Introduction
The complex entity termed as Mineral and Bone Disorder (MBD) may contribute to the development of vascular calcification and adverse clinical outcomes among patients with kidney failure1. By the time of dialysis start, most patients have hyperplasia of the parathyroid glands2 and markedly elevated parathyroid hormone (PTH) levels3; these tend to rise further with longer duration of kidney replacement therapy4.5. Findings of most observational studies have described associations between high PTH levels and increased mortality in this population6–14, although this association was not found in a recent meta-analysis15. Reflecting the lack of strong evidence and the heterogeneity of PTH assays, recent clinical practice guidelines published in 2009 and 201016,17 suggested a PTH target level that was more liberal than what had previously been recommended18, citing 2 to 9 times the upper limit of normal for the assay used rather than 150 to 300 pg/ml. Furthermore, in the United States (US), MBD practices implemented in response to the 2011 Prospective Payment System (PPS) may have contributed to changes in PTH levels. Based on updated data from the US Dialysis Outcomes and Practice Patterns Study (DOPPS), we report trends in PTH levels and in secondary hyperparathyroidism (SHPT) therapies over the past 4 years, including differences by race.
The DOPPS Practice Monitor (DPM) was launched in 2010 to report trends in dialysis care after PPS implementation.19 The DPM is updated quarterly with the data lagged 2–4 months. DPM data here include an average of 12,334 hemodialysis patients per month in 168 facilities over a four year period from August 2010-August 2014. Sampling design for the DPM has previously been published20,21. We report on levels of PTH and MBD-related treatments including oral and intravenous vitamin D agents (paricalcitol, doxercalciferol, calcitriol) and cinacalcet. Race was based on the patient’s medical record. Data regarding facility PTH targets were answered by an average of 71 facilities in the DOPPS medical director’s survey from 2010–2014. A separate analysis using the patient sample and methods described in Tentori et al.14 assessed whether the association of PTH with mortality differed by race.
Results
Trends in PTH levels and association with mortality
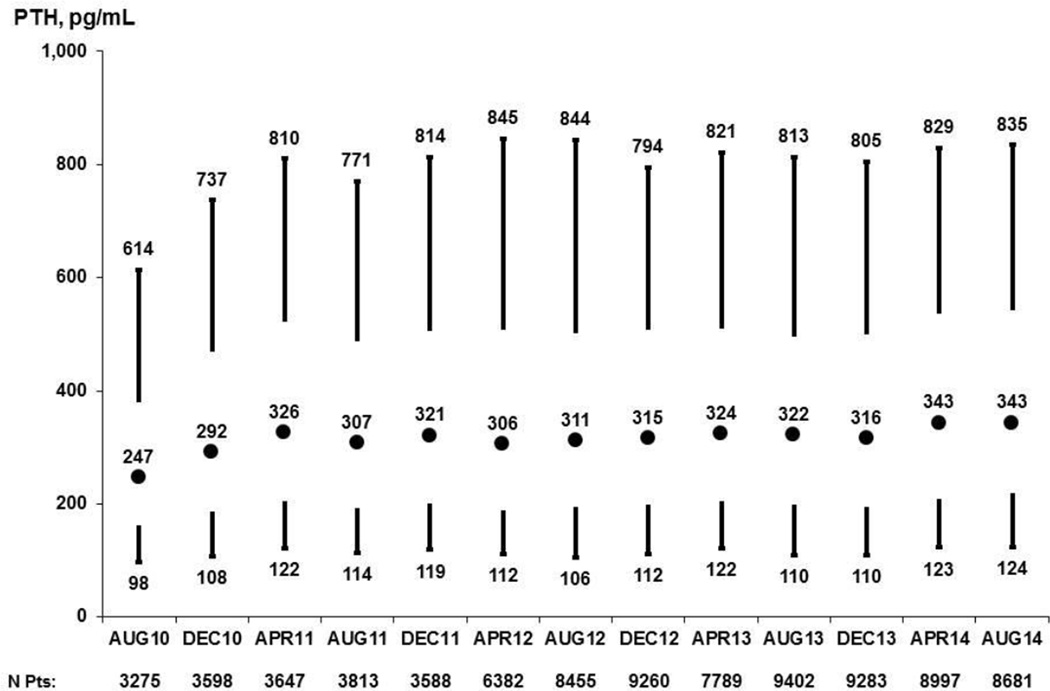
Among DOPPS participants in the United States14, PTH levels increased from 2010 to 2014 (Figure 1). The largest increase in PTH was observed between August 2010 and April 2011, when the median PTH increased by 32% (from 247 to 326 pg/ml) and the 90th percentile also increased by 32% (from 614 to 810 pg/ml). Between April 2011 and August 2014, however, PTH levels remained fairly constant with the median PTH levels increasing 5% (from 326 to 343 pg/ml).
