Abstract
Troxerutin is a bioflavonoid, which can be used to treat venous disorders, thrombosis and cerebrovascular diseases. Recent studies have demonstrated that it may also be used to prevent edemas. However, it is not known whether troxerutin protects against the cardiomyopathic complications of diabetes. In the present study, a rat model of type 2 diabetes was used to investigate the potential for troxerutin to protect against diabetic cardiomyopathy, through changes to nuclear factor-κB (NF-κB) expression. Troxerutin administration significantly reduced heart rate, blood pressure, blood glucose and plasma triglyceride levels across all measured time points. Furthermore, troxerutin significantly reduced reactive oxygen species levels, NF-κB protein expression, and suppressed the phosphorylated forms of AKT, insulin receptor substrate 1 (IRS1) and c-Jun N-terminal kinase (JNK). These results suggested that troxerutin protects against cardiomyopathy via alterations in NF-κB, AKT and IRS1 signaling, in a rat model of type 2 diabetes.
Keywords: troxerutin, cardiomyopathy, type 2 diabetes, nuclear factor-κB, AKT, insulin receptor substrate 1
Introduction
The morbidity of diabetes has increased steadily in recent years. Previous epidemiological data has indicated that the total number of patients with diabetes may surpass 400 million by 2030 (1). Diabetes, which has become the fifth major cause of worldwide mortality after infectious disease, cardiovascular disease, cancer and trauma, is a progressive disease that is characterized by hyperglycemia (2), and may be classified into types 1 and 2 (2). The development of type 2 diabetes is associated with a high fat diet, sedentary lifestyle and obesity. Type 2 diabetes, which is caused by insulin resistance, accounts for ~90% of adult diabetes (3) and ~5.2% of cases of global mortality (4).
Diabetes mellitus (DM) can cause capillary and vessel lesions, resulting in diabetic retinopathy, diabetic nephropathy, cerebrovascular diseases and diabetic cardiomyopathy (DCM) (5). Besides vasculopathy, myocardial cell injuries may also occur, and these contribute to the development of DCM (6). Notably, DCM can develop independently of coronary artery disease, hypertension and valvular disease (7). The major features of DCM include oxidative stress, cardiomyocyte hypertrophy and apoptosis, myocardial interstitial fibrosis and decreasing heart function (8).
Troxerutin is a natural flavonoid drug, which has demonstrated an ability to: Inhibit the agglomeration of blood platelets and red blood cells; prevent thrombogenesis; protect endothelial cells; and enhance microcirculation (9). Previous research has demonstrated that troxerutin, which is anti-inflammatory and anti-apoptotic, can also improve cognition and resist oxidation (10). It has been reported that troxerutin can effectively cure venous diseases (11). Due to the ability of troxerutin to inhibit platelet aggregation and prevent thrombogenesis, it has also been reported that troxerutin can be used to treat sequelae of apoplexy (12). The present study investigated whether troxerutin can protect against DCM, in a rat model of type 2 diabetes.
Materials and methods
Animals, materials, experimental design and induction of diabetes
Adult male Wistar rats weighing 250–300 g (n=30) were purchased form the Animal Experiment Center of Shandong University (Jinan, China) and used in the present study. The rats had ad libitum access to food and water, and were kept at 22±1°C and 55±5% relative humidity. The study protocol, including the procedures for animal handling and husbandry, was reviewed and approved by the Animal Care and Use Committee of Linzi District People's Hospital (Zibo, China). After acclimation to their environment for 7 days, all Wistar rats were randomly allocated into three groups (n=10/group): Vehicle, DM and troxerutin. Animals in the vehicle and DM groups were given physiological saline; animals in the troxerutin group were given 150 mg/kg of troxerutin (Sigma-Aldrich; Merck Merck, Darmstadt, Germany) once daily for 4 weeks. The DM model was induced in the DM and troxerutin groups by intraperitoneal injection of streptozotocin (STZ; 50 mg/kg, pH 4.5; Sigma-Aldrich; Merck KGaA). The chemical structure of troxerutin is shown in Fig. 1.
