Abstract
Despite a high prevalence of pancreatic endocrine and exocrine insufficiency in cystic fibrosis (CF), pancreas transplantation is rarely reported. United Network for Organ Sharing (UNOS) data were used to examine utilization of pancreas transplant and posttransplant outcomes in CF patients. Between 1987–2014, CF patients (N = 4600) underwent 17 liver–pancreas, three lung–pancreas, one liver–lung pancreas, four kidney–pancreas, and three pancreas-only transplants. Of the 303 CF patients who received liver transplantation, 20% had CF-related diabetes (CFRD) before transplantation, and nine of those received a liver–pancreas transplant. Of 4241 CF patients who underwent lung transplantation, 33% had CFRD before transplantation, and three of those received a pancreas transplant. Of 49 CF patients who received a liver–lung transplant, 57% had CFRD before transplantation and one received a pancreas transplant. Posttransplantation diabetes developed in 7% of CF pancreas transplant recipients versus 24% of CF liver and 29% of CF lung recipients. UNOS has no data on pancreas exocrine insufficiency. Two-year post-transplantation survival was 88% after liver–pancreas transplant, 33% after lung–pancreas transplant, and 100% after pancreas–kidney and pancreas-only transplants. Diabetes is common pretransplantation and posttransplantation in CF solid organ transplant recipients, but pancreas transplantation remains rare. Further consideration of pancreas transplant in CF patients undergoing other solid organ transplant may be warranted.
Introduction
Cystic fibrosis (CF) is an autosomal recessive disorder caused by defects in the CF transmembrane conductance regulator (CFTR) channel. While commonly considered a disease of the lungs, it can impair the function of several organs, and occasionally leads to a need for solid organ transplantation.
Pancreatic manifestations of CF are very common. Ninety percent of CF patients have pancreatic exocrine insufficiency by age 10 (1), which contributes to malnutrition and poor pulmonary outcomes (2). Pancreatic exocrine insufficiency requires lifelong supplementation with pancreatic enzyme replacement. Pancreatic endocrine insufficiency, in the form of CF-related diabetes (CFRD), develops in 26% of CF patients over the age of 10 years (1). These patients become insulin dependent. The combination of pancreatic exocrine and endocrine dysfunction can substantially complicate nutritional management, which is crucial to minimize CF-related morbidity and mortality (3–7).
As there have been improvements in long-term survival with CF and transplant outcomes, options for solid organ transplantation in CF have also expanded. Lung and liver transplants as treatments for the chronic lung disease and cirrhosis associated with CF, respectively, are well described (8–11).
Pancreas transplantation, on the other hand, either combined with other solid-organ transplant or in isolation, has not been as well studied. Pancreas transplantation could improve quality of life as well as improve diabetes control and nutritional status through adequate pancreatic exocrine function. Case series are limited to single-center reports of patients undergoing pancreas transplantation in combination with lung (two series) or liver (six series) (Table 1, (12–18)).
Table 1.
Published literature on pancreas transplantation in CF patients
| Simultaneous pancreas with: | Publications (n) | Patients (n) | Outcomes | References |
|---|---|---|---|---|
| Liver | 7 | 17 | 1 year: 100% patient/graft survival 17/17 exocrine and endocrine sufficient at 4–60 months | (12,14–19) |
| Lung | 1 | 3 | 100% patient/graft survival 3/3 exocrine and endocrine sufficient at 4–14 months | (13) |
| Liver–lung | 0 | 0 | N/A |
N/A, not applicable.
We analyzed the United Network for Organ Sharing (UNOS) database to describe utilization and outcomes associated with pancreas transplantation among CF patients receiving other solid organ transplants. This is the first analysis of pancreas transplantation in CF patients from a comprehensive national database.
Materials and Methods
We conducted a retrospective review of deidentified data from the UNOS Standard Transplant Analysis and Research (STAR) files on liver, thoracic, kidney, and pancreas organ transplants in the United States from October 1987 to December 2014. Institutional review board approval (Study 15-16583) for this study was obtained from the University of California, San Francisco.