Figure 1.
Patient PTH levels in the prior 3 months, 2010–2014. Values at each month are based on the most recent measurement obtained within the prior 3 months; vertical lines extend from 10th to 25th (lower) and 75th to 90th (upper) percentiles; circle represents median.
Adapted with permission of Arbor Research Collaborative for Health from the December 2014 update to the DOPPS Practice Monitor.
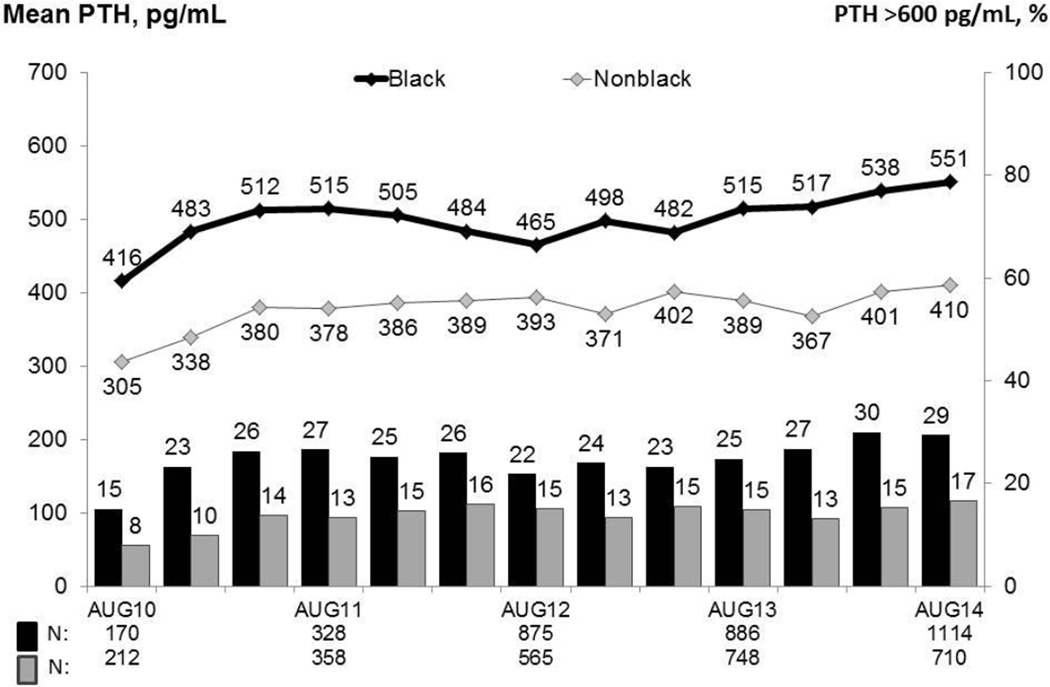
The rise in PTH levels over the past 4 years was similar between blacks and nonblacks (p = 0.26), leading to a higher percentage of patients with very high PTH levels (> 600 pg/ml) in both groups (Figure 2). However, because black patients had higher PTH levels at the DPM baseline (416 vs. 305 pg/ml), the percentage of patients with PTH > 600 pg/ml in August 2014 was much higher among blacks vs. nonblacks (29% vs. 17%). In an analysis including US patients in DOPPS 1 – 4 (1996 – 2011)14, PTH values above 600 pg/ml were associated with increased mortality (HR=1.17 (1.05 1.32), p=0.007 with no effect modification identified by race.
Figure 2.
Mean PTH levels and the percentage of patients with PTH ≥ 600 pg/ml, by race, 2010–2014. Values at each month are based on the most recent measurement obtained within the prior 3 months; vertical bars represent the percent of patients with PTH > 600 pg/mL; horizontal lines represent the mean PTH.
