Abstract
Corpus callosotomy is a palliative procedure performed to reduce the severity of drug-resistant epilepsy. We assessed its efficacy on different seizure types in 20 subjects (age range 5–19 years); eight with active vagus nerve stimulator (VNS). Fourteen had complete callosotomy, 4 had anterior 2/3 and two had anterior 2/3 followed later by complete callosotomy. Ten had endoscopic approach. Eighty-five percent had ≥ 50% reduction of generalized seizures leading to falls (atonic, tonic, myoclonic); 35% became seizure-free (follow-up period: 6 months-9 years; mean 3 years). Seizure outcome distribution was better for generalized than for partial seizures (p=0.003). Endoscopic approach was as effective as transcranial approach. Seven subjects who failed VNS therapy responded with ≥50% seizure reduction. Corpus callosotomy is an effective treatment for intractable generalized epilepsy leading to falls with significant seizure-reduction or even elimination of seizures, in the majority of children.
Keywords: Drug-resistant epilepsy, Palliative surgery, Pediatric epilepsy surgery, Endoscopic surgery, Lennox-Gastaut Syndrome
INTRODUCTION
In general, children with medically intractable epilepsy are first evaluated for curative resection of the epileptic focus. However, children who have non-localizing or generalized epilepsy based on seizure semiology, ictal electroencephalography (EEG) and neuroimaging findings are not candidates for resective surgery and, therefore, palliative surgical options should be explored.
At our institution, in some children with multifocal ictal onset where seizures emanate from bilateral cortical foci at different frequencies, we have sometimes performed palliative surgical resections in which the major epileptogenic zone is removed.1 However, for children with disabling seizures resulting from atonic, tonic and myoclonic seizures leading to debilitating falls, corpus callosotomy is considered. This procedure is based on the hypothesis that the corpus callosum is the major pathway for the interhemispheric spread of ictal discharges,2 and its disconnection leads to a disruption of rapid seizure spread.
Another form of palliative epilepsy surgery is vagus nerve stimulation (VNS) placement. Since VNS approval by the United States Food and Drug Administration (FDA) in July 19973, physicians’ attention was drawn to VNS more than corpus callosotomy, as reflected in the number of presentations in the Annual Meeting of the American Epilepsy Society (Figure 1).4 During this time, we have continued to perform other palliative procedures (i.e., corpus callosotomy, palliative resections) in addition to VNS for patients not approved for curative surgery. Here, we present our experience at the Children’s Hospital of Michigan (Detroit) on corpus callosotomy for children with medically intractable seizures. Specifically, we analyzed their seizure outcome based on seizure types. We revisited and tested the generally accepted notion that the postoperative outcome would differ among different seizure types. We also determined if corpus callosotomy would be effective in patients who had failed or had poor response to VNS therapy. Furthermore, we determined if endoscopic corpus callosotomy is as effective as the transcranial approach.
Figure 1.
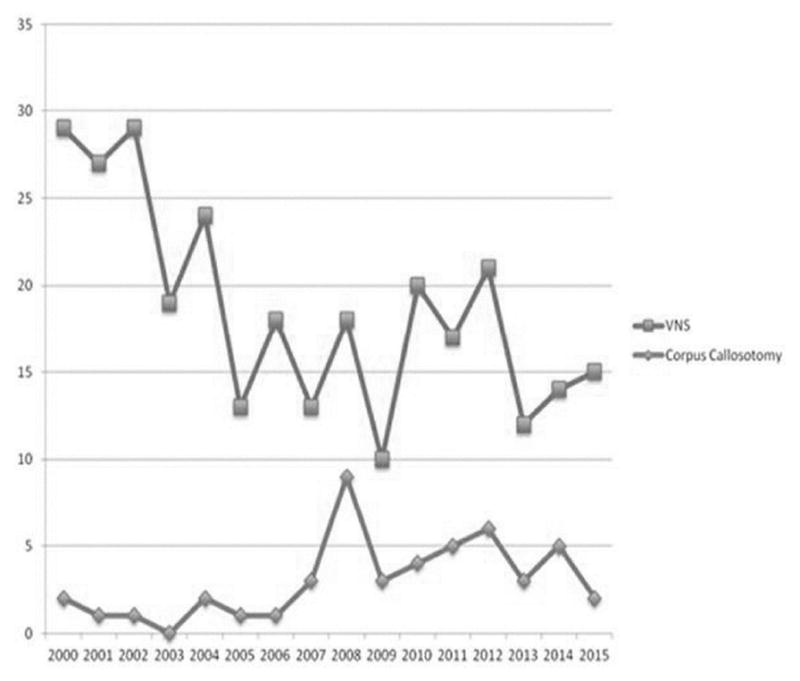
The number of abstracts accepted in the Annual Meeting of American Epilepsy Society from 2000 to 2015. Between 2000 and 2015, the number of abstracts including a keyword “callosotomy” was less compared to the number of abstracts including a keyword “vagus nerve stimulation” or “VNS”.
