Abstract
We present a phase 2, single-arm study evaluating 800 mg daily venetoclax, a highly selective, oral small-molecule B-cell leukemia/lymphoma-2 (BCL-2) inhibitor in patients with high-risk relapsed/refractory acute myelogenous leukemia (AML) or unfit for intensive chemotherapy. Responses were evaluated following revised International Working Group (IWG) criteria. The overall response rate was 19%; an additional 19% of patients demonstrated anti-leukemic activity not meeting IWG criteria (partial bone marrow response and incomplete hematologic recovery). Twelve (38%) patients had isocitrate dehydrogenase 1/2 mutations, of whom 4 (33%) achieved complete response/complete response with incomplete blood count recovery. Six (19%) patients had BCL-2–sensitive protein index at screening, which correlated with time on study. BH3 profiling was consistent with on-target BCL-2 inhibition and identified potential resistance mechanisms. Common adverse events included nausea, diarrhea and vomiting (all grades), and febrile neutropenia and hypokalemia (grade 3/4). Venetoclax demonstrated activity and acceptable tolerability in patients with AML and adverse features.
Keywords: acute myelogenous leukemia, BCL-2, biomarker, clinical trial, venetoclax
INTRODUCTION
Acute myelogenous leukemia (AML) is a heterogeneous disease characterized by impaired differentiation of hematopoietic stem cells and subsequent clonal expansion of myeloid blasts in the bone marrow, peripheral blood, and extramedullary tissue. Patients older than 65 years derive less benefit from standard intensive chemotherapy and poorly tolerate toxicities (1, 2).
B-cell leukemia/lymphoma-2 (BCL-2), an anti-apoptotic protein commonly expressed in hematologic malignancies, has been shown to be involved in tumor survival and chemoresistance (3). BH3 mimetics are an emerging group of agents that bind and inhibit anti-apoptotic proteins, freeing pro-apoptotic proteins to initiate apoptosis (4). Venetoclax (ABT-199/GDC-0199) is a highly selective, orally bioavailable BCL-2 inhibitor that has shown activity in BCL-2–dependent leukemia and lymphoma cell lines (5, 6). Venetoclax induced cell death in AML cell lines and primary patient samples in vitro and in mouse xenograft models (7, 8). BCL-2 inhibition induced cell death in AML cell lines and in leukemic blasts, progenitor, and stem cells from patients with AML (9, 10). The unique role of BCL-2 in leukemia stem cell survival suggests that BCL-2 inhibition has the potential to eliminate chemotherapy-resistant leukemia stem cells while sparing normal hematopoietic stem cells (9–11). These data provide rationale for targeted BCL-2 inhibition with venetoclax in AML.
Venetoclax has demonstrated promising clinical activity as a single agent in the treatment of chronic lymphocytic leukemia (CLL) (12). This is the first clinical study of venetoclax monotherapy in patients with relapsed/refractory AML or untreated AML unfit for intensive therapy. The primary objective was efficacy; secondary objectives included safety and pharmacokinetics. Exploratory objectives evaluated biomarkers, including protein expression patterns of BCL-2, BCL-2–like 1 (BCL-XL) and myeloid cell leukemia sequence 1 (MCL-1), and BH3 profiling. BCL-2 family protein expression and BH3 profiling using synthetic oligopeptides derived from BH3 domains of pro-death BH3 peptides that are applied to mitochondria (4) have been retrospectively evaluated as potential biomarkers of clinical response to venetoclax.
RESULTS
Patient Characteristics and Disposition
Thirty-two patients received at least 1 one dose of venetoclax and 26 patients had at least 4 weeks of therapy. Thirteen (41%) were reported to have an antecedent hematologic disorder or myeloproliferative neoplasms, and 4 (13%) patients had therapy-related AML (from prior malignancies) with complex cytogenetics. Two previously untreated patients were considered unfit for intensive chemotherapy by the treating physician and were treatment-naïve at study entry. Thirty (94%) patients had received at least 1 prior therapy and 13 (41%) patients had received at least 3 prior treatment regimens. Seventeen (53%) had received standard 7+3 induction therapy (cytarabine + anthracycline) and 23 (72%) had received at least 1 hypomethylating agent. The median age was 71 years (19–84); baseline characteristics are summarized in Table 1.
Table 1.
Patient demographics and baseline characteristics
| Characteristic | N = 32 |
|---|---|
| Median age (range), years | 71 (19–84) |
|
| |
| Sex, n (%) | |
| Female | 16 (50) |
| Male | 16 (50) |
|
| |
| Diagnosis, n (%) | |
| Relapsed/refractory | 30 (94) |
| Newly diagnosed | 2 (6) |
|
| |
| Ethnicity, n (%) | |
| White | 25 (78) |
| Black | 4 (13) |
| Asian | 3 (9) |
|
| |
| ECOG performance score, n (%)a | |
| 0 | 3 (9) |
| 1 | 14 (44) |
| 2 | 14 (44) |
| Missing | 1 (3) |
|
| |
| Any prior therapy, n (%) | 30 (94) |
| Prior regimens ≥3 | 13 (41) |
| Prior standard induction (3+7) therapy | 17 (53) |
| Prior hypomethylating agents | 24 (75) |
| Prior allogeneic stem cell transplant | 4 (13) |
| Treatment naïve | 2 (6) |
|
| |
| Prior myeloid disorder, n (%) | |
| Prior myelodysplastic syndromeb | 11 (35) |
| Prior myeloproliferative neoplasm | 2 (6) |
|
| |
| Molecular markersc, n (%) | |
| IDH mutationsd | 12 (38) |
| FLT3-ITDe | 4 (13) |
| BCR/ABL | 1 (3) |
| JAK2 | 1 (3) |
| KRAS | 1 (3) |
| MLL | 1 (3) |
| NPM1 | 4 (13) |
| CEBPα | 2 (6) |
|
| |
| Cytogenetics, n (%) | |
| del(7q) | 10 (31) |
| Complex | 10 (31) |
| None | 2 (6) |
ECOG = Eastern Cooperative Oncology Group.
Percentage based on known values.
Includes n = 4 with therapy-related myelodysplastic syndrome with transformation to AML.
Cytogenetics were evaluated for all patients at the investigator sites. Not all patients had molecular marker analysis; 8/32 did not have IDH mutational analysis performed at the sites. Data included patients with more than one marker expression.
Two were IDH1 mutations and 10 were IDH2 mutations. Of the 12, 11 were in a known site that leads to (R)-2-hydroxyglutarate and one mutation was not in the putative hotspot (exon 3; D76frameshift).