Our cohort included all waitlisted candidates in the United States submitted to the Organ Procurement and Transplantation Network (OPTN) between October 1987 and December 2014. Adult and pediatric subjects with CF were identified from listing and transplant diagnoses using diagnostic code 4285 (liver), 5004 (kidney–pancreas), and 1602 (thoracic) as well as through a text search of typed diagnoses using “CF” and “cystic fibrosis.” If a patient underwent a combined organ transplant and a diagnosis of CF in one organ database was found, then the patient was included. Recipients undergoing organ retransplantation were identified and counted only once. Patients were followed until December 2014 or until death, retransplantation, or loss to follow up. Pretransplantation and posttransplantation recipient data, including demographics, illness severity, characteristics of the recipient and donor, and outcomes from baseline and annual follow-up files, were assessed.
Pretransplantation and posttransplantation diabetes, patient survival, and waitlist time were analyzed by stratified cohort groupings. CFRD was defined as diabetes before transplantation, as documented in pretransplantation databases. CFRD is treated with insulin, so these patients are assumed to have insulin-dependent diabetes. If a subject underwent combined organ transplantation with pretransplantation diabetes in one organ database, then the subject was considered to have CFRD. Patients were considered to have posttransplantation diabetes if they did not have diabetes documented before transplantation but had diabetes documented in follow-up after transplantation. The follow-up providers documented insulin-dependent diabetes separately. There was inconsistency in documentation of diabetes at follow-up visits. As such, patients who had diabetes documented at their last follow-up visit were counted as a separate outcome of interest as well. Posttransplantation diabetes was not collected in the STAR lung files until April 1, 1994, or in the STAR liver, kidney, or pancreas files until June 30, 2004.
Statistical Analysis
Descriptive analysis was performed with calculation of medians and interquartile ranges for continuous variables and proportions for categorical variables. Comparative analyses were performed using t-test or Wilcoxon rank-sum test for continuous variables and χ2 or Fisher’s exact test for categorical variables as appropriate. A time-to event analysis was performed to evaluate the effect of type of organ on survival after transplantation. The log-rank test statistic was used to compare survival estimates among groups. A p value of <0.05 was considered statistically significant in all analyses. Analyses were performed using statistical software (STATA 13.1, SE; StataCorp, College Station, TX).
Results
From 1987 through 2014, there were 303 liver transplantations, 4241 lung transplantations, and 49 combined liver–lung transplantations in CF patients. Of these, there were 21 combined with a pancreas transplant. In addition, there were three pancreas–kidney and four isolated pancreas transplants reported in CF patients (Figure 1). Of the 28 total pancreas transplantations, 11 recipients were children and 17 were adults. Twenty-four of the pancreas transplantations were performed in combination with another solid organ. The most common combined organ transplant was liver with pancreas (17 patients) (Figure 1). The majority of pancreas transplants were implanted with enteric drainage (21 of 28). Three had bladder drainage, and the remaining four did not report how the pancreatic duct was drained.
Figure 1. Pretransplantation and posttransplantation diabetes in first-time liver, lung, lung–liver, and pancreas transplant recipients for an indication of CF divided according to other solid organ transplant.
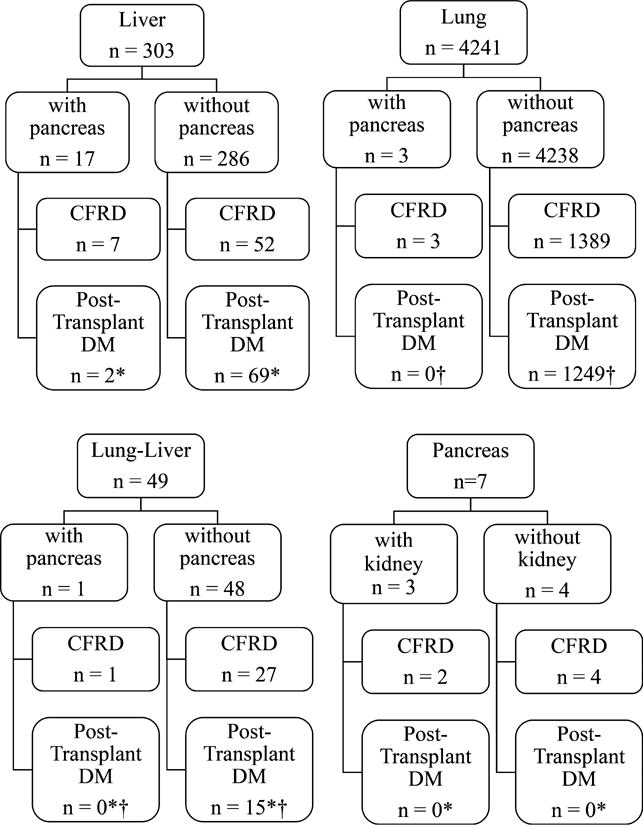
DM, diabetes mellitus. CFRD refers to pretransplantation CF-related diabetes. De novo diabetes in patients without CFRD. *Follow-up of posttransplantation diabetes not started until June 30, 2004, for pancreas and liver transplants. †Follow-up of posttransplantation diabetes not started until April 1, 1994, for lung transplants.