MATERIALS AND METHODS
Subjects’ seizure types’ classification and selection criteria for corpus callosotomy
The subjects’ individual seizure types based on parental history and as documented by the video electroencephalography (EEG) monitoring were analyzed and classified according to the 2010 International League Against Epilepsy (ILAE) classification system.5 The presence of electro-clinical syndrome was also identified.
The following selection criteria for corpus callosotomy were utilized: presence of medically refractory seizures not responsive to more than 2 appropriately chosen antiepileptic drugs, absence of resectable seizure focus, generalized seizure type leading to sudden falls and injuries such as atonic, tonic and myoclonic seizures. Subjects with rapidly spreading non-lateralizing focal seizures that lead to falls were also considered.
Twenty-five subjects underwent corpus callosotomy between January 1, 2000 and January 31, 2016. Four out of the 25 were excluded because their corpus callosotomy was combined with focal resection; one other subject was excluded due to lack of follow up data. The remaining 20 subjects were included in the analysis. All underwent preoperative epilepsy surgery evaluation, which included video-EEG monitoring, magnetic resonance imaging (MRI), 2-deoxy-2-(18F)-fluoro-D-glucose (FDG) positron emission tomography (PET) and neuropsychological evaluation, if feasible. Prior to surgery, each case was discussed in our Epilepsy Surgery Conference to determine if he/she would benefit from resective surgery. Nineteen of the 20 had debilitating generalized seizures leading to sudden falls (atonic, tonic, and/or myoclonic seizures) and 15 of them had associated other seizure types (generalized tonic-clonic [GTC], atypical absence and dyscognitive focal seizures). One of the 20 had rapidly spreading focal seizures leading to falls secondary to a right porencephaly from an old intracranial hemorrhage due to hemophilia could have been a candidate for hemispherectomy. However, due to his bleeding disorder and the very high risk of related complications, the family elected to proceed with corpus callosotomy instead. A report on this unique patient has been previously published by our group.6
Brain MRI and FDG-PET
Each subject underwent brain MRI and FDG-PET imaging.7,8 MRI was performed using either a 1.5 or 3 Tesla GE scanner. MRI sequences included T1-W with and without contrast, T2-W and T2 fluid attenuation inversion recovery (FLAIR) images. FDG-PET studies were performed using either the GE Discovery STE PET/CT scanner (Milwaukee, WI) or the Siemens/CTI EXACT/HR (Knoxville, TN) whole-body positron tomograph. The isotropic image resolution for both scanners was 5 mm full width half maximum for the FDG scans. Scalp EEG was monitored during and after the tracer injection. Subsequently, a dynamic emission scan of the brain (7 × 5 minutes) was acquired in 3D-mode, generating 47 image planes with 3.125 mm slice thickness.
Corpus Callosotomy
The decision whether to perform anterior 2/3 corpus callosotomy versus complete/total corpus callosotomy was based on the degree of cognitive impairment and developmental delay, and was also guided intraoperatively in some subjects by the presence of EEG activity desynchronization or transformation of generalized epileptiform discharges to asynchronized (lateralized) epileptiform discharges during the surgical course of the corpus callosotomy procedure. Ten subjects underwent the traditional craniotomy approach through the coronal suture dissecting through the interhemispheric fissure below the falx to reach the corpus callosum. In the remaining 10 subjects, an endoscopic approach from the anterior, as described by our group9, was utilized. In the 2 most recent patients, we utilized a posterior interhemispheric endoscopic approach to perform a complete corpus callosotomy.10 The posterior approach bypasses the need to perform interhemispheric dissection since the falx is invariably in close proximity to the corpus callosum in this region. Both these endoscopic approaches were accomplished through a small 3 cm incision. Anterior commissurotomy was also performed in 11 subjects.