Three with FLT3-ITD and a concomitant IDH mutation were confirmed at study entry and one site reported FLT3-ITD mutation was not detected at study entry (assay sensitivity was 0.1%).
Molecular markers and cytogenetics were evaluated locally for all patients. Additionally, AML associated genetic mutations were assessed in pretreatment specimens using two next generation sequencing panels: the Trusight Myeloid panel for 31 of 32 patients and the FoundationOne Heme™ panel, which assesses genomic aberrations in over 400 genes, for 15 of 32 patients. Key molecular markers and cytogenetics data are highlighted in Table 1. Mutations in isocitrate dehydrogenase (IDH) 1 or 2 were identified in 12 (38%) patients (n = 2 IDH1 and n = 10 in IDH2). Eleven of the 12 IDH1/2 mutations were in a known site that leads to production of (R)-2-hydroxyglutarate (e.g., IDH1-R132, IDH2-R140, and IDH2-R172) (13–15). One patient had a mutation in exon 3 of IDH2 (D76frameshift), the functional significance of this mutation is unknown. FMS-like tyrosine kinase-3–internal tandem duplication (FLT3-ITD) mutations were reported in 4 (13%) patients (3 of which were confirmed in pretreatment specimens). These 3 patients had concomitant mutations in IDH. Ten (31%) patients had chromosome 7q deletions [del(7q)] and 10 (31%) had complex cytogenetics.
Overall Activity
The objective response rate by International Working Group (IWG) criteria was 19% (6/32), with 6% (2) achieving a complete response (CR) and 13% (4) achieving a CR with incomplete blood count recovery (CRi) (Table 2). Except for 1 CRi, all objective responses were achieved by the week 4 assessment. All IWG-defined responses were observed in patients who had been previously treated for AML. Three of the 6 responders had an antecedent hematologic disorder. Of the 24 patients who received prior hypomethylating agents, 25% (6/24) achieved CR/CRi. Among the 12 patients with IDH1/2 mutations, 4 (33%) achieved CR/CRi. One patient with an IDH2 mutation (not in a putative hotspot) achieved a CRi at week 24 following a 20-day dose interruption after week 4. The median duration of venetoclax therapy in responders was 144.5 days (83–256), and the median duration of CR was 48 days (Table 3). Thirty-four percent (11/32) of patients escalated to 1200 mg daily venetoclax after lack of objective response at the first assessment on 800 mg and 9% (3/32) after relapse following CR/CRi on 800 mg dose. The 1200 mg dose did not demonstrate additional activity. Median time on study was 63.5 days (14–256). All patients have discontinued venetoclax: 29 due to progressive disease, 1 due to an adverse event (AE) (terminal ileitis), 1 withdrew consent, and 1 proceeded to allogeneic hematopoietic stem cell transplant after achieving stable disease.
Table 2.
Overall activity of venetoclax in AML patients
| All N = 32 (%) |
IDH mutation n = 12 (%) |
|
|---|---|---|
| Objective response rate (CR + CRi) by IWG criteria | 6 (19) | 4 (33) |
| CR | 2 (6) | 2 (17) |
| CRia | 4 (13) | 2 (17) |
|
| ||
| Anti-leukemic activity that did not meet IWG criteria | 6 (19) | 2 (17) |
| ≥50% bone marrow blast reduction with two cell line recovery and transfusion independenceb | 2 (6) | 0 |
| ≥50% bone marrow blast reduction with one cell line recoveryc | 2 (6) | 2 (17) |
| ≥50% bone marrow blast reduction with no hematologic recovery | 2 (6) | 0 |
|
| ||
| Treatment failured | 20 (63) | 6 (50) |
|
| ||
| Overall activitye | 12 (38) | 6 (50) |
One with IDH mutation in exon 3; dose interruption for 20 days after week 4 and achieved CRi at week 24.
One with ≥50% bone marrow blast reduction at week 5 and recovery of hemoglobin and platelets, and one with ≥50% bone marrow blast reduction at week 12 and recovery of neutrophils and hemoglobin.
Both with ≥50% bone marrow blast reduction and recovery of a single cell line (hemoglobin).
Treatment failure includes progressive disease and less than partial response by IWG criteria.
Overall activity includes objective responses by IWG criteria and anti-leukemic activity that does not meet IWG criteria.
Table 3.
Overall activity and predictors of response
| Response | Time on venetoclax, days | Cytogeneticsa | IDH mutation | FLT3-ITD | BCL-2 family protein index at screening | |
|---|---|---|---|---|---|---|
| Response that met IWG criteria | CR | 247 | Normal | – | – | – |
| CRi | 256 | Normal | Y | – | Sensitive | |
| CRi | 170 | Complex; del(7q) | – | – | Resistant | |
| CRi | 119 | Normal | Y | – | – | |
| CR | 107 | Normal | Y | – | – | |
| CRi | 83 | Normal | Y | – | – | |
|
| ||||||
| Median (range) = | 144.5 (83–256) | |||||
|
| ||||||
| Anti-leukemic activity that did not meet IWG criteria | SDb | 247 | Normal | – | – | – |
| SDc | 143 | Complex; del(7q) | – | – | – | |
| SDd | 106 | del(7q) | Y | – | Sensitive | |
| SDe | 104 | Normal | Y | – | Sensitive | |
| SD | 128 | Complex; t(8;12) | – | – | Resistant | |
| SDf | 86 | Normal | – | Y | Sensitive | |
|
| ||||||
| Median (range) = | 117 (86–247) | |||||
Most common cytogenetic abnormalities were evaluated for all patients at the investigator sites.
≥50% bone marrow blast reduction at week 5 and recovery of hemoglobin and platelets.
≥50% bone marrow blast reduction at week 12 and recovery of neutrophils and hemoglobin.
Both with ≥50% bone marrow blast reduction and recovery of a single cell line (hemoglobin).
≥50% bone marrow blast reduction with no hematologic recovery.
FLT3-ITD mutation not confirmed at study entry, assay sensitivity was less than 0.1%..
