novel protein family of cyclophilin-RNA interacting proteins (CRIPs) conserved from fission yeast to man. Mol Microbiol. 2001;42:257–267. doi: 10.1046/j.1365-2958.2001.02634.x. [DOI] [PubMed] [Google Scholar]
- 19.Leverson JD, Ness SA. Point mutations in v-Myb disrupt a cyclophilin-catalyzed negative regulatory mechanism. Mol Cell. 1998;1:203–211. doi: 10.1016/S1097-2765(00)80021-0. [DOI] [PubMed] [Google Scholar]
- 20.Lin DT, Lechleiter JD. Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization. J Biol Chem. 2002;277:31134–31141. doi: 10.1074/jbc.M112035200. [DOI] [PubMed] [Google Scholar]
- 21.O'Connell S, Tuite N, Slattery C, Ryan MP, McMorrow T. Cyclosporine A-induced oxidative stress in human renal mesangial cells: A role for ERK 1/2 MAPK signaling. Toxicol Sci. 2012;126:101–113. doi: 10.1093/toxsci/kfr330. [DOI] [PubMed] [Google Scholar]
- 22.de Oliveira e Silva AM, Vidal-Novoa A, Batista-González AE, Pinto JR, Mancini DA Portari, Reina-Urquijo W, Mancini-Filho J. In vivo and in vitro antioxidant activity and hepatoprotective properties of polyphenols from Halimeda opuntia (Linnaeus) Lamouroux. Redox Rep. 2012;17:47–53. doi: 10.1179/1351000212Y.0000000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ismail A, Jr, Tan S. Antioxidant activity of selected commercial seaweeds. Malays J Nutr. 2002;8:167–177. [PubMed] [Google Scholar]
- 24.Chandini SK, Ganesan P, Bhaskar N. In vitro antioxidant activities of three selected brown seaweeds of India. Food Chem. 2008;107:707–713. doi: 10.1016/j.foodchem.2007.08.081. [DOI] [Google Scholar]
- 25.Kumar SR, Hosokawa M, Miyashita K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar Drugs. 2013;11:5130–5147. doi: 10.3390/md11125130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sathya R, Kanaga N, Sankar P, Jeeva S. Antioxidant properties of phlorotannins from brown seaweed Cystoseira trinodis (Forsskål) C. Agardh. Arab J Chem. 2013 doi: 10.1016/j.arabjc.2013.09.039. [DOI] [Google Scholar]
- 27.Yuan YV, Bone DE, Carrington MF. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005;91:485–494. doi: 10.1016/j.foodchem.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 28.Balboa EM, Conde E, Moure A, Falqué E, Domínguez H. In vitro antioxidant properties of crude extracts and compounds from brown algae. Food Chem. 2013;138:1764–1785. doi: 10.1016/j.foodchem.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 29.Ganesan P, Kumar CS, Bhaskar N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour Technol. 2008;99:2717–2723. doi: 10.1016/j.biortech.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Hwang ES, Thi ND. Effects of extraction and processing methods on antioxidant compound contents and radical scavenging activities of laver (Porphyra tenera) Prev Nutr Food Sci. 2014;19:40–48. doi: 10.3746/pnf.2014.19.1.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fathi-Roudsari M, Akhavian-Tehrani A, Maghsoudi N. Comparison of three Escherichia coli strains in recombinant production of reteplase. Avicenna J Med Biotechnol. 2016;8:16–22. [PMC free article] [PubMed] [Google Scholar]
- 32.Abu-Reidah IM, Arráez-Román D, Quirantes-Piné R, Fernández-Arroyo S, Segura-Carretero A, Fernández-Gutiérrez A. HPLC-ESI-Q-TOF-MS for a comprehensive characterization of bioactive phenolic compounds in cucumber whole fruit extract. Food Res Int. 2012;46:108–117. doi: 10.1016/j.foodres.2011.11.026. [DOI] [Google Scholar]
- 33.Schmidt AJ, Heiser P, Hemmeter UM, Krieg JC, Vedder H. Effect of antidepressants on mRNA levels of antioxidant enzymes in human monocytic U-937 cells. Prog Neuro-Psychoph. 2008;32:1567–1573. doi: 10.1016/j.pnpbp.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Aguilar-Melero P, Prieto-Álamo MJ, Jurado J, Holmgren A, Pueyo C. Proteomics in HepG2 hepatocarcinoma cells with stably silenced expression of PRDX1. J Proteomics. 2013;79:161–171. doi: 10.1016/j.jprot.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Dinkova-Kostova AT, Talalay P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol Nutr Food Res. 2008;52(Suppl 1):S128–S138. doi: 10.1002/mnfr.200700195. [DOI] [PubMed] [Google Scholar]
- 36.Liao JC, Chiu CS, Chen HJ, Huang SS, Hou WC, Lin WC, Lin YH, Huang GJ. Characterization of a novel Cyclophilin-type peptidylprolyl isomerase protein from sweet potato storage roots. Botanical Studies. 2012;53:315–324. [Google Scholar]
- 37.Lee SP, Hwang YS, Kim YJ, Kwon KS, Kim HJ, Kim K, Chae HZ. Cyclophilin A binds to peroxiredoxins and activates its peroxidase activity. J Biol Chem. 2001;276:29826–29832. doi: 10.1074/jbc.M101822200. [DOI] [PubMed] [Google Scholar]
- 38.Glanemann C, Loos A, Gorret N, Willis LB, O'Brien XM, Lessard PA, Sinskey AJ. Disparity between changes in mRNA abundance and enzyme activity in Corynebacterium glutamicum: Implications for DNA microarray analysis. Appl Microbiol Biotechnol. 2003;61:61–68. doi: 10.1007/s00253-002-1191-5. [DOI] [PubMed] [Google Scholar]