Figure 1.

Chemical structure of troxerutin.
Body weight and biochemical analysis
Blood glucose levels were monitored in all rats using a glucometer, and a diagnosis of DM was made when these rose >300 mg/dl. Following the completion of troxerutin treatment, heart rate and blood pressure were measured. All rats were sacrificed by decapitation following anesthesia with 0.3% pentobarbital sodium (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China). Body weight was recorded, and heart samples were immediately acquired and weighed. Venous blood was acquired and centrifuged at 1,000 × g for 10 min at 4°C to collect the serum. Plasma triglyceride levels were measured in the collected serum using a Triglycerides assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and measuring absorbance on a multimode microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) at a wavelength of 450 nm.
Reactive oxygen species (ROS) assay
Serum was incubated with 20 µM 2′,7′-dichlorofluorescin (DCF) diacetate (Beyotime Institute of Biotechnology, Haimen, China) for 20 min at 37°C in the dark. Subsequently, miscible liquids were lysed with radioimmunoprecipitation assay lysis buffer (Beyotime Institute of Biotechnology) and carboxy-DCF fluorescence was detected. Reactive oxygen species (ROS) levels were measured using a multimode microplate reader (Bio-Rad Laboratories, Inc.) at 488 nm (excitation) and at 530 nm (emission).
Western blot analysis
Heart samples were immediately acquired and homogenized in 1:3 (w/v) ice-cold radioimmunoprecipitation assay lysis buffer containing a protease inhibitor mixture. Protein concentrations were determined using a Bicinchoninic Acid Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc., Waltham, MA, USA). Protein samples (50 µg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a 10–12% (w/v) gel, and transferred to a polyvinylidene difluoride membrane (Roche Diagnostics Corporation, Indianapolis, IN, USA) by electrophoretic transfer. The membranes were incubated overnight at 4°C with primary antibodies: Anti-NF-κB (catalog no. 8242; 1:2,000; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-phosphorylated (p)-AKT (catalog no. 13038; 1:4,000; Cell Signaling Technology, Inc.), anti-p-insulin receptor substrate 1 (p-IRS1; catalog no. 2381; 1:3,000; Cell Signaling Technology, Inc.), anti-p-c-Jun N-terminal kinase 1 (p-JNK1; catalog no. 4668; 1:2,000; Cell Signaling Technology, Inc.) and anti-β-actin (1:3,000; catalog no. sc-7210; Santa Cruz Biotechnology, Inc., Dallas, TX, USA). Membranes were incubated with a mouse anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (catalog no. sc-2357; Santa Cruz Biotechnology, Inc.), and protein bands were visualized with an Enhanced Chemiluminescence Prime kit (GE Healthcare Lifesciences, Ltd., Shanghai, China). Blots were analyzed using Scion Image Analysis software version 4.03 (Scion Corp., Frederick, MD, USA).
Statistical analysis
Data are presented as the mean ± standard error of three replicates. Differences in biochemical parameters between the groups were analyzed using one-way analysis of variance (ANOVA), followed by Tukey's test. P<0.05 was considered to indicate a statistically significant difference.
Results
Troxerutin reduces blood glucose levels in a rat model of type 2 diabetes
To estimate the ability of troxerutin to reduce blood glucose levels, glucose measurements were performed at weekly intervals. The blood glucose levels of the DM rats were consistently higher than those of the vehicle-treated control group (Fig. 2). In the troxerutin-treated DM model group, glucose levels were similar to those of the vehicle-treated DM group in week 1, however these levels reduced steadily between weeks 2 and 4 (Fig. 2).
Figure 2.