Patients receiving pancreas transplants were older, more often female, and more likely to be Hispanic than were other CF transplant recipients (Table 2). Seventy-one percent of pancreas transplantations were performed after 2000. Nine of 28 pancreas transplantations were performed in Region 10, and three transplantations each were performed in Regions 2, 3, 4, 5, 7, and 8.
Table 2.
CF solid organ transplant recipients with and without concomitant pancreas transplant
| Recipient characteristics | Pancreas transplant (n = 28) | No pancreas transplant (n = 4572) | p-value |
|---|---|---|---|
| Age at transplantation, y (mean ± SD) | 27.6 ± 10.9 | 22.4 ± 11.4 | 0.01 |
| Sex, male (%) | 35.7 | 52.2 | 0.08 |
| Race (%) | 0.06 | ||
| Non-Hispanic white | 82.1 | 94.3 | |
| African American | 3.6 | 1.4 | |
| Hispanic | 14.3 | 3.9 | |
| Other | 0.4 | 0 | |
| Body mass index, kg/m2 (median, IQR) | 20.3 (18.5–22.3) | 18.7 (17.1–20.6) | 0.009 |
| Transplant decade (%) | 0.5 | ||
| 1987–1996 | 14.3 | 17.5 | |
| 1997–2006 | 53.6 | 42.3 | |
| 2006–2014 | 32.1 | 40.2 | |
| Time on waitlist (median, IQR) | 170 (92–413) | 187 (53–488) | 0.9 |
Among all 303 CF patients who received their first liver transplant, 20% (n = 61) had CFRD (Figure 1); 11 of those were listed for combined pancreas transplant and nine actually received it. There were eight additional combined liver–pancreas transplantations: one patient had CFRD but was not listed for combined transplantation, and four did not have CFRD but were listed for combined transplantation for CF without diabetes. The remaining three patients did not have documented CFRD and were not listed for pancreas transplantation but did receive a pancreas with their liver for CF without diabetes.
Among the 4241 CF patients who received their first lung transplant, 33% (n = 1392) had CFRD. Only four CF/CFRD patients were listed for pancreas transplantation, and three received a pancreas transplant. Of the 49 CF patients who underwent combined liver–lung transplantation, 57% had CFRD. Only one patient was listed for and received a pancreas transplant.
There were seven pancreas transplantations performed independent of liver or lung transplantation for a listed indication of CF. Four were combined kidney–pancreas transplantations, and three of those patients had CFRD. Three were pancreas-only transplantations, and two of those patients had CFRD.
Posttransplantation survival
Two-year survival in CF liver–pancreas recipients was 88% (n = 17). Among CF lung–pancreas transplant recipients (n = 3), one died after 6 months of viral septicemia, one died after 20 months of Pseudomonas pneumonia, and the final patient died after 32 months of follow-up of acute respiratory distress syndrome. All three of the CF pancreas–kidney and all four of the CF pancreas-only transplant recipients were alive at 2 years.
Two-year survival in CF liver transplant recipients, with or without pancreas transplant, was very similar in those with CFRD (83%) and those without (85%, p = 0.22). There was no difference in 2-year survival for lung transplant patients with and without CFRD (84% for both). However, among CF patients with liver–lung transplant, 2-year survival in the CFRD group was worse (67%) than in those without CFRD (82%, p = 0.051). Two-year survival in CF solid organ transplant recipients was 81.5% in those also receiving a pancreas transplant and 75.3% in those without a pancreas transplant (p = 0.27). Among CF liver transplant recipients, 2-year survival was 87.5% in those with a pancreas transplant compared with 84.4% in those without.