Intraoperative EEG monitoring
During the entire procedure, intraoperative scalp EEG was monitored in 19/20 subjects. A total of 10 scalp electrodes were utilized (F3, C3, P3, F7, T3, F4, C4, P4, F8 and T4) and the recording was made on the Nihon Kohden digital system. The level of isoflurane was kept at <1% and the level of ETCO2 was kept below 40%.7 Visual analysis was done in the interpretation of the EEG findings. The extent of corpus callosum disconnection was confirmed in all cases by a post-operative brain MRI.
Seizure outcome
We have identified the seizure outcome of all individual seizure types by classifying the seizure frequency reduction from baseline seizure frequency using the following scale: <50%; 50–75%; 76–99%, 100% seizure reduction and no seizure reduction or seizure worsening. Seizure outcomes were based on the parental reports of seizure frequency reduction for each seizure type during the patients’ follow-up visit or through telephone interview and supported by data from follow-up video-EEG in some patients.
Statistical Analysis
Quantitative values were expressed as mean ± standard deviation whereas qualitative values were given as numbers or percentages. To determine whether the distribution of seizure outcome differs across the different seizure types, Chi square test was performed. One-way analysis of variance (ANOVA) was used to determine the presence of statistical difference in the outcome between seizure types. Chi square test was used to compare the seizure outcome and complication rate between the transcranial and endoscopic corpus callosotomy groups and the seizure outcome of the subjects with or without anterior commissurotomy and to determine the association between the intraoperative EEG findings and the surgical outcome.
SPSS 23.0 (SPSS Inc. Armonk, NY, U.S.A.) was used for data analysis. P value of <0.05 was considered as statistically significant.
RESULTS
Clinical and seizure data (Table 1)
Table 1.
Demographic data.
| A total of 20 subjects were included.
| |
|---|---|
| Total number of subjects | 20 |
|
| |
| Males | 12 (60%) |
|
| |
| Females | 8 (40%) |
|
| |
| Age at surgery (mean; range) | 12 years; 5.1 years to 19 years |
|
| |
| Seizure onset (mean; range) | 4.0 years; 2 weeks to 10 years |
|
| |
| Etiology | |
| Unknown | 14 |
| Structural/metabolic | 4 |
| Genetics | 2 |
|
| |
| Seizure types- number of patients (percentage) | |
| seizures leading to falls | 13 (65%) |
| Tonic | 9 (45%) |
| Atonic | 5 (25%) |
| Myoclonic | 1 (5%) |
| Rapidly spreading focal seizure | 9 (45%) |
| Generalized tonic clonic seizure | 10 (50%) |
| Atypical absence | 1 (5%) |
| Epileptic spasms | 4 (20%) |
| Dyscognitive focal seizure | |
|
| |
| With vagus nerve stimulator | 9 (45%) |
|
| |
| Duration of epilepsy prior to corpus callosotomy | 8.41 years; 1 year to 18 years |
|
| |
| Extent of corpus callosotomy | |
| Total | 15 (75%) |
| Two-third | 3 (15%) |
| Two-third followed by Total | 2 (10%) |
|
| |
| Technique | |
| Craniotomy | 10 |
| Endoscopy | 10 |
| Anterior approach | 8 |
| Posterior approach | 2 |
The mean age at surgery was 12 years (range 5 to 19 years) and the mean age of seizure onset was 4 years (range 2 weeks to 10 years). Twelve of the 20 were males. Seizure duration prior to surgery ranged from 1 year to 18 years with a mean duration of 8.4 years.