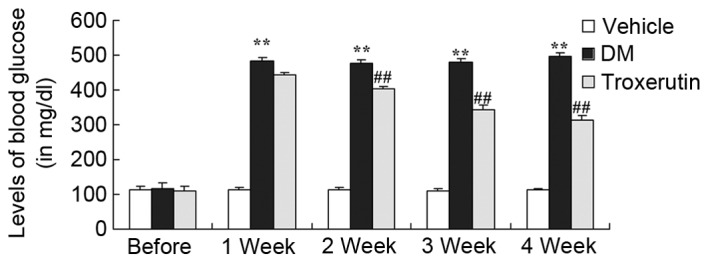
Troxerutin suppresses DM-induced increases in blood glucose. **P<0.01 compared with vehicle group; ##P<0.01 compared with DM group. Vehicle, vehicle control group; DM, diabetes mellitus model group; Troxerutin, troxerutin-treated group.
Troxerutin does not affect the heart/body weight ratio
The effects of troxerutin on the heart/body weight ratio were evaluated (Fig 3). The results demonstrated that there was no significant difference between the vehicle control, DM and troxerutin groups (P>0.05).
Figure 3.
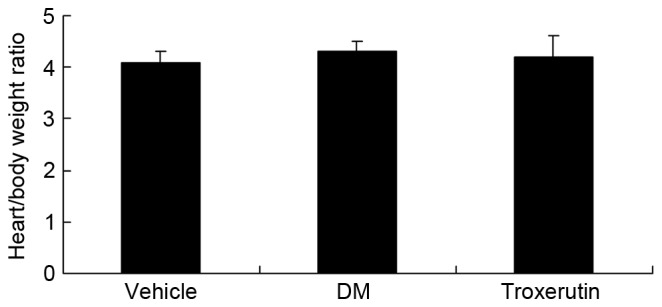
Troxerutin has no effect on heart/body weight ratio in a rat model of type 2 diabetes. Vehicle, vehicle control group; DM, diabetes mellitus model group; Troxerutin, troxerutin-treated group.
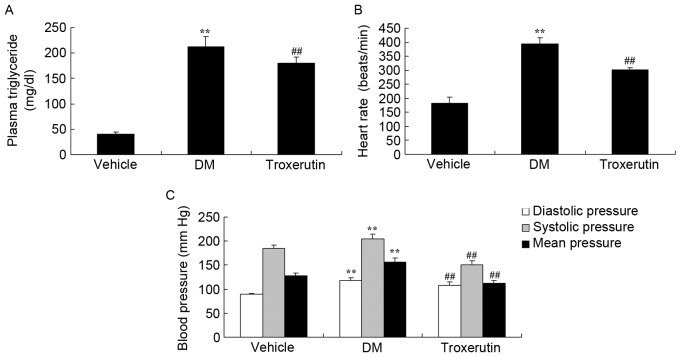
Troxerutin reduces heart rate, blood pressure and plasma triglyceride levels in a rat model of type 2 diabetes
Basal heart rate, blood pressure and plasma triglyceride levels were all significantly increased in the DM rats, compared with the controls. However, levels of these clinical features were all lowered in the DM group treated with troxerutin, compared with the vehicle-treated DM group (Fig. 4).
Figure 4.
Troxerutin reduces heart rate, blood pressure and plasma triglyceride levels. Troxerutin reduces (A) plasma triglyceride levels, (B) heart rate, and (C) blood pressure in a rat model of type 2 diabetes. **P<0.01 compared with vehicle group; ##P<0.01 compared with DM group. Vehicle, vehicle control group; DM, diabetes mellitus model group; Troxerutin, troxerutin-treated group.
Troxerutin reduces levels of ROS in a rat model of type 2 diabetes
The DM model rats exhibited a significant increase in ROS levels, compared with the vehicle control group; however, this increase was lower in the rats treated with troxerutin (Fig. 5).
Figure 5.