Posttransplantation diabetes
Insulin dependent posttransplantation diabetes was documented in 2 of the 28 CF pancreas transplant recipients. Both subjects had undergone combined liver-pancreas transplantation and did not have pretransplantation diabetes. One had diabetes at their last follow up visit, and the other had documented diabetes for two consecutive follow-ups then no further documentation of diabetes. The median follow-up time for all pancreas transplant recipients was 4.5 years (interquartile range [IQR] 1.9–8.8).
Posttransplantation diabetes for those CF patients who did not receive a pancreas transplant developed in 24% of liver recipients (69/286), 29% of lung recipients (1249/4238), and 31% of combined liver–lung recipients (15/48). Insulin-dependent diabetes developed in many of the posttransplantation diabetics: 87% (60/69) of cases in liver transplant recipients, 89% (1110/1249) of cases in lung transplant recipients, and 67% (10/15) of the cases in lung–liver recipients. In those without a pancreas transplant who developed posttransplantation diabetes, it persisted through last follow-up in 22% of diabetic liver transplants (median follow-up 5.2 years, IQR 1–9.7), 36% of diabetic lung transplants (median follow-up 3 years, IQR 1–6.7), and 31% of diabetic liver–lung transplants (median follow up 2.5 years, IQR 0.5–5.3 years).
Waitlist times
There was no difference in waitlist times in subjects who received a pancreas transplant (median 170 days, IQR 92–413 days) and those who did not (median 187 days, IQR 53–489 days, p = 0.06). Overall, in the CF cohort, median waitlist time for liver transplantation was 124 days (IQR 42–348 days), and median waitlist time for lung transplantation was 190 days (IQR 54–505 days). There was no statistically significant difference in waitlist time between CF liver–pancreas recipients (median 189 days, IQR 95–412 days) and CF liver-only recipients (median 120 days, IQR 40–342 days; p = 0.2).
Discussion
Pancreas transplantation remains rare in patients with CF, either isolated or in combination with other organ transplants, despite the high prevalence of pancreatic exocrine and endocrine insufficiency in CF. Among the small number of pancreas transplantations reported in UNOS, overall survival after 2 years was excellent. Pancreas transplantation did not appear to impact survival significantly. Pancreas graft function also appeared to be excellent with only 7.1% of all CF patients who received pancreas transplants developing documented posttransplantation diabetes.
With advances in surgical technique, immunosuppression, donor and recipient selection, and graft surveillance, there have been significant improvements in pancreas graft survival (19). Nonetheless, the procedure is underused in the CF population. Several reasons could account for this. Pancreas transplantation is a technically difficult surgery and associated with significant posttransplantation complications, and the procedure is not considered life-saving because organ function can be replaced by exogenous medications. In combined pancreas–lung transplantation, there are increased complications of entering the abdomen in addition to the chest cavity. In this cohort of patients, all three lung–pancreas transplant patients did not survive to 3 years posttransplantation, indicating either an increased risk to the combination of these procedures or increased disease severity before transplantation. Finally, there are significant logistical and donor-related issues that may increase the complexity of a combined procedure.
It is difficult to glean from the data available in the UNOS registry why pancreas transplantation was used in some cases and not others. Bandasma et al recently published an international survey of 81 pediatric transplantation centers characterizing subjects who underwent simultaneous liver–pancreas transplantation and the attitudes of transplant physicians. Only eight cases of combined liver–pancreas transplantation were reported. The survey revealed that “lack of experience with this procedure” ranked as one of the most prevalent reasons for not considering the procedure (20). Limited experience may also increase the risk of complications. In this series, four patients experienced surgical complications: graft thrombosis in two, anastomotic biliary stricture in one, and pancreatic leak in another. Two patients died at 4 and 8 years posttransplantation from worsening lung function. Region 10 accounted for one-third of the pancreas transplantations, suggesting that increased experience might lead to higher utilization for specific cases.