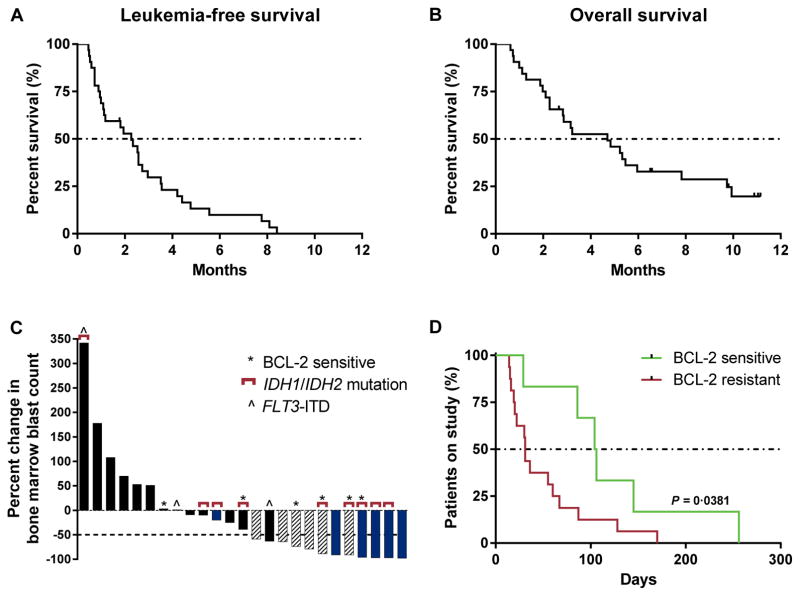
Anti-leukemic activity evidenced by bone marrow blast reduction from baseline and incomplete hematologic recovery, which did not meet IWG response criteria, was observed in an additional 19% (6/32) of patients. Two patients had persistent anti-leukemic activity: 1 patient had ≥50% bone marrow blast reduction at week 5 and recovery of hemoglobin and platelets (until discontinuing venetoclax after 247 days), and the other patient had ≥50% bone marrow blast reduction at week 12 and recovery of neutrophils and hemoglobin (until discontinuing venetoclax after 143 days). Two patients with IDH1/2 mutations achieved ≥50% bone marrow blast reduction and recovery of a single cell line (hemoglobin), and 2 patients achieved ≥50% bone marrow blast reduction without evidence of hematologic recovery (Table 2). The median duration of venetoclax therapy was 117 days (86–247) in the 6 patients with reductions in bone marrow blasts and transfusion independence not meeting IWG response criteria versus 30 days (14–145) in patients who had treatment failure (Table 3 and Supplementary Table S1). Five of the patients with observed anti-leukemic activity not meeting IWG response criteria had relapsed/refractory AML patients and 1 patient was treatment-naïve. None of the 4 patients with therapy-related AML experienced anti-leukemic activity on venetoclax. The median time to progression was 2.5 months (1–3). The 6-month leukemia-free survival rate was 10% (95% confidence interval [CI] 2.5–23.3), and the median leukemia-free survival was 2.3 months (1.0–2.7) (Fig. 1A). The 6-month overall survival estimate was 36% (95% CI 20–53), and median overall survival was 4.7 months (2.3–6.0) (Fig. 1B). One patient is in follow-up for survival as of March 04, 2016, who proceeded to transplant after achieving stable disease on venetoclax.
Figure 1.
Biologic activity of venetoclax. A and B, the median leukemia-free survival was 2.3 months (range 1.0–2.7) and the median overall survival was 4.7 months (range 2.3–6.0), in relapsed/refractory AML patients or those unfit for intensive chemotherapy treated with venetoclax monotherapy. C, 25 (78%) patients had bone marrow blast counts evaluable at the first assessment (week 4). The best percent change in bone marrow counts at the first assessment (week 4) is shown. Six patients came off study before the first assessment and 1 patient had unevaluable bone marrow (aplasia). Patients who achieved an objective response by the IWG criteria (complete response/complete response with incomplete blood count recovery) are indicated with blue, and patients who had anti-leukemic activity that did not meet IWG criteria are indicated with hashed bars. Patients with a BCL-2 family protein sensitive index at screening and/or IDH1/2 mutations are annotated with asterisks and brackets, respectively. D, 6 patients with a BCL-2 family sensitive index (green) achieved longer durations on venetoclax therapy than the 16 patients with a BCL-2 family resistant index (red) (P = 0.0381 by Wilcoxon signed rank test). The median number of days on venetoclax was 96 days for patients with a BCL-2 sensitive index vs 31 days for patients with a BCL-2 resistant index.
Twenty-five (78%) patients had bone marrow blast counts evaluable at the first assessment after 4 weeks of therapy (6 patients came off study before the first assessment and 1 patient had unevaluable bone marrow) (Fig. 1C). Twelve (38%) patients had ≥50% blast count reduction at the week 4 assessment. Of these, 5 patients achieved a CR/CRi as a best objective response (3/5 patients had IDH1/2 mutation and none had FLT3-ITD mutation).
Biomarker Correlates
Peripheral blood specimens were collected from 31 of the 32 patients at screening for biomarker studies. BCL-2 family protein expression was evaluated before first dose in the 22 patients who had samples of sufficient quality for analysis. Specimens from the other 9 patients failed analysis owing to either low quality (n = 6, 3 of which were CR/CRi patients) or lack of tumor cells present (n = 3, 1 of which was a CR/CRi patient). The percent of tumor cells expressing BCL-2, BCL-XL and the ratio of BCL-2/BCL-XL in the blasts were retrospectively assessed with time on study and reduction in bone marrow blasts with the goal of identifying BCL-2 family sensitive or resistant indices based on protein expression. Patients were categorized as having a BCL-2 family sensitive protein index if ≥35% of tumor cells expressed BCL-2 and <40% of tumor cells expressed BCL-XL protein (Supplementary Fig. S1). Patients with <35% of tumor cells having detectable BCL-2 protein expression and/or >40% having detectable BCL-XL protein expression were categorized as having a BCL-2 family resistant protein index. Six of 22 (27%) patients had a BCL-2 family sensitive protein index; of these, 1 patient achieved CRi (the other CRi patient with an evaluable specimen had a BCL-2 resistant profile) and 3 patients achieved anti-leukemic activity not meeting IWG response criteria (Fig. 1C and Table 3). Patients with a BCL-2 family sensitive protein index at screening experienced longer durations on venetoclax therapy (P = 0.0381 by Wilcoxon signed rank test; Fig. 1D and Table 3).