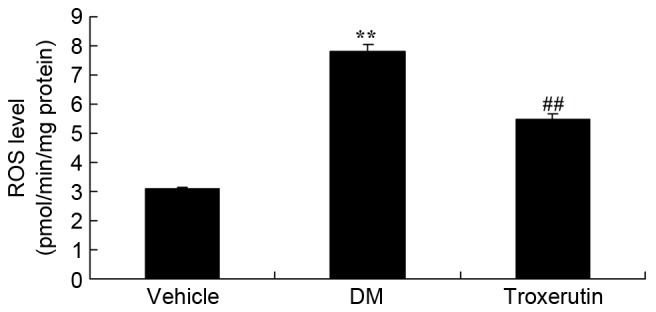
Troxerutin suppresses changes in ROS levels in a rat model of type 2 diabetes. **P<0.01 compared with vehicle group; ##P<0.01 compared with DM group. Vehicle, vehicle control group; DM, diabetes mellitus model group; Troxerutin, troxerutin-treated group; ROS, reactive oxygen species.
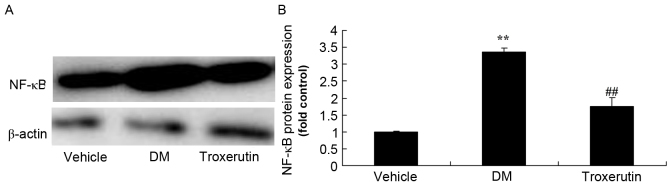
Troxerutin reduces NF-κB protein expression in a rat model of type 2 diabetes
Diabetes induction with STZ resulted in a significant increase in NF-κB protein expression, compared with the control group. However, treatment with troxerutin significantly reduced the NF-κB protein expression, compared with the vehicle-treated DM group (Fig. 6).
Figure 6.
Troxerutin suppresses changes in NF-κB protein expression in a rat model of type 2 diabetes. (A) NF-κB protein expression was determined by western blot analysis. (B) Statistical analysis of NF-κB protein expression. **P<0.01 compared with vehicle group; ##P<0.01 compared with DM group. Vehicle, vehicle control group; DM, diabetes mellitus model group; Troxerutin, troxerutin-treated group; NF-κB, nuclear factor-κB.
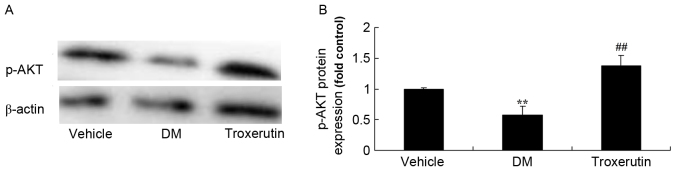
Troxerutin reverses DM-induced changes to p-AKT protein expression
Western blot analysis demonstrated that p-AKT expression in the DM rats was lower than that of the vehicle control group (Fig. 7). Troxerutin treatment resulted in a significant increase in p-AKT expression, compared with the vehicle-treated DM group (Fig. 7).
Figure 7.
Troxerutin suppresses changes in p-AKT protein expression in a rat model of type 2 diabetes. (A) p-AKT protein expression was determined by western blot analysis. (B) Statistical analysis of p-AKT protein expression, in a rat model of type 2 diabetes. **P<0.01 compared with vehicle group; ##P<0.01 compared with DM group. Vehicle, vehicle control group; DM, diabetes mellitus model group; Troxerutin, troxerutin treated group; p, phosphorylated.
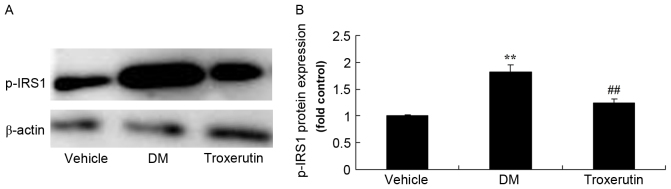
Troxerutin reduces DM-induced changes in p-IRS1 protein expression
The DM rats exhibited higher protein expression levels of p-IRS1 compared with the vehicle control group. However, troxerutin treatment reduced p-IRS1 protein expression, compared with the vehicle-treated DM group (Fig. 8).