Adapted with permission of Arbor Research Collaborative for Health from the December 2014 update to the DOPPS Practice Monitor
Prescription of secondary hyperparathyroidism (SHPT)-related treatments
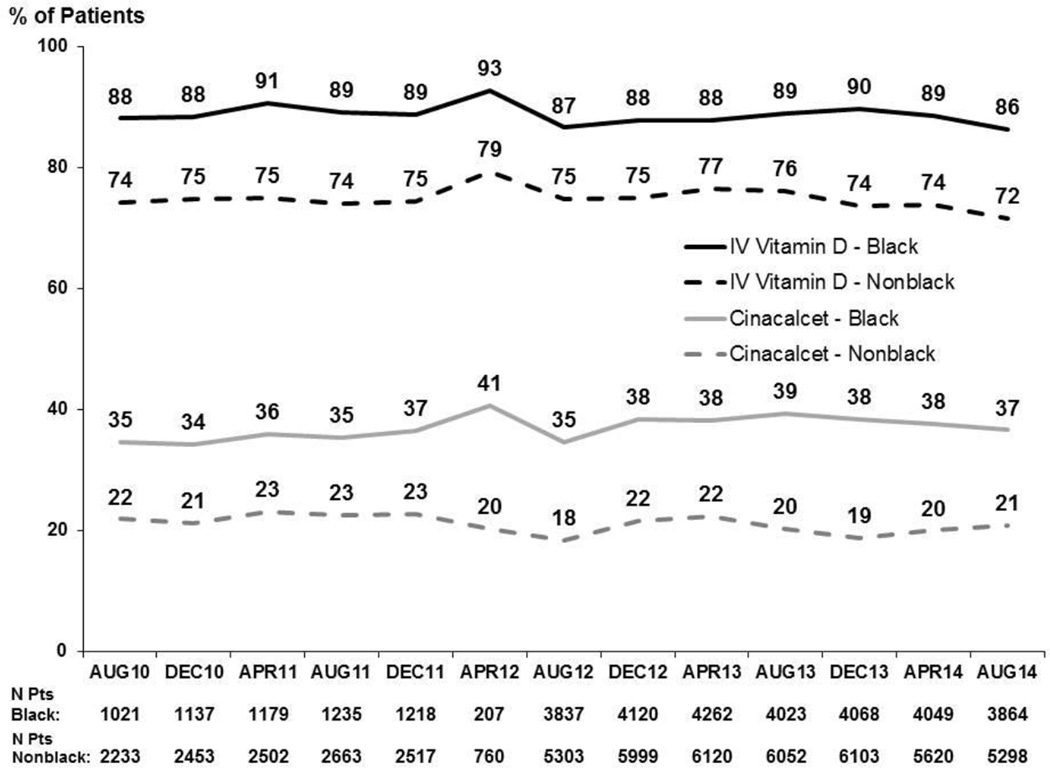
Overall, there was little change during the study period in the percentage of patients prescribed SHPT-related treatments, including IV vitamin D (from 79% in August 2010 to 76% in August 2014), oral vitamin D receptor agonists (~6–7% over the four year period) and cinacalcet (24–28%). A higher percentage of black patients were prescribed IV vitamin D and cinacalcet compared to nonblack patients. However, the trend in the prescription of MBD related therapies from August 2010 to August 2014 has remained stable for both black and nonblack patients (Figure 3). IV vitamin D dose by product type has not changed over the 4 year period (data not shown).
Figure 3.
Prescription of MBD related therapies in the prior 3 months, by race, 2010–2014. Values for each month reflect any prescription during prior three months. Prescription of oral vitamin D (not shown) showed a similar trend in both Black and Nonblack patients.