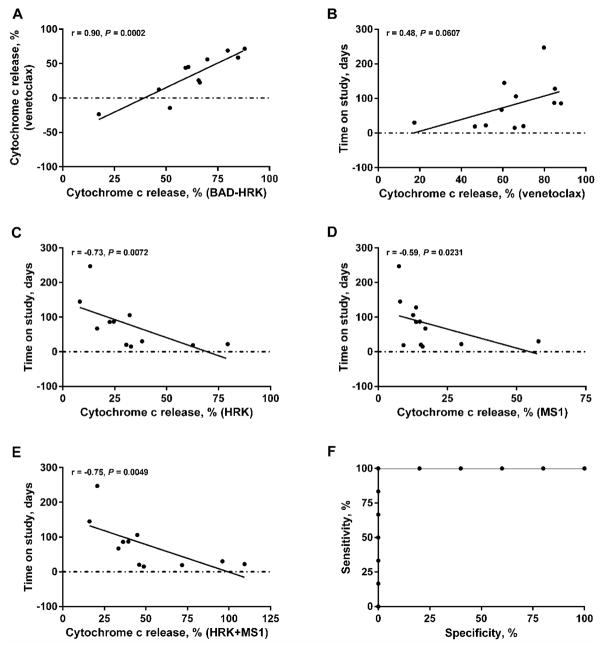
BH3 profiling is a functional assay with utility for probing interactions between anti-apoptotic BCL-2 family proteins and their pro-apoptotic counterparts to determine BCL-2 family dependence (4). We assessed whether BH3 profiling results would correlate with clinical response to venetoclax (16). BH3 profiling with a series of BH3 peptides was performed on 18 pretreatment bone marrow biopsies or peripheral blood samples; 12 samples passed quality control criteria for data analysis (>50% viability on thaw; >5% AML blasts) (Fig. 2). Mitochondrial cytochrome c release in response to HRK peptide indicated BCL-XL dependence, to MS1 peptide indicated MCL-1 dependence, and to BAD peptides indicated BCL-2 and/or BCL-XL dependence. Subtracting cytochrome c release caused by HRK from that caused by BAD (BAD-HRK) provides a rough index of selective BCL-2 dependence (17). Mitochondrial cytochrome c release in response to venetoclax correlated with release caused by BAD-HRK (P = 0.0002; Fig. 2A), indicating on-target activity within myeloblast mitochondria for venetoclax. Cytochrome c release caused by direct ex vivo exposure of myeloblast mitochondria to venetoclax correlated weakly with duration on venetoclax (P = 0.0607; Fig. 2B). Although not statistically significant, this result is consistent with a mitochondrial mechanism of action for venetoclax in vivo. Negative correlation was observed between AML blast dependence on the anti-apoptotic protein BCL-XL (using the HRK peptide, P = 0.0072; Fig. 2C) or the anti-apoptotic protein MCL-1 (using the MS1 peptide, P = 0.0231; Fig. 2D) correlated with days on venetoclax. Because dependence on MCL-1 or BCL-XL predicted short duration of venetoclax therapy, we asked whether we could make a superior predictor by arithmetically adding response to HRK to that of MS1 (P = 0.0049; Fig. 2E). This post hoc metric was a good binary predictor of staying on venetoclax more than 30 days; the area under the curve (AUC) of the receiver operating characteristic was 1.0 (Fig. 2F).
Figure 2.
Venetoclax acts through on-target BCL-2 inhibition. Twelve samples were analyzed (>50% viability on thaw; >5% AML blasts). A, mitochondrial BCL-2 dependence correlated with release caused by BAD-HRK. Background BCL-XL dependence was removed by subtraction of HRK for a specific measure of mitochondrial BCL-2 dependence. B, mitochondrial response to venetoclax was weakly related to days on venetoclax therapy. C and D, AML blast dependence on the anti-apoptotic protein BCL-XL (using the HRK peptide) or the anti-apoptotic protein MCL-1 (using the MS1 peptide) negatively correlated with days on venetoclax. E, an index combining AML blast dependence on the anti-apoptotic protein BCL-XL and the anti-apoptotic protein MCL-1 negatively correlated with days on venetoclax therapy. F, ROC analysis of dependence on MCL-1 or BCL-XL, by arithmetically adding response to HRK peptide to that of MS1 peptide, was a perfect binary predictor of staying on venetoclax therapy more than 30 days (area under the curve of the ROC = 1.0). Correlations (r) and P-values based on one-tailed Spearman rank-order correlation tests.
Safety
Venetoclax monotherapy was generally well tolerated in patients with AML. Treatment-emergent AEs were reported for all patients and are summarized in Table 4. The most common AEs of any grade were nausea, diarrhea, hypokalemia, vomiting, and headache. The most common grade 3/4 AEs were febrile neutropenia, hypokalemia, pneumonia, hypotension, and urinary tract infection. Serious AEs were reported in 27 (84%) patients, with febrile neutropenia being the most common (10/32, 31%) (Table 4). There were no events of tumor lysis syndrome (TLS). The incidence of infection was consistent with expectations for this patient population. Electrolyte abnormalities were related to concomitant supportive therapy for TLS prophylaxis and antifungal agents.
Table 4.
Treatment-emergent adverse events
| Adverse event, n (%)a | Any grade adverse event | Grade 3/4 adverse event |
|---|---|---|
| Any adverse event | 32 (100) | 26 (81) |
| Nausea | 19 (59) | 2 (6) |
| Diarrhea | 18 (56) | 2 (6) |
| Hypokalemia | 13 (41) | 7 (22) |
| Vomiting | 13 (41) | 0 |
| Fatigue | 11 (34) | 0 |
| Headache | 11 (34) | 1 (3) |
| Hypomagnesemia | 11 (34) | 0 |
| Febrile neutropenia | 10 (31) | 10 (31) |
| Hypophosphatemia | 10 (31) | 2 (6) |
| Abdominal pain | 9 (28) | 2 (6) |
| Cough | 9 (28) | 0 |
| Epistaxis | 8 (25) | 2 (6) |
| Hyperphosphatemia | 8 (25) | 0 |
| Hypocalcemia | 8 (25) | 3 (9) |
| Pneumonia | 8 (25) | 6 (19) |
| Dyspnea | 7 (22) | 0 |
| Hypotension | 7 (22) | 4 (13) |
| Peripheral edema | 7 (22) | 0 |
| Pyrexia | 7 (22) | 1 (3) |
| Insomnia | 5 (16) | 1 (3) |
| Urinary tract infection | 5 (16) | 4 (13) |
| Serious adverse event, n (%)b | Total | |
| Any serious adverse event | 27 (84) | |
| Febrile neutropenia | 9 (28) | |
| Pneumonia | 5 (16) | |
| Abdominal pain | 2 (6) | |
| Acute renal failure | 2 (6) | |
| Failure to thrive | 2 (6) | |
| Hypotension | 2 (6) | |
| Sepsis | 2 (6) | |
| Urinary tract infection | 2 (6) | |
Adverse events occurring in ≥10% of patients regardless of cause; excludes 9 adverse events of disease progression of AML.
Serious adverse events occurring in at least 2 patients regardless of cause; excludes serious adverse events of disease progression of AML.
There were no AEs that required reduction of venetoclax dose. Venetoclax interruptions occurred in 8/32 (25%) patients. Of these, 6 patients had AEs; 4 were grade 3/4, 2 were grade 1/2. There were 2 grade 3/4 events of diarrhea and 2 grade 3/4 events of febrile neutropenia managed by interruption of venetoclax dosing. In addition, one patient had bone marrow failure and one patient had disease progression. Other events of febrile neutropenia were managed by standard of care (n = 9).