Figure 8.
Troxerutin suppresses changes in p-IRS1 protein expression in a rat model of type 2 diabetes. (A) p-IRS1 protein expression was determined by western blot analysis. (B) Statistical analysis of p-IRS1 protein expression. **P<0.01 compared with vehicle group; ##P<0.01 compared with DM group. Vehicle, vehicle control group; DM, diabetes mellitus model group; Troxerutin, troxerutin-treated group; p-IRS1, phosphorylated insulin receptor substrate 1.
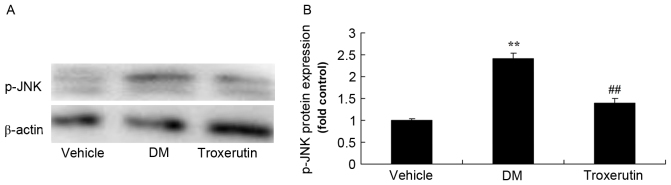
Troxerutin reduces p-JNK1 protein expression in a rat model of type 2 diabetes
DM-induced rats exhibited higher levels of p-JNK1, compared with controls (Fig. 9), however troxerutin treatment significantly suppressed p-JNK1 protein expression, compared with the untreated DM rats.
Figure 9.
Troxerutin suppresses changes in p-JNK protein expression in a rat model of type 2 diabetes. (A) p-JNK protein expression was determined by western blot analysis. (B) Statistical analysis of p-JNK protein expression. **P<0.01 compared with vehicle group; ##P<0.01 compared with DM group. Vehicle, vehicle control group; DM, diabetes mellitus model group; Troxerutin, troxerutin-treated group; p-JNK, phosphorylated c-Jun N-terminal kinase.
Discussion
Due to the improvement of living standards, lifestyle changes, unfavorable dietary habits and various harmful factors, the number of patients with diabetes is increasing annually (13). DCM is a major complication of DM, and it contributes to the mortality of diabetes due to its ability to result in cardiac failure. Pre-existing cardiovascular risk factors, such as coronary artery disease and hypertension, are not necessary for the development of DCM; pathogenesis can occur independently of these factors (14). The pathogenic mechanisms of DCM are relatively complex; they include insulin resistance and cardiomyocyte hypertrophy, an increased apoptotic rate of cardiomyocytes, myocardial fibrosis and remodeling of myofibrils, cardiovascular autonomic neuropathy and calcium ion overloading in myocardial cells (15). Currently, there are no specific therapeutic interventions for the treatment of DCM, and therapy generally focuses on improving cardiac function. Attention should therefore focus on the identification of drugs and targets to treat DCM (15). The present study investigated the effects of troxerutin treatment, and demonstrated that it significantly inhibited DM-induced increases in blood glucose, heart rate, blood pressure and plasma triglyceride in rats.
NF-κB serves an essential role in signal transmission and in the induction of gene expression (16). It regulates the expression of cytokines, chemokines, growth factors, cell adhesion molecules and some acute phase proteins (17). These factors have a key role in the occurrence, pathogenesis and progression of myocardial lesions. A previous study demonstrated that a key step of NF-κB activation is the phosphorylation of IκB, which is catalyzed by IκB kinase (IKK) (18). According to the findings of the present study, treatment with troxerutin effectively inhibited the DM-induced levels of ROS and NF-κB protein expression in a rat model of type 2 diabetes. Lu et al (10) reported that troxerutin inhibits domoic acid-induced memory deficits via IKKβ/NF-κB signaling suggesting that troxerutin may regulate NF-κB signaling in DM.
AKT kinase has an important role in the protection of pancreatic β cells, and therefore enhancing its secretion in the early stages of diabetes may reduce damage to these cells (19). Another important organ in glucose metabolism is the liver, where activated AKT may transform glucose into a different energy reserve during the early and mid stages of diabetes pathogenesis (20). However, complications caused by diabetes, metabolic syndrome or obesity can reduce the activity of AKT (21), thereby limiting the AKT-induced utilization of glycogen. The present study demonstrated that troxerutin treatment reversed the STZ-induced inhibition of p-AKT protein expression in DM rats. Lu et al (22) observed that troxerutin also attenuated cognitive impairment and oxidative stress through the activation of AKT and the extracellular signal-regulated kinase 1/2 signaling pathway.