The majority of the subjects did not have an underlying etiology for their epilepsy. Four showed structural abnormalities on MRI, including lissencephaly, bilateral subcortical band heterotopia, diffuse cortical dysplasia, and bilateral hippocampal sclerosis from post-infectious cause. Two had known genetic diagnoses including Down syndrome and SCN1A gene mutation, clinically compatible with a diagnosis of Dravet syndrome. Sixteen of the 20 had associated multiple seizure types: 13 subjects with tonic seizures, 9 with GTC, 9 with atonic, 5 with myoclonic, 10 with atypical absence, 4 with dyscognitive focal seizures and 1 with rapidly spreading focal seizure leading to falls. A single subject had epileptic spasms. Sixteen satisfied the electro-clinical diagnosis of Lennox-Gastaut Syndrome (LGS).
Forty-five percent (9/20) had VNS for a mean of 2.9 years (range: 6 months to 8.8 years) with mean device output current of 2.125 milliamperes (mA) (range: 1.25 to 2.4 mA), 30 hertz (Hz) signal frequency; 500 microseconds pulse width; on time of 30 seconds with mean off time of 1.5 minute (range: 0.8 to 1.8 minute). Corpus callosotomy was performed due to failed or poor response to VNS which was defined using the McHugh VNS seizure outcome classification11 as <50% seizure reduction from baseline seizure frequency (Class III), magnet benefit only (Class IV) or no improvement (Class V). Eight out of the nine had active VNS. The majority (15 subjects) had total corpus callosotomy, 3 had anterior 2/3 corpus callosotomy and two had two-staged corpus callosotomy (anterior 2/3 followed by total callosotomy due to inadequate response from the first procedure).
Seizure outcome data
After a mean follow up period of 3 years (range: 6 months to 9 years), 7 out of the 20 (35%) subjects became free from the targeted seizures leading to falls (due to tonic, atonic, myoclonic and rapidly spreading focal seizure); 10 had 50–75% reduction, with the remaining three benefiting from 76–99% seizure reduction. In addition, among the 9 subjects with GTCs, 4 had 50% seizure reduction, 2 had 76–99% reduction, and 3 became GTC seizure-free. Among the 10 subjects with atypical absence seizure, 4 had 50% seizure reduction, 4 had 76–99% seizure reduction, and 2 became absence seizure-free. One subject with generalized seizures had increased frequency of focal seizures following corpus callosotomy. Among the four subjects with associated dyscognitive focal seizures, three did not show positive response to corpus callosotomy, and one had increased frequency of seizures. The latter subject had 50–75% reduction of generalized seizure leading to falls which led to much improved quality of life.
After analysis of the outcome of the different seizure types of the 20 subjects, we noted that the distribution of seizure outcome differed significantly across the different seizure types (p value=0.001) (Figure 2), and seizure outcome distribution was better for generalized than for focal seizures (p=0.003). Among the 8 subjects with active VNS, 7 responded with at least 50% seizure reduction following corpus callosotomy. The generalized seizure outcome of the group with transcranial (n=10) and endoscopic corpus callosotomy approach (n=10) did not show significant difference (p value =0.215). Likewise, no significant difference in the generalized seizure outcome was noted between the group who also underwent anterior commissurotomy (n=11) and those without (n=9) (p value =0.59).
Figure 2.