Pharmacokinetics
Venetoclax plasma concentration-time profile at week 6 day 1 from 13 patients who received the 800-mg dose is shown in Supplementary Fig. S2. Following multiple-dose administrations of 800 mg venetoclax, plasma concentrations peaked at 6 hours (4–8), and the mean (CV%) maximum plasma concentration (Cmax), trough plasma concentration and AUC over a 24-hour dose interval (AUC0-24) were 3.74 (48) μg/mL, 1.43 (134) μg/mL and 61.6 (69) μg•h/mL, respectively. Venetoclax exposures in the current study are consistent with those observed in the first-in-human venetoclax studies in patients with relapsed/refractory CLL or non-Hodgkin lymphoma (12, 18). Venetoclax IC50 was <10 nM (0.0087 μg/mL) in primary AML patient myeloblasts samples and <100 nM (0.087 μg/mL) in sensitive AML cell lines (7).
DISCUSSION
Single-agent venetoclax demonstrated a 19% objective response rate by IWG criteria in patients with heavily pretreated AML. An additional 19% derived had anti-leukemic activity demonstrated by partial bone marrow response and incomplete hematologic recovery that did not meet standard response criteria. For the majority of patients, activity was observed at the first assessment (end of week 4). Activity was observed in patients pretreated with standard induction therapy and hypomethylating agents; 25% of those previously treated with hypomethylating agents achieved an objective response.
Venetoclax was tolerable in these heavily pretreated AML patients, with AEs consistent with expectations in this population (19, 20). There were no events of TLS, and no new safety signals were identified compared with other venetoclax monotherapy trials in hematologic malignancies (12, 18).
Mutations in IDH1/2 are present in approximately 15%–20% of patients with AML (15, 21); in the current study, 12 (38%) patients had IDH1/2 mutations. These mutations are acquired early in progression to leukemia (22, 23) and appear stable during disease evolution (24, 25). Mutant IDH1/2 proteins catalyze production of the oncometabolite (R)-2-hydroxyglutarate and elicit epigenetic changes, dysregulated mitochondrial function, and increased BCL-2 dependence in AML cells (26). Four (33%) patients with IDH1/2 mutations achieved objective responses with venetoclax. Three patients with IDH1/2 mutations had concomitant FLT3-ITD mutations, a known adverse prognostic factor; these patients did not achieve response and had shorter durations on venetoclax (Supplementary Table S1). Our data provide evidence that AML with IDH1/2 mutations exhibit BCL-2 dependence and validates preclinical data that suggests suppression of cytochrome c oxidase activity in IDH1/2 mutant AML lowers the mitochondrial threshold to trigger apoptosis upon BCL-2 inhibition (7, 8, 26). However, activity observed in patients with wild-type IDH1/2 suggests targeting BCL-2 with venetoclax should not be restricted to patients with mutations in IDH1/2. The number of patients in our study is relatively small and further studies are required to draw full conclusions regarding the clinical activity of venetoclax in patients with IDH1/2 mutations.
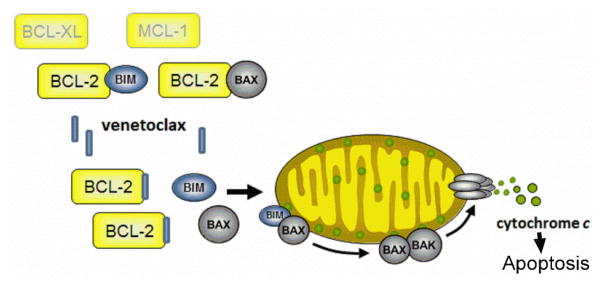
BCL-2 family protein expression has been previously evaluated as a predictor of clinical response to BH3 mimetics with mixed success (27–30). We analyzed the predictive capacity of protein expression of BCL-2, BCL-XL and MCL-1, and BH3 profiling, and evaluated putative predictors of response. The findings of the current study provide evidence that venetoclax acts as originally described, through on-target BCL-2 inhibition and subsequent induction of apoptosis (Fig. 3) (5). BCL-2 family protein expression at screening identified patients with a BCL-2 family sensitive or resistant index. Four patients with a BCL-2 family sensitive index experienced clinical benefit, either by achieving an objective response, anti-leukemic activity that did not meet IWG criteria, or longer durations on venetoclax therapy.
Figure 3.
Mechanism of action of venetoclax. Venetoclax acts as a specific inhibitor of BCL-2 and upon binding, releases pro-apoptotic proteins to induce apoptosis. BIM, BCL-2–like 11; BAX, BCL-2–associated X protein; BAK, BCL-2 antagonist/killer 1.
BH3 profiling has been used in AML to predict response to novel agents (31, 32) and to identify primary cells from patients with AML sensitive to BCL-2 inhibition (7, 11). In this study, BH3 profiling identified patient samples most sensitive to BCL-2 inhibition by venetoclax. The performance of the post hoc receiver operating characteristic analysis indicates that BH3 profiling warrants further testing as a predictive assay for venetoclax sensitivity. Dependence on BCL-2 does not seem to be sufficient for prolonged clinical sensitivity to venetoclax; rather, AML blasts also need to lack dependence on resistance factors BCL-XL and MCL-1. This suggests that the best predictor of sustained response to a BCL-2 inhibitor as a single agent is the lack of readily accessible resistance mechanisms provided by BCL-XL and MCL-1. These results also suggest that dependence on individual anti-apoptotic proteins is more heterogeneous in AML than in CLL, in which dependence on BCL-2 is relatively homogeneous (33).
Relapsed/refractory AML in the elderly population has an extremely poor prognosis, with overall survival less than 6 months. The 19% objective response rate plus additional examples of myeloblast reduction observed in this study, with a relatively well tolerated oral single agent, is a promising clinical achievement. Response rates in this population vary greatly based on prognostic factors, ranging from 10%–85%. However, remission rates greater than 20% are rarely obtained without the application of intense cytotoxic chemotherapy regimens requiring extended inpatient care (34–36). There is potential for improvement in the response rate with the use of predictive biomarkers to identify and select patients responsive to venetoclax therapy.