Activated JNK1 not only serves a role in the mitogen-activated protein kinase signal transduction pathway, but also phosphorylates IRS1, and JNK1 therefore potentially regulates this protein (23). IRS1, which is stimulated by insulin or insulin-like growth factor-1, then activates phosphoinositide 3-kinase, which in turn activates the AKT pathway, and cardiac hypertrophy and cardiac failure subsequently occur (24,25). The present study demonstrated that troxerutin treatment significantly inhibited p-IRS1 protein expression. Sampath and Karundevi (26) demonstrated that troxerutin improved insulin signaling via reduction in phosphorylation of IRS1, and thus glucose utilization, in the skeletal muscle of type 2 diabetic adult male rats.
Also known as stress-activated protein kinase, the JNKs serve an important role in the stress response and can be activated by several proapoptotic stimuli, including ultraviolet radiation, osmotic pressure changes, inflammatory factors and withdrawal of growth factors (27). The JNK signaling pathway is associated with insulin resistance, type 2 diabetes and obesity (28), and its activity is increased in patients with type 2 diabetes, obesity in animal models and diabetic animal models (24). It has been suggested that the activity of JNK can lead to insulin resistance by blocking insulin synthesis, as JNK is a serine/threonine kinase, JNK may increase the serine phosphorylation of IRS-1, thereby interfering with the normal tyrosine phosphorylation of this protein, and subsequently affecting insulin signal transduction (23). The present study indicated that treatment with troxerutin significantly suppressed p-JNK protein expression in type 2 diabetic rats. Furthermore, Zhang et al (12) reported that troxerutin inhibits 2,2′, 4,4′-tetrabromodiphenyl ether-induced hepatocyte apoptosis via suppression of tumor necrosis factor receptor-associated factor 2/apoptosis signal-regulating kinase 1/JNK signaling.
In conclusion, troxerutin appears to protect against DCM through inhibition of NF-κB and ROS, and activation of the AKT/IRS/JNK signaling pathway, in a rat model of type 2 diabetes.
References
- 1.Fukuda T, Fukui M, Tanaka M, Senmaru T, Iwase H, Yamazaki M, Aoi W, Inui T, Nakamura N, Marunaka Y. Effect of Brazilian green propolis in patients with type 2 diabetes: A double-blind randomized placebo-controlled study. Biomed Rep. 2015;3:355–360. doi: 10.3892/br.2015.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han L, Tang L, Wang C, Chen Z, Zhang T, Chen S, Liu S, Peng X, Mai Y, Duan S. Fat mass and obesity-associated gene rs11642015 polymorphism is significantly associated with prediabetes and type 2 diabetes subsequent to adjustment for body mass index. Biomed Rep. 2014;2:681–686. doi: 10.3892/br.2014.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staimez LR, Weber MB, Narayan KM, Oza-Frank R. A systematic review of overweight, obesity, and type 2 diabetes among Asian American subgroups. Curr Diabetes Rev. 2013;9:312–331. doi: 10.2174/15733998113099990061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yamada Y, Matsui K, Takeuchi I, Oguri M, Fujimaki T. Association of genetic variants of the α-kinase 1 gene with type 2 diabetes mellitus in a longitudinal population-based genetic epidemiological study. Biomed Rep. 2015;3:347–354. doi: 10.3892/br.2015.