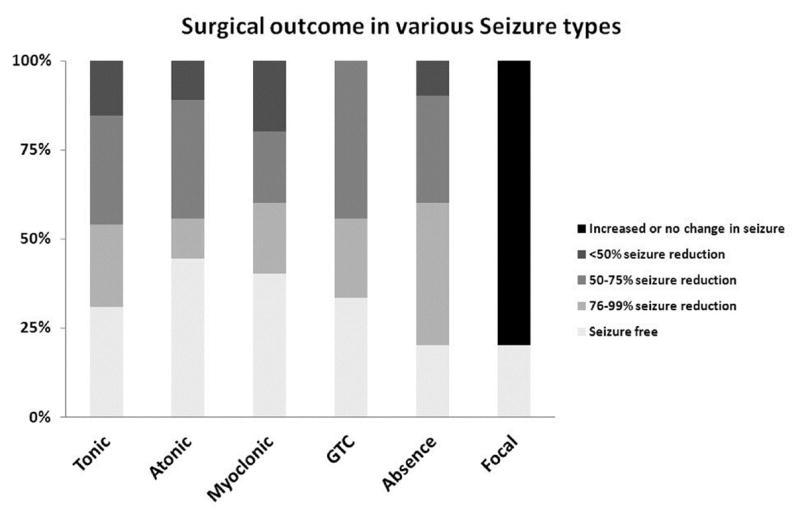
Analysis of the outcome of the different seizure types of the 20 subjects showed that the distribution of seizure outcome significantly differs across the different seizure types (p value=0.001). GTC – Generalized tonic clonic seizure
Intraoperative EEG during corpus callosotomy
Nineteen out of the 20 subjects had intraoperative scalp EEG monitoring during the entire surgery. Prior to corpus callosotomy, 15 out of 19 showed generalized spike wave discharges and 3 of them also showed multifocal spike wave discharges in both hemispheres. In four subjects, no interictal spikes were seen. EEG change immediately following corpus callosotomy was categorized into four groups: (a) decreased and desynchronized (lateralized) spikes in 12, (b) decreased spikes but still generalized in 2, (c) still frequent but asynchronous (lateralized) spikes in 1, (d) no spikes pre and post-corpus callosotomy in 4. We found no association between immediate post-operative EEG findings and seizure outcome (p value =0.72), suggesting that immediate post-operative EEG findings failed to predict the surgical outcome in our cohort.
Complications
Transient neurologic deficits related to acute disconnection were noted in five out of the 20 (25%) subjects regardless of the technique (p value=0.41). Among those who had endoscopy, one patient had transient decreased speech, one had left upper extremity weakness, and one developed unsteadiness. Among those who had craniotomy, one had transient gait instability and another had decreased speech. All of the aforementioned symptoms subsided within four weeks following the operation. Permanent neurologic deficits were noted in two patients following callosotomy via the craniotomy. A subject with SCN1A gene mutation and a diagnosis of Dravet syndrome became non-ambulatory. Another subject, preoperatively suspected of having a neurodegenerative disorder, had a sustaining and progressive memory decline following surgery. Medical complications were seen in the craniotomy group. One child developed pneumonia and one subject with Down syndrome developed atelectasis post-operatively.
Discussion
Our findings demonstrated that corpus callosotomy is an effective treatment option for intractable generalized seizures leading to debilitating falls (atonic, tonic, myoclonic and rapidly spreading focal seizures) since 85% of our 20 subjects had at least 50% seizure reduction and, indeed, 35% of the subjects became seizure-free. In addition, a single patient who also had epileptic spasms became free of the spasms. All subjects with GTC had at least 50% seizure-reduction following corpus callosotomy. Conversely, this surgical procedure does not appear to be as effective in dyscognitive focal seizures. In our series, dyscognitive focal seizures were not ameliorated by corpus callosotomy; they either persisted or even increased in frequency. Indeed, one patient with generalized seizures developed new focal seizures.
The present series highlights the novel approach of endoscopic anterior and posterior interhemispheric corpus callosotomy. A posterior endoscopic approach is particularly useful if the patient has a high-riding falx or deficient falx anteriorly. It is one of the authors’ experience (SS) that patients with brain malformations often have a deficient falx anteriorly or a high-riding falx making the dissection of the corpus callosum more difficult as the gyri of each hemisphere may interdigitate across the midline into the other hemisphere. The endoscopic approach offers an advantage of minimal incision and smaller craniotomy and fewer post-operative complications. Complications are rare and are usually transient.9,10 In this study, the endoscopic approach was as effective as the transcranial approach in seizure control.