Adapted with permission of Arbor Research Collaborative for Health from the December 2014 update to the DOPPS Practice Monitor
Facility-level PTH targets
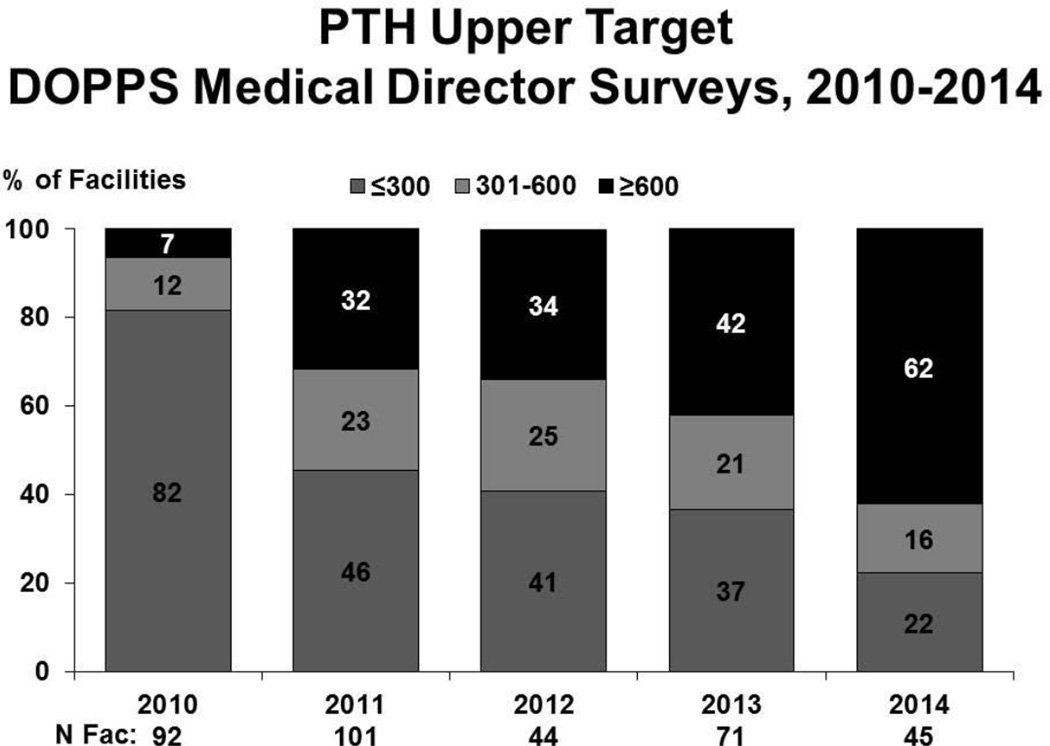
From 2010 to 2014, medical directors at US DOPPS dialysis units reported targeting higher PTH levels14. The facility upper PTH target has shifted with more facilities indicating an upper target ≥600 pg/ml. The percentage of facilities with an upper PTH target of ≤300 pg/ml has sharply declined from 82% in 2010 to 22% in 2014, while the percentage of facilities indicating a PTH upper target of ≥600 pg/ml increased from 7% in 2010 to 62% in 2014 (Figure 4).
Figure 4.
PTH Upper Target DOPPS Medical Director Surveys, 2010–2014. Facility reported upper targets of PTH, 2010–2014.
Summary
In the US DPM sample, median PTH levels increased 32%, from August 2010 through April 2011 and have remained relatively stable since then. Black patients carry a disproportionate burden of PTH levels above 600 pg/ml (29%; vs. 17% among nonblacks). This finding warrants particular attention, since such high PTH levels have consistently been associated with adverse outcomes6–14 and the association between severe hyperparathyroidism and mortality appeared consistent in both racial groups. While we had postulated that the PPS may have led to lower utilization of MBD-related intravenous drugs, vitamin D prescription remained stable over time. Rather, the increase in PTH levels seem to have been driven mainly by adoption of more liberal PTH targets, as reported in the medical director survey. However, PTH levels >600 are above any currently recommended guidelines and likely represent a modifiable risk factor for adverse outcomes. Clinicians should take this into account especially when treating black patients, since nearly a third of them presented such high levels in the US DOPPS sample.
Acknowledgment
Support: The Dialysis Outcomes and Practice Patterns Study Program is supported by Amgen, Kyowa Hakko Kirin, AbbVie Inc., Sanofi Renal, Baxter Healthcare, and Vifor Fresenius Medical Care Renal Pharma, Ltd. Additional support for specific projects and countries is also provided by Amgen, BHC Medical, Janssen, Takeda, and Kidney Foundation of Canada (for logistics support) in Canada; Hexal, Deutsche Gesellschaft fur Nephrologie (DGfN), Shire, and WiNe Institute in Germany; and the Japanese Society for Peritoneal Dialysis for Peritoneal Dialysis Outcomes and Practice Patterns Study in Japan. F.T. is supported, in part, by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Award K01DK087762. All support is provided without restrictions on publications.
F.T. has received honoraria from Amgen, Dialysis Clinic Inc., and Renal Research Institute. R.L.P. has received speaker fees from Amgen, Kyowa Hakko Kirin, and Vifor; served as a consultant for Pursuit Vascular; and served on an advisory panel for Merck. B.M.R. has received speaker fees for Kyowa Hakko Kirin.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK or the National Institutes of Health.