In summary, this phase 2 study demonstrated pharmacologic activity of venetoclax as a single agent in AML with an acceptable safety profile. These results, along with preclinical data with venetoclax in combination with other agents (9, 37, 38) and observations that AML stem cells may be dependent on BCL-2 (7, 9, 10), support evaluating venetoclax in combination with other agents in patients with AML. Venetoclax is currently being investigated in phase 1 studies in combination with low-dose cytarabine (NCT02287233) and decitabine or azacitidine (NCT02203773). Preliminary results from clinical trials in treatment naïve elderly AML with combinations of venetoclax and hypomethylating agents suggest that response rates comparable to standard induction therapy can be obtained with tolerable toxicity (39).
METHODS
Patients
Eligible patients had relapsed/refractory AML by the World Health Organization classification or untreated AML unfit for intensive therapy; Eastern Cooperative Oncology Group Performance score 0–2; adequate organ function (creatinine clearance ≥50 mL/min, aspartate aminotransferase and alanine aminotransferase ≤3.0 × ULN [upper limit of normal], bilirubin ≤1.5 × ULN); and did not meet exclusion criteria including white blood cell count >25 × 109/L (hydroxyurea permitted to lower count), unresolved ≥grade 2 clinically significant nonhematologic toxicities from prior anti-cancer therapy, other active malignancy within 1 year before study entry, major organ dysfunction, active infections, and pregnancy or breastfeeding.
Study Design and Treatment
Phase 2, open-label, single-arm, multicenter study of venetoclax monotherapy in AML patients, enrolled between December 31, 2013 and April 5, 2014 (NCT01994837). The study used a Simon two-stage optimal design (Supplementary Fig. S3). Nineteen patients were to be enrolled and treated with venetoclax monotherapy in the first stage. If ≥5 patients achieved an objective response by revised IWG criteria (CR, CRi, or partial response) (40) in an interim analysis after at least 12 weeks of therapy, the second stage would begin and enroll an additional 35 patients. The sample size was determined with 90% power at a type 1 error rate of 0.05 with an uninteresting response rate of 20% and an interesting response rate of 40%. During execution of the trial, 13 eligible patients were in the screening process when the interim analysis of stage one began. Patients were allowed to initiate treatment before completion of the stage one interim analysis due to the disease severity and prognosis of these patients without available options for therapy. A total of 32 patients were enrolled. Enrollment stopped after the first stage, and no additional patients were screened or treated after the interim analysis was completed.
To mitigate risk of TLS (12), a stepwise ramp-up of venetoclax dosing was used to achieve the target venetoclax dose (800 mg). Oral venetoclax was administered daily beginning with 20 mg on week 1 day 1, and escalated daily to 50 mg on day 2, 100 mg on day 3, 200 mg on day 4, 400 mg on day 5, and 800 mg on day 6 (and 800 mg daily thereafter). Patients were hospitalized the first week of therapy (day 1 until at least 24 hours after reaching the target dose), and received hydration (oral and intravenous) and treatment with a uric acid-reducing agent. Blood chemistries were performed on the first day of dosing and each day of a new dose at 0 (within 4 hours before dosing), 8, and 12 hours, and at 24, 48, and 72 hours after the first 800-mg dose.
Dose interruption, reduction, or continuation at the current level was permitted if toxicities were observed. Dose escalation to 1200 mg daily was allowed if CR or CRi was not achieved at first assessment (end of week 4) and 800 mg was tolerable. Patients continued daily venetoclax until unacceptable toxicity or progression. Supportive treatment, including anti-infection prophylaxis and growth factor support, was allowed at the investigator’s discretion.
Assessments
Responses were evaluated using the revised IWG guidelines for AML; hematologic responses were also evaluated (40). The overall response rate by IWG criteria included CR/CRi. Patients without clinical or cytological progressive disease who did not achieve IWG response were considered to have stable disease. Bone marrow aspirate and biopsy were performed at screening, end of week 4, and every 8 weeks thereafter. AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE version 4.0). Physical exam, vital signs, and clinical laboratory tests were performed at screening and throughout the study. Clinical and laboratory evidence of adverse events were monitored routinely throughout the study and for 30 days following discontinuation of study drug.
Pharmacokinetic samples were collected 8 hours post-dose after the first dose and the first day of each new dose level. Intensive venetoclax pharmacokinetic samples (0–8 hours) were collected at week 6 day 1. For the intensive pharmacokinetic days, venetoclax Cmax, the time to Cmax (peak time), and AUC0-24 were determined using noncompartmental methods.
BCL-2 family protein analysis
Pre-treatment bone marrow aspirates or peripheral blood samples for BCL-2 family protein mutational analysis were collected and analyzed. The presence of mutations in genes associated with hematological malignancies was determined in baseline specimens using 2 next-generation sequencing panels: the Trusight Myeloid panel (Illumina, San Diego, CA, USA) for 31 of 32 patients, and the FoundationOne Heme™ panels (Foundation Medicine, Cambridge, MA, USA) for 15 patients. Two milliliters of peripheral blood were added to freshly prepared 1× Lyse/Fix buffer (BD Biosciences, San Jose, CA, USA) in a 1:40 ratio. The sample was inverted 5–8 times, incubated at 37°C for 10 minutes, and frozen at −70°C. Prior to testing, the specimens were thawed at 37°C, filtered through a 45-micron, centrifuged at 1500 rpm for 5 minutes, re-suspended in PBS, and the number of peripheral blood mononuclear cells (PBMCs) recovered were determined. Twenty-five specimens had sufficient integrity and cell number to determine the expression of BCL-2, BCL-XL, and MCL-1 protein in the tumor cells. The PBMCs were permeabilized with 1× Phosflow Perm Wash buffer 1 (BD Biosciences, San Jose, CA, USA) and incubated with fluorescently labelled antibodies to cell surface markers (CD45, BD Biosciences, San Jose, CA, USA) and intracellular proteins (BCL-2 and MCL-1, BD Biosciences, San Jose, CA, USA, and BCL-XL, Cell Signaling, Danvers, MA, USA).
BH3 profiling
Pre-treatment bone marrow aspirates or peripheral blood samples were obtained and mononuclear cells were isolated using a Ficoll gradient and viably frozen at a central repository. Twelve of 18 specimens were sufficient for analysis. Thawed cells were washed once with PBS and stained with 1:100 Invitrogen Live/Dead – aqua stain (#423102, BioLegend, San Diego, CA, USA) in PBS for 20 minutes on ice, washed with PBS, and subsequently stained with 1:100 CD45-BV421 (clone HI30, #563879; BD Biosciences, San Jose, CA, USA) and 1:100 CD33-PE (clone WM53, #561816; BD Biosciences, San Jose, CA, USA) in FACS buffer (2% FBS in PBS) on ice for 20 minutes with 1:100 human FcR block (#130-059-901; Miltenyi Biotec, San Diego, CA, USA). Cells were BH3 profiled as described in Pan et al (7) and samples were analyzed using the LSRII flow cytometer and data analysis performed using BD FACSDiva Software (BD Biosciences, San Jose, CA, USA). Cytochrome c loss was measured by a gating strategy, in which a gate was drawn around the DMSO negative control to depict 100% cytochrome c retention. DMSO was used as a negative control for cytochrome c retention, whereas a control without the cytochrome c antibody was used as a positive control for 100% cytochrome c release. Cytochrome c loss was calculated using the equation: [Cytochrome c loss = 100 – (% of cells within cytochrome c retention gate)].