Seizure outcome in our patients is similar to previously reported seizure outcomes of corpus callosotomy in children, as summarized in the recent meta-analysis performed by Graham et al.12 Furthermore, among our 8 subjects with active VNS prior to corpus callosotomy, 7 had at least 50% seizure reduction and one became seizure-free. It was unclear if it was the combination of VNS and corpus callosotomy in these patients that contributed to their seizure reduction. It has, however, been demonstrated that corpus callosotomy is more effective than VNS in the reduction of tonic and atonic seizures in LGS and is more likely to provide atonic seizure-freedom.13
Since all except two subjects had a complete corpus callosotomy, comparison of the efficacy between anterior 2/3 and complete corpus callosotomy was not feasible due to the small sample size. However, several studies have suggested that complete corpus callosotomy is more effective and its efficacy is sustainable with less relapse rates compared to anterior corpus callosotomy14–17. In two of our subjects, anterior 2/3 corpus callosotomy was followed by complete corpus callosotomy after 5 and 7 years, respectively, and one of them had significant improvement after complete corpus callosotomy whereas no further improvement was noted with the second subject. Our series also suggested that anterior commisurotomy does not offer additional advantage of improvement in seizure outcome.
In our series, we noted that persistence of spike-and-wave discharges on intraoperative scalp EEG post-corpus callosotomy did not necessarily predict a poor seizure outcome. Similar to the findings of Kwan et al.18, our subjects’ immediate post-op EEG findings did not predict their surgical outcome. Fiol et al19 however demonstrated that the degree of lateralization of generalized epileptiform discharges did not correlate with the degree of reduction of tonic-atonic seizures.
Transient neurological deficits relate to acute disconnection occurred in 25% of the patients. One subject with SCN1A mutation and clinical features of Dravet syndrome became non-ambulatory. It is possible that his gait dysfunction was related to his underlying neurologic syndrome rather than due to the surgical disconnection, although the acuteness of gait decline following surgery makes this less likely. It would be of interest to see how other patients with SCN1A mutation have fared following corpus callosotomy.
Limitations
An important limitation of our study is the small number of subjects included and short follow up period (<1 year in four subjects). Although assessment of reduction of seizure leading to falls by parents are reliable, the assessment of reduction of other seizure types with milder manifestations, such as myoclonic and atypical absence may be difficult to ascertain by the caregivers. Tonic seizures that might have occurred during sleep may also be missed. We were not able to differentiate the outcome between subjects who had complete or anterior 2/3 corpus callosotomy due to the limited number of subjects who had anterior 2/3 corpus callosotomy. We were not able to perform neuropsychological testing in all our subjects and, therefore, the neurocognitive effect of the procedure was not adequately assessed.
Conclusion
Our study provides additional support for the efficacy of corpus callosotomy as a palliative treatment approach for children with intractable generalized seizures, particularly in those with seizures leading to falls. Further, patients who have failed to respond to VNS may respond well to subsequent corpus callosotomy. Surgical complications were rare and acute disconnection related deficits were transient in most cases. Finally, we demonstrated that corpus callosotomy can be effectively done using an endoscopic approach.
Acknowledgments
Funding
This work was supported by NIH Grants NS064033 (EA) and RO1 NS064989 (HTC).
We thank the faculties, staff and EEG technologists of the Pediatric Epilepsy Program and EEG department of Children’s Hospital of Michigan.
Footnotes
Declaration of Conflicts of Interests
The authors declare no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions
AFL contributed in the patient care, collection and analysis of data, writing of the initial manuscript draft, manuscript revision and writing. EA contributed in the data collection and analysis and critical manuscript revisions and writing. HTC contributed to patient care and selection for surgery and in the critical manuscript revisions and writing. AK performed data analysis and provided figures. SS performed the surgeries, contributed to data collection and in the critical manuscript revisions and writing.
Ethical approval
The study was approved by the Wayne State University Human Investigation Committee and written informed consent was obtained from all patients/guardians in compliance with the committee’s regulations.
Reference List
- 1.Ilyas M, Sivaswamy L, Asano E, Sood S, Zidan M, Chugani H. Seizure control following palliative resective surgery for intractable epilepsy-a pilot study. Pediatr Neurol. 2014;51(3):330–335. doi: 10.1016/j.pediatrneurol.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Asadi-Pooya AA, Sharan A, Nei M, Sperling MR. Corpus callosotomy. Epilepsy & behavior : E&B. 2008;13(2):271–278. doi: 10.1016/j.yebeh.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Food and Drug Administration (FDA) [Accessed August 13, 2016]; Available at: https://www.accessdata.fda.gov/scripts/cdrh/devicesatfda/index.cfm.