Our analysis also highlights how common CFRD is in CF patients listed for solid organ transplants. CFRD has features of both type 1 and type 2 diabetes. It is mostly characterized by insulin insufficiency, but fluctuating levels of insulin resistance related to acute and chronic illness play a role (21,22). CFRD can often be clinically silent with prolonged periods of abnormal glucose metabolism before diagnosis (22).
In CF patients who underwent liver or lung transplantation without accompanying pancreas transplantation, posttransplantation diabetes was common. Progressive CFRD and immunosuppression after transplantation may accelerate the development of insulin-dependent diabetes in children and adults with CF (23). Rates of new-onset diabetes after lung transplantation in CF patients in other cohorts range from 16% to 53% (24–26). It is hypothesized that, along with a predisposition for development of CFRD, corticosteroids and calcineurin inhibitors contribute to this high prevalence. The UNOS database started tracking posttransplantation diabetes midway through our study period; thus, we may not have fully captured posttransplantation diabetes.
We unfortunately could not assess pancreatic exocrine function pretransplantation or posttransplantation, as there was no information on pancreatic enzyme use or fecal elastase. In addition, there were no data on the anatomic location of enteric drainage. En bloc liver–pancreas transplantation in CF patients undergoing liver transplantation would provide proximal pancreatic enzymes that could be functionally helpful, but distal enteric drainage or drainage into the urinary bladder would not provide a functional exocrine benefit. There is at least one case series that reported proximal enteric drainage in combined pancreas–lung transplantation for CF patients were able to discontinue all pancreatic enzyme supplements (13). Similarly, there are multiple case reports of recipients’ en bloc liver–pancreas transplant recipients who became independent of pancreatic enzyme supplements (12,14–18). Given these findings, it may be beneficial to attempt proximal enteric drainage in pancreas transplants performed in the CF population.
A less invasive option for patients with CFRD may be simultaneous or sequential islet transplantation. Recent studies have suggested that this may be an important and viable alternative (27–30). The patients in these published case reports had difficult-to-control diabetes before islet transplantation. All tolerated the islet infusion and had improved glycemic control after the procedure, although many still required insulin (27–30). Use of this approach is limited by its experimental nature, varying long-term insulin independence, and current lack of reimbursement. Future improvements in islet manufacture and transplantation, along with improved insurance coverage, may increase the feasibility of this option.
In summary, this study represents the first comprehensive analysis of pancreas transplantation in CF patients in the United States. This is the largest reported cohort of CF pancreas transplant recipients and represents a comprehensive database of US solid organ transplants. Pancreas transplantation in CF patients undergoing other solid organ transplantation is rare but potentially successful. Posttransplantation diabetes is common among CF patients receiving other solid organ transplants. This may be a valid indication for consideration of pancreas transplantation in CF solid organ transplant recipients. These findings represent an opportunity for additional discussion and collaboration between transplant surgeons and CF multidisciplinary teams assessing patient candidacy for pancreas transplantation. More-detailed studies will be required to determine the potential benefits and risks of these procedures in the CF population.
Acknowledgments
Disclaimer
Dr. Usatin was supported by T32 DK007762 and a Cystic Fibrosis Foundation Fellowship Grant for her training. Dr. Perito was supported by National Institute of Diabetes and Digestive and Kidney Diseases K23 DK099253-01A1. This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. The content is the responsibility of the authors alone and does not necessarily reflect the views or policies of the Department of Health and Human Services nor does mention of trade names, commercial products or organizations imply endorsement by the US government.