Acute myelogenous leukemia (AML) blasts were identified by CD45low-mid/CD33mid-high/SSC-Alow. Cells from the DHL4 cell line were BH3 profiled with the AML patient samples as an internal control for peptide function.
Statistical Analysis
The data cutoff for this report was January 22, 2015, when all patients had discontinued. Analyses were specified in a statistical analysis plan. Descriptive statistics including medians, ranges, and SDs were calculated. Efficacy, safety, and pharmacokinetic analyses were performed on all patients who received at least 1 dose of venetoclax. Assessments at screening served as baseline unless repeated on week 1 day 1 before dosing. All statistical analyses were performed using SAS version 9 (Cary, NC, USA) and all plots were generated using GraphPad Prism Software version 6.05 (La Jolla, CA, USA). The Kaplan-Meier method was used for time-to-event analyses. Data for leukemia-free survival were censored at the date of last disease assessment or date of first dose plus 1 day for patients without any disease assessments. Overall survival was censored at the date of last study visit or last known date to be alive, whichever was later. Correlations were analyzed with one-tailed Spearman rank-order correlation tests.
Supplementary Material
SIGNIFICANCE.
Venetoclax monotherapy demonstrated clinical activity in patients with relapsed/refractory AML or those unfit for intensive chemotherapy and had a tolerable safety profile in this phase 2 study. Moreover, biomarker correlates were consistent with BCL-2 dependence and potential predictive markers of response to venetoclax were identified. These findings, together with preclinical data, provide compelling rationale to evaluate venetoclax in combination with other agents in patients with AML.
Acknowledgments
Financial support: This study was supported by AbbVie in collaboration with Genentech/Roche.
AbbVie and Genentech provided financial support for the study and participated in the design, study conduct, analysis and interpretation of data, as well as the writing, review, and approval of the manuscript. Venetoclax (ABT-199/GDC-0199) is being developed in collaboration between AbbVie and Genentech.
We thank the patients who participated in this trial and their families; the study coordinators and the support staff at the clinical sites; and AbbVie and Genentech venetoclax team members. We also thank Jingwen Jia, Vinay Tavva, and Ruiling Zhang for statistical programming support, Relja Popovic, for genetic mutations analysis support and Leanne Lash, for writing assistance; all are employees of AbbVie. Editorial and administrative assistance was provided by Evidence Scientific Solutions (Philadelphia, PA, USA) and funded by AbbVie, Inc.
Footnotes
Authors’ Contributions
Study conception and design: M. Konopleva, D.A. Pollyea, T. Busman, E. McKeegan, M. Zhu, J. Ricker, W. Blum, J. Leverson, R. Humerickhouse, M. Mabry, H. Kantarjian, A. Letai.
Patient enrollment and care of patients: M. Konopleva, D.A. Pollyea, W. Blum, C.D. DiNardo, T. Kadia, R. Stone, H. Kantarjian, A. Letai.
Data collection, analysis, and interpretation: M. Konopleva, D.A. Pollyea, J. Potluri, B. Chyla, L. Hogdal, T. Busman, E. McKeegan, A.H. Salem, M. Zhu, J. Ricker, W. Blum, C.D. DiNardo, T. Kadia, M. Dunbar, R. Kirby, N. Falotico, J. Leverson, R. Humerickhouse, M. Mabry, R. Stone, H. Kantarjian, A. Letai.
Manuscript preparation and final approval: M. Konopleva, D.A. Pollyea, J. Potluri, B. Chyla, L. Hogdal, T. Busman, E. McKeegan, A.H. Salem, M. Zhu, J. Ricker, W. Blum, C.D. DiNardo, T. Kadia, M. Dunbar, R. Kirby, N. Falotico, J. Leverson, R. Humerickhouse, M. Mabry, R. Stone, H. Kantarjian, A. Letai.
Disclosure of Potential Conflicts of Interest
M. Konopleva has been a consultant to and received research funding from AbbVie and Genentech. D.A. Pollyea is an advisory board member for Agios, Ariad, Celgene, Karyopharm, and Pfizer. L. Hogdal has nothing to disclose. W. Blum has been a consultant to Genentech. C. DiNardo has received research funding from Abbvie, Agios, Celgene, CTI, Daiichi-Sankyo, and Novartis. T. Kadia has received research funding from BMS and Celgene, and has been a consultant to Ariad and Novartis. R. Stone has been a consultant to AbbVie, Agios, Amgen, Arrog, Celator, Celgene, Janssen, Karyopharm, Merck, Novartis, Pfizer, Genentech, and Sunesis. H. Kantarjian has received research funding from Ariad, BMS, Novartis, and Pfizer. A. Letai has been an advisor to and his laboratory has received research funds from AbbVie, AstraZeneca, Tetralogic, and XrX. J. Potluri, B. Chyla, T. Busman, E. McKeegan, A.H. Salem, M. Zhu, J.L. Ricker, M. Dunbar, R. Kirby, N. Falotico, J. Leverson, R. Humerickhouse, and M. Mabry are AbbVie employees and may own stock.
Study Oversight and Role of Funding Source
The study protocol was designed by the sponsors (AbbVie and Genentech) in collaboration with the investigators and approved by local institutional review boards. The study was conducted according to the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice. Each patient provided signed informed consent. Study investigators and their research teams collected clinical data; AbbVie confirmed and compiled the data. Authors had access to the data and agreed to submit the manuscript for publication. Manuscript drafts were prepared with assistance from a professional medical writer employed by AbbVie.