- 4.American Epilepsy Society. [Accessed August 13, 2016]; Available at: https://www.aesnet.org/annual_meeting/abstract_search.
- 5.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010;51(4):676–685. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 6.Rajpurkar M, Callaghan M, Frey MJ, Set K, Chugani H, Sood S. Management of intracranial surgery for refractory epilepsy in severe factor VII deficiency: choosing the optimal dosing regimen. Haemophilia. 2014;20(3):e234–237. doi: 10.1111/hae.12397. [DOI] [PubMed] [Google Scholar]
- 7.Asano E, Benedek K, Shah A, et al. Is intraoperative electrocorticography reliable in children with intractable neocortical epilepsy? Epilepsia. 2004;45(9):1091–1099. doi: 10.1111/j.0013-9580.2004.65803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kumar A, Juhasz C, Asano E, Sood S, Muzik O, Chugani HT. Objective detection of epileptic foci by 18F-FDG PET in children undergoing epilepsy surgery. J Nucl Med. 2010;51(12):1901–1907. doi: 10.2967/jnumed.110.075390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sood S, Marupudi NI, Asano E, Haridas A, Ham SD. Endoscopic corpus callosotomy and hemispherotomy. J Neurosurg Pediatr. 2015;16(6):681–686. doi: 10.3171/2015.5.PEDS1531. [DOI] [PubMed] [Google Scholar]
- 10.Sood S, Asano E, Altinok D, Luat A. Endoscopic posterior interhemispheric complete corpus callosotomy. J Neurosurg Pediatr. 2016:1–4. doi: 10.3171/2016.6.PEDS16131. [DOI] [PubMed] [Google Scholar]
- 11.McHugh JC, Singh HW, Phillips J, Murphy K, Doherty CP, Delanty N. Outcome measurement after vagal nerve stimulation therapy: proposal of a new classification. Epilepsia. 2007;48(2):375–378. doi: 10.1111/j.1528-1167.2006.00931.x. [DOI] [PubMed] [Google Scholar]
- 12.Graham D, Tisdall MM, Gill D. Corpus callosotomy outcomes in pediatric patients: A systematic review. Epilepsia. 2016;57(7):1053–1068. doi: 10.1111/epi.13408. [DOI] [PubMed] [Google Scholar]
- 13.Lancman G, Virk M, Shao H, et al. Vagus nerve stimulation vs. corpus callosotomy in the treatment of Lennox-Gastaut syndrome: a meta-analysis. Seizure. 2013;22(1):3–8. doi: 10.1016/j.seizure.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunaga S, Shimizu H, Sugano H. Long-term follow-up of seizure outcomes after corpus callosotomy. Seizure. 2009;18(2):124–128. doi: 10.1016/j.seizure.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Maehara T, Shimizu H. Surgical outcome of corpus callosotomy in patients with drop attacks. Epilepsia. 2001;42(1):67–71. doi: 10.1046/j.1528-1157.2001.081422.x. [DOI] [PubMed] [Google Scholar]
- 16.Pinard JM, Delalande O, Chiron C, et al. Callosotomy for epilepsy after West syndrome. Epilepsia. 1999;40(12):1727–1734. doi: 10.1111/j.1528-1157.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 17.Kawai K, Shimizu H, Yagishita A, Maehara T, Tamagawa K. Clinical outcomes after corpus callosotomy in patients with bihemispheric malformations of cortical development. Journal of neurosurgery. 2004;101(1 Suppl):7–15. doi: 10.3171/ped.2004.101.2.0007. [DOI] [PubMed] [Google Scholar]
- 18.Kwan SY, Lin JH, Wong TT, Chang KP, Yiu CH. Prognostic value of electrocorticography findings during callosotomy in children with Lennox-Gastaut syndrome. Seizure. 2005;14(7):470–475. doi: 10.1016/j.seizure.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 19.Fiol ME, Gates JR, Mireles R, Maxwell RE, Erickson DM. Value of intraoperative EEG changes during corpus callosotomy in predicting surgical results. Epilepsia. 1993;34(1):74–78. doi: 10.1111/j.1528-1157.1993.tb02378.x. [DOI] [PubMed] [Google Scholar]