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knabl J, Hüttenbrenner R, Hutter S, Günthner-Biller M, Riedel C, Hiden U, Kainer F, Desoye G, Jeschke U. Gestational diabetes mellitus upregulates vitamin D receptor in extravillous trophoblasts and fetoplacental endothelial cells. Reprod Sci. 2015;22:358–366. doi: 10.1177/1933719114542020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicolucci A, Cercone S, Chiriatti A, Muscas F, Gensini G. A Randomized trial on home telemonitoring for the management of metabolic and cardiovascular risk in patients with type 2 diabetes. Diabetes Technol Ther. 2015;17:563–570. doi: 10.1089/dia.2014.0355. [DOI] [PubMed] [Google Scholar]
- 7.Gu Y, Warren J, Walker N, Kennelly J. Gender differences in cardiovascular disease risk management for Pacific Islanders in primary care. Qual Prim Care. 2013;21:275–285. [PubMed] [Google Scholar]
- 8.Saisho Y. Glycemic variability and oxidative stress: A link between diabetes and cardiovascular disease? Int J Mol Sci. 2014;15:18381–18406. doi: 10.3390/ijms151018381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geetha R, Radika MK, Priyadarshini E, Bhavani K, Anuradha CV. Troxerutin reverses fibrotic changes in the myocardium of high-fat high-fructose diet-fed mice. Mol Cell Biochem. 2015;407:263–279. doi: 10.1007/s11010-015-2474-3. [DOI] [PubMed] [Google Scholar]
- 10.Lu J, Wu DM, Zheng YL, Hu B, Cheng W, Zhang ZF, Li MQ. Troxerutin counteracts domoic acid-induced memory deficits in mice by inhibiting CCAAT/enhancer binding protein β-mediated inflammatory response and oxidative stress. J Immunol. 2013;190:3466–3479. doi: 10.4049/jimmunol.1202862. [DOI] [PubMed] [Google Scholar]
- 11.Schmeck-Lindenau HJ, Naser-Hijazi B, Becker EW, Henneicke-von Zepelin HH, Schnitker J. Safety aspects of a coumarin-troxerutin combination regarding liver function in a double-blind placebo-controlled study. Int J Clin Pharmacol Ther. 2003;41:193–199. doi: 10.5414/CPP41193. [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZF, Shan Q, Zhuang J, Zhang YQ, Wang X, Fan SH, Lu J, Wu DM, Hu B, Zheng YL. Troxerutin inhibits 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47)-induced hepatocyte apoptosis by restoring proteasome function. Toxicol Lett. 2015;233:246–257. doi: 10.1016/j.toxlet.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Yu X, Zhang Q, Cui W, Zeng Z, Yang W, Zhang C, Zhao H, Gao W, Wang X, Luo D. Low molecular weight fucoidan alleviates cardiac dysfunction in diabetic Goto-Kakizaki rats by reducing oxidative stress and cardiomyocyte apoptosis. J Diabetes Res. 2014;2014:420929. doi: 10.1155/2014/420929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel BM, Raghunathan S, Porwal U. Cardioprotective effects of magnesium valproate in type 2 diabetes mellitus. Eur J Pharmacol. 2014;728:128–134. doi: 10.1016/j.ejphar.2014.01.063. [DOI] [PubMed] [Google Scholar]
- 15.Stolen TO, Høydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, Larsen T, Rolim N, Condorelli G, Smith GL, Wisløff U. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res. 2009;105:527–536. doi: 10.1161/CIRCRESAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- 16.Tan HW, Xing SS, Bi XP, Li L, Gong HP, Zhong M, Zhang Y, Zhang W. Felodipine attenuates vascular inflammation in a fructose-induced rat model of metabolic syndrome via the inhibition of NF-kappaB activation. Acta Pharmacol Sin. 2008;29:1051–1059. doi: 10.1111/j.1745-7254.2008.00843.x. [DOI] [PubMed] [Google Scholar]