References
- 1.Kantarjian H, O’Brien S, Cortes J, Giles F, Faderl S, Jabbour E, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–8. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian H, Ravandi F, O’Brien S, Cortes J, Faderl S, Garcia-Manero G, et al. Intensive chemotherapy does not benefit most older patients (age 70 years or older) with acute myeloid leukemia. Blood. 2010;116:4422–9. doi: 10.1182/blood-2010-03-276485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, et al. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–65. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 5.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–8. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 6.Vogler M, Dinsdale D, Dyer MJ, Cohen GM. ABT-199 selectively inhibits BCL2 but not BCL2L1 and efficiently induces apoptosis of chronic lymphocytic leukaemic cells but not platelets. Br J Haematol. 2013;163:139–42. doi: 10.1111/bjh.12457. [DOI] [PubMed] [Google Scholar]
- 7.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4:362–75. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015;7:279ra40. doi: 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- 9.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12:329–41. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vo TT, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151:344–55. doi: 10.1016/j.cell.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–22. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gross S, Cairns RA, Minden MD, Driggers EM, Bittinger MA, Jang HG, et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J Exp Med. 2010;207:339–44. doi: 10.1084/jem.20092506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mardis ER, Ding L, Dooling DJ, Larson DE, McLellan MD, Chen K, et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N Engl J Med. 2009;361:1058–66. doi: 10.1056/NEJMoa0903840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel JP, Gonen M, Figueroa ME, Fernandez H, Sun Z, Racevskis J, et al. Prognostic relevance of integrated genetic profiling in acute myeloid leukemia. N Engl J Med. 2012;366:1079–89. doi: 10.1056/NEJMoa1112304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Gaizo Moore V, Letai A. BH3 profiling--measuring integrated function of the mitochondrial apoptotic pathway to predict cell fate decisions. Cancer Lett. 2013;332:202–5. doi: 10.1016/j.canlet.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chonghaile TN, Roderick JE, Glenfield C, Ryan J, Sallan SE, Silverman LB, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 2014;4:1074–87. doi: 10.1158/2159-8290.CD-14-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davids MS, Seymour JF, Gerecitano JF, Kahl BS, Pagel JM, Wierda WG, et al. Phase I study of ABT-199 (GDC-0199) in patients with relapsed/refractory non-Hodgkin lymphoma: responses observed in diffuse large B-cell (DLBCL) and follicular lymphoma (FL) at higher cohort doses. Haematologica. 2014;99(suppl 1) Abstract S1348. [PubMed] [Google Scholar]
- 19.Appelbaum FR, Gundacker H, Head DR, Slovak ML, Willman CL, Godwin JE, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinto A, Zagonel V, Ferrara F. Acute myeloid leukemia in the elderly: biology and therapeutic strategies. Crit Rev Oncol Hematol. 2001;39:275–87. doi: 10.1016/s1040-8428(00)00122-0. [DOI] [PubMed] [Google Scholar]
- 21.DiNardo CD, Ravandi F, Agresta S, Konopleva M, Takahashi K, Kadia T, et al. Characteristics, clinical outcome, and prognostic significance of IDH mutations in AML. Am J Hematol. 2015;90:732–6. doi: 10.1002/ajh.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan SM, Majeti R. Role of DNMT3A, TET2, and IDH1/2 mutations in pre-leukemic stem cells in acute myeloid leukemia. Int J Hematol. 2013;98:648–57. doi: 10.1007/s12185-013-1407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corces-Zimmerman MR, Hong WJ, Weissman IL, Medeiros BC, Majeti R. Preleukemic mutations in human acute myeloid leukemia affect epigenetic regulators and persist in remission. Proc Natl Acad Sci U S A. 2014;111:2548–53. doi: 10.1073/pnas.1324297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou WC, Hou HA, Chen CY, Tang JL, Yao M, Tsay W, et al. Distinct clinical and biologic characteristics in adult acute myeloid leukemia bearing the isocitrate dehydrogenase 1 mutation. Blood. 2010;115:2749–54. doi: 10.1182/blood-2009-11-253070. [DOI] [PubMed] [Google Scholar]
- 25.Chou WC, Lei WC, Ko BS, Hou HA, Chen CY, Tang JL, et al. The prognostic impact and stability of Isocitrate dehydrogenase 2 mutation in adult patients with acute myeloid leukemia. Leukemia. 2011;25:246–53. doi: 10.1038/leu.2010.267. [DOI] [PubMed] [Google Scholar]
- 26.Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong WJ, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015;21:178–84. doi: 10.1038/nm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–96. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kipps TJ, Eradat H, Grosicki S, Catalano J, Cosolo W, Dyagil IS, et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56:2826–33. doi: 10.3109/10428194.2015.1030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–9. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merino D, Khaw SL, Glaser SP, Anderson DJ, Belmont LD, Wong C, et al. Bcl-2, Bcl-x(L), and Bcl-w are not equivalent targets of ABT-737 and navitoclax (ABT-263) in lymphoid and leukemic cells. Blood. 2012;119:5807–16. doi: 10.1182/blood-2011-12-400929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishizawa J, Kojima K, McQueen T, Ruvolo V, Chachad D, Nogueras-Gonzalez GM, et al. Mitochondrial profiling of acute myeloid Leukemia in the assessment of response to apoptosis modulating drugs. PLoS One. 2015;10:e0138377. doi: 10.1371/journal.pone.0138377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierceall WE, Kornblau SM, Carlson NE, Huang X, Blake N, Lena R, et al. BH3 profiling discriminates response to cytarabine-based treatment of acute myelogenous leukemia. Mol Cancer Ther. 2013;12:2940–9. doi: 10.1158/1535-7163.MCT-13-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–21. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, Verdonck LF, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–78. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Chevallier P, Labopin M, Turlure P, Prebet T, Pigneux A, Hunault M, et al. A new Leukemia Prognostic Scoring System for refractory/relapsed adult acute myelogeneous leukaemia patients: a GOELAMS study. Leukemia. 2011;25:939–44. doi: 10.1038/leu.2011.25. [DOI] [PubMed] [Google Scholar]
- 36.Thol F, Schlenk RF, Heuser M, Ganser A. How I treat refractory and early relapsed acute myeloid leukemia. Blood. 2015;126:319–27. doi: 10.1182/blood-2014-10-551911. [DOI] [PubMed] [Google Scholar]
- 37.Bogenberger JM, Delman D, Hansen N, Valdez R, Fauble V, Mesa RA, et al. Ex vivo activity of BCL-2 family inhibitors ABT-199 and ABT-737 combined with 5-azacytidine in myeloid malignancies. Leuk Lymphoma. 2015;56:226–9. doi: 10.3109/10428194.2014.910657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsao T, Shi Y, Kornblau S, Lu H, Konoplev S, Antony A, et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol. 2012;91:1861–70. doi: 10.1007/s00277-012-1537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiNardo C, Pollyea D, Pratz K, Thirman MJ, Letai A, Frattini M, et al. A phase 1b study of venetoclax (ABT-199/GDC-0199) in combination with decitabine or azacitidine in treatment-naive patients with acute myelogenous leukemia who are ≥ to 65 years and not eligible for standard induction therapy. Blood. 2015;126:327. [Google Scholar]
- 40.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.