Abbreviations
- CF
cystic fibrosis
- CFRD
cystic fibrosis–related diabetes
- CFTR
cystic fibrosis transmembrane conductance receptor
- IQR
interquartile range
- OPTN
Organ Procurement and Transplantation Network
- STAR
Standard Transplant Analysis and Research
- UNOS
United Network for Organ Sharing
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Cystic Fibrosis Foundation. Patient Registry 2011: Annual Report. Bethesda, MD: Cystic Fibrosis Foundation; 2011. [Google Scholar]
- 2.Somaraju UR, Solis-Moya A. Pancreatic enzyme replacement therapy for people with cystic fibrosis. Cochrane Database Syst Rev. 2014;10:CD008227. doi: 10.1002/14651858.CD008227.pub2. [DOI] [PubMed] [Google Scholar]
- 3.Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth, and pulmonary function in patients with cystic fibrosis in Boston and Toronto. J Clin Epidemiol. 1988;41(6):583–591. doi: 10.1016/0895-4356(88)90063-7. [DOI] [PubMed] [Google Scholar]
- 4.Steinkamp G, Wiedemann B. Relationship between nutritional status and lung function in cystic fibrosis: Cross sectional and longitudinal analyses from the German CF quality assurance (CFQA) project. Thorax. 2002;57(7):596–601. doi: 10.1136/thorax.57.7.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vedam H, Moriarty C, Torzillo PJ, McWilliam D, Bye PT. Improved outcomes of patients with cystic fibrosis admitted to the intensive care unit. J Cyst Fibros. 2004;3(1):8–14. doi: 10.1016/j.jcf.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr. 2013;162(3):530–535.e1. doi: 10.1016/j.jpeds.2012.08.040. [DOI] [PubMed] [Google Scholar]
- 7.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153(4):345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnon R, Annunziato RA, Miloh T, et al. Liver and combined lung and liver transplantation for cystic fibrosis: Analysis of the UNOS database. Pediatr Transplant. 2011;15(3):254–264. doi: 10.1111/j.1399-3046.2010.01460.x. [DOI] [PubMed] [Google Scholar]
- 9.Mendizabal M, Reddy KR, Cassuto J, et al. Liver transplantation in patients with cystic fibrosis: Analysis of United Network for Organ Sharing data. Liver Transpl. 2011;17(3):243–250. doi: 10.1002/lt.22240. [DOI] [PubMed] [Google Scholar]
- 10.Desai CS, Gruessner A, Habib S, Gruessner R, Khan KM. Survival of cystic fibrosis patients undergoing liver and liver-lung transplantations. Transplant Proc. 2013;45(1):290–292. doi: 10.1016/j.transproceed.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Hadjiliadis D. Special considerations for patients with cystic fibrosis undergoing lung transplantation. Chest. 2007;131(4):1224–1231. doi: 10.1378/chest.06-1163. [DOI] [PubMed] [Google Scholar]
- 12.Fridell JA, Vianna R, Kwo PY, et al. Simultaneous liver and pancreas transplantation in patients with cystic fibrosis. Transplant Proc. 2005;37(8):3567–3569. doi: 10.1016/j.transproceed.2005.09.091. [DOI] [PubMed] [Google Scholar]
- 13.Fridell JA, Wozniak TC, Reynolds JM, et al. Bilateral sequential lung and simultaneous pancreas transplant: A new approach for the recipient with cystic fibrosis. J Cyst Fibros. 2008;7(4):280–284. doi: 10.1016/j.jcf.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Henn C, Kapellen T, Prenzel F, et al. Combined heterotopic liver-pancreas transplantation as a curative treatment for liver cirrhosis and diabetes mellitus in cystic fibrosis. Pediatr Transplant. 2014;18(1):E6–E9. doi: 10.1111/petr.12157. [DOI] [PubMed] [Google Scholar]
- 15.Mekeel KL, Langham MR, Jr, Gonzalez-Perralta R, Reed A, Hemming AW. Combined en bloc liver pancreas transplantation for children with CF. Liver Transpl. 2007;13(3):406–409. doi: 10.1002/lt.21070. [DOI] [PubMed] [Google Scholar]