Financial Disclosure: The other authors declare that they have no other relevant financial interests.
References
- 1.Goodman WG, Goldin J, Kuizon BD, et al. Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–1483. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 2.de Francisco AM, Ellis HA, Owen JP, Cassidy MJ, Farndon JR, Ward MK, Kerr DN. Parathyroidectomy in chronic renal failure. Q J Med. 1985;55:289–315. [PubMed] [Google Scholar]
- 3.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Plone M, Dillon MA, Burke SK, Slatopolsky E. Hyperparathyroidism and dialysis vintage. Clin Nephrol. 2000;54:295–300. [PubMed] [Google Scholar]
- 5.Malberti F, Marcelli D, Conte F, Limido A, Spotti D, Locatelli F. Parathyroidectomy in patients on renal replacement therapy: an epidemiologic study. J Am Soc Nephrol. 2001;12:1242–1248. doi: 10.1681/ASN.V1261242. [DOI] [PubMed] [Google Scholar]
- 6.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 7.Slinin YFR, Collins AJ. Calcium, phosphorus, parathyroid hormone, and cardiovascular disease in hemodialysis patients: the USRDS waves 1, 3, and 4 study. J Am Soc Nephrol. 2005;16:1788–1793. doi: 10.1681/ASN.2004040275. [DOI] [PubMed] [Google Scholar]
- 8.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 67:1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 9.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70:771–780. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 10.Kimata N, Albert JM, Akiba T, Yamazaki S, Kawaguchi T, Fukuhara S, Akizawa T, Saito A, Asano Y, Kurokawa K, Pisoni RL, Port FK. Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: the Japan dialysis outcomes and practice patterns study. Hemodial Int. 2007;11:340–348. doi: 10.1111/j.1542-4758.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- 11.Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, Young EW, Akizawa T, Akiba T, Pisoni RL, Robinson BM, Port FK. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2008;52:519–530. doi: 10.1053/j.ajkd.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Lacson E, Jr, Wang W, Hakim RM, Teng M, Lazarus JM. Associates of mortality and hospitalization in hemodialysis: potentially actionable laboratory variables and vascular access. Am J Kidney Dis. 2009;53:79–90. doi: 10.1053/j.ajkd.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 13.Floege JKJ, Ireland E, Chazot C, Drueke T, de Francisco A, Kronenberg F, Marcelli D, Passlick-Deetjen J, Schernthaner G, Fouqueray B, Wheeler DC ARO Investigators. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26:1948–1955. doi: 10.1093/ndt/gfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tentori F, Wang M, Bieber BA, Karaboyas A, Li Y, Jacobson SH, Andreucci VE, Fukagawa M, Frimat L, Mendelssohn DC, Port FK, Pisoni RL, Robinson BM. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol. 2015;10(1):98–109. doi: 10.2215/CJN.12941213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, Strippoli GF. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305:1119–1127. doi: 10.1001/jama.2011.308. [DOI] [PubMed] [Google Scholar]
- 16.Kidney Disease: Improving Global Outcomes CKD MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009:S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 17.Uhlig K, Berns JS, Kestenbaum B, Kumar R, Leonard MB, Martin KJ, Sprague SM, Goldfarb S. KDOQI US commentary on the 2009 KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of CKD-Mineral and Bone Disorder (CKD-MBD) Am J Kidney Dis. 2010;55(5):773–799. doi: 10.1053/j.ajkd.2010.02.340. [DOI] [PubMed] [Google Scholar]
- 18.K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:S1–S201. [PubMed] [Google Scholar]
- 19.Tentori F, Fuller D, Bieber B, et al. The DOPPS Practice Monitor for US Dialysis Care: Potential Impact of Recent Guidelines and Regulatory Changes on Management of Mineral and Bone Disorder Among US Hemodialysis Patients. Am J Kidney Dis. 2014;63(5):851–854. doi: 10.1053/j.ajkd.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): An international hemodialysis study. Kidney Int. 2000;57(suppl 74):S-74–S-81. [Google Scholar]
- 21.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner M, Wolfe RA. The Dialysis Outcomes and Practice Patterns Study: Design, data elements, and methodology. Am J Kidney Dis. 2004;44(Suppl 2):S7–S15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]