- 17.Asare Y, Shagdarsuren E, Schmid JA, Tilstam PV, Grommes J, El Bounkari O, Schütz AK, Weber C, de Winther MP, Noels H, Bernhagen J. Endothelial CSN5 impairs NF-κB activation and monocyte adhesion to endothelial cells and is highly expressed in human atherosclerotic lesions. Thromb Haemost. 2013;110:141–152. doi: 10.1160/TH13-02-0155. [DOI] [PubMed] [Google Scholar]
- 18.Hattori Y, Suzuki K, Tomizawa A, Hirama N, Okayasu T, Hattori S, Satoh H, Akimoto K, Kasai K. Cilostazol inhibits cytokine-induced nuclear factor-kappaB activation via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovasc Res. 2009;81:133–139. doi: 10.1093/cvr/cvn226. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Bei Y, Lu Y, Sun W, Liu Q, Wang Y, Cao Y, Chen P, Xiao J, Kong X. Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1α and Akt activation. Cell Physiol Biochem. 2015;35:2159–2168. doi: 10.1159/000374021. [DOI] [PubMed] [Google Scholar]
- 20.Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D, Guo S, Ming Z, Liu C. Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One. 2012;7:e52013. doi: 10.1371/journal.pone.0052013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Z, Ballou LM, Jiang YP, Cohen IS, Lin RZ. Restoration of defective L-type Ca2+ current in cardiac myocytes of type 2 diabetic db/db mice by Akt and PKC-i. J Cardiovasc Pharmacol. 2011;58:439–445. doi: 10.1097/FJC.0b013e318228e68c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Wu DM, Hu B, Zheng YL, Zhang ZF, Wang YJ. NGF-Dependent activation of TrkA pathway: A mechanism for the neuroprotective effect of troxerutin in D-galactose-treated mice. Brain Pathol. 2010;20:952–965. doi: 10.1111/j.1750-3639.2010.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lakshmanan AP, Harima M, Sukumaran V, Soetikno V, Thandavarayan RA, Suzuki K, Kodama M, Nagata M, Takagi R, Watanabe K. Modulation of AT-1R/AMPK-MAPK cascade plays crucial role for the pathogenesis of diabetic cardiomyopathy in transgenic type 2 diabetic (Spontaneous Diabetic Torii) rats. Biochem Pharmacol. 2012;83:653–660. doi: 10.1016/j.bcp.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Reinking BE, Wedemeyer EW, Weiss RM, Segar JL, Scholz TD. Cardiomyopathy in offspring of diabetic rats is associated with activation of the MAPK and apoptotic pathways. Cardiovasc Diabetol. 2009;8:43. doi: 10.1186/1475-2840-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez AB, Young L, Doll JA, Morgan GM, Crawford SE, Plunkett BA. Elevated neonatal insulin-like growth factor I is associated with fetal hypertrophic cardiomyopathy in diabetic women. Am J Obstet Gynecol. 2014;211:290 e1–e7. doi: 10.1016/j.ajog.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Sampath S, Karundevi B. Effect of troxerutin on insulin signaling molecules in the gastrocnemius muscle of high fat and sucrose-induced type-2 diabetic adult male rat. Mol Cell Biochem. 2014;395:11–27. doi: 10.1007/s11010-014-2107-2. [DOI] [PubMed] [Google Scholar]
- 27.Pan Y, Wang Y, Zhao Y, Peng K, Li W, Wang Y, Zhang J, Zhou S, Liu Q, Li X, et al. Inhibition of JNK phosphorylation by a novel curcumin analog prevents high glucose-induced inflammation and apoptosis in cardiomyocytes and the development of diabetic cardiomyopathy. Diabetes. 2014;63:3497–3511. doi: 10.2337/db13-1577. [DOI] [PubMed] [Google Scholar]
- 28.Kuo WW, Wang WJ, Tsai CY, Way CL, Hsu HH, Chen LM. Diallyl trisufide (DATS) suppresses high glucose-induced cardiomyocyte apoptosis by inhibiting JNK/NFκB signaling via attenuating ROS generation. Int J Cardiol. 2013;168:270–280. doi: 10.1016/j.ijcard.2012.09.080. [DOI] [PubMed] [Google Scholar]