- 16.Miguel M, Andres AM, Lopez-Santamaria M, et al. Liver transplantation in children with cystic fibrosis: Experience in our centre and preliminary results with a combined en bloc liver-pancreas graft. Eur J Pediatr Surg. 2012;22(1):60–66. doi: 10.1055/s-0031-1291288. [DOI] [PubMed] [Google Scholar]
- 17.Stern RC, Mayes JT, Weber FL, Jr, Blades EW, Schulak JA. Restoration of exocrine pancreatic function following pancreas-liver-kidney transplantation in a cystic fibrosis patient. Clin Transplant. 1994;8(1):1–4. [PubMed] [Google Scholar]
- 18.Young AL, Peters CJ, Toogood GJ, et al. A combined liver-pancreas en-bloc transplant in a patient with cystic fibrosis. Transplantation. 2005;80(5):605–607. doi: 10.1097/01.tp.0000167007.58199.9b. [DOI] [PubMed] [Google Scholar]
- 19.Kandaswamy R, Stock PG, Skeans MA, et al. OPTN/SRTR 2011 Annual Data Report: Pancreas. Am J Transplant. 2013;13(Suppl 1):47–72. doi: 10.1111/ajt.12020. [DOI] [PubMed] [Google Scholar]
- 20.Bandsma RH, Bozic MA, Fridell JA, et al. Simultaneous liver-pancreas transplantation for cystic fibrosis-related liver disease: A multicenter experience. J Cyst Fibros. 2014;13(4):471–477. doi: 10.1016/j.jcf.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Marshall BC, Butler SM, Stoddard M, Moran AM, Liou TG, Morgan WJ. Epidemiology of cystic fibrosis-related diabetes. J Pediatr. 2005;146(5):681–687. doi: 10.1016/j.jpeds.2004.12.039. [DOI] [PubMed] [Google Scholar]
- 22.Moran A, Brunzell C, Cohen RC, et al. Clinical care guidelines for cystic fibrosis-related diabetes: A position statement of the American Diabetes Association and a clinical practice guideline of the Cystic Fibrosis Foundation, endorsed by the Pediatric Endocrine Society. Diabetes Care. 2010;33(12):2697–2708. doi: 10.2337/dc10-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hadjiliadis D, Madill J, Chaparro C, et al. Incidence and prevalence of diabetes mellitus in patients with cystic fibrosis undergoing lung transplantation before and after lung transplantation. Clin Transplant. 2005;19(6):773–778. doi: 10.1111/j.1399-0012.2005.00420.x. [DOI] [PubMed] [Google Scholar]
- 24.van Belle Meerkerk G, van de Graaf EA, van Kwakkel Erp JM, et al. Diabetes before and after lung transplantation in patients with cystic fibrosis and other lung diseases. Diabet Med. 2012;29(8):e159–e162. doi: 10.1111/j.1464-5491.2012.03676.x. [DOI] [PubMed] [Google Scholar]
- 25.Lama R, Alvarez A, Santos F, et al. Long-term results of lung transplantation for cystic fibrosis. Transplant Proc. 2001;33(1–2):1624–1625. doi: 10.1016/s0041-1345(00)02618-x. [DOI] [PubMed] [Google Scholar]
- 26.Ye X, Kuo HT, Sampaio MS, Jiang Y, Bunnapradist S. Risk factors for development of new-onset diabetes mellitus after transplant in adult lung transplant recipients. Clin Transplant. 2011;25(6):885–891. doi: 10.1111/j.1399-0012.2010.01383.x. [DOI] [PubMed] [Google Scholar]
- 27.Cretin N, Buhler L, Fournier B, et al. Results of human islet allotransplantation in cystic fibrosis and type I diabetic patients. Transplant Proc. 1998;30(2):315–316. doi: 10.1016/s0041-1345(97)01285-2. [DOI] [PubMed] [Google Scholar]
- 28.Kessler L, Bakopoulou S, Kessler R, et al. Combined pancreatic islet-lung transplantation: A novel approach to the treatment of end-stage cystic fibrosis. Am J Transplant. 2010;10(7):1707–1712. doi: 10.1111/j.1600-6143.2010.03143.x. [DOI] [PubMed] [Google Scholar]
- 29.Spijker HS, Wolffenbuttel BH, van der Bij W, Engelse MA, Rabelink TJ, de Koning EJ. Islet-after-lung transplantation in a patient with cystic fibrosis-related diabetes. Diabetes Care. 2014;37(7):e159–e160. doi: 10.2337/dc14-0639. [DOI] [PubMed] [Google Scholar]
- 30.Tschopp JM, Brutsche MH, Frey JG, et al. End-stage cystic fibrosis: Improved diabetes control 2 years after successful isolated pancreatic cell and double-lung transplantation. Chest. 1997;112(6):1685–1687. doi: 10.1378/chest.112.6.1685. [DOI] [PubMed] [Google Scholar]


