Abstract
Alzheimer's disease (AD) is a type of dementia which is known as one of a major problem in elderly. Clinicians commonly use mini-mental state examination (MMSE) score to determine the severity of cognitive decline, but MMSE has some limitations such as more subjective, influenced by age, educational degree, and local culture. F-18 fluorodeoxyglucose positron emission tomography (F-18 FDG PET) can be used to assess the process of glucose metabolism in posterior cingulate cortex (PCC) area which endures a central role in supporting cognitive function directly. The purpose of this study is to observe a correlation between metabolic activity value of PCC and MMSE score in predicting the severity of AD. A cross-sectional study was done to 30 subjects suspect AD disease with aged 60 years and older. Characteristic data including gender, age, and education, MMSE scoring by psychiatrist, and imaging of F-18 FDG PET were established. The results of correlation test between the value of FDG metabolic activity and MMSE score shows that the value of metabolic activity in the PCC area tends to increase along with the increase of MMSE score (rs = 0.411, P = 0.024). While from the results of multiple regression test with predictor variable consisting of F-18 FDG metabolic activity in the PCC, gender, age, education level, and the interaction between the metabolic activity of F-18 FDG at PCC and gender, a regression model was obtained. There is a significant correlation observed between the captured of F-18 FDG radioactivity with MMSE score in PCC area which can be used as a tool to predict the severity of AD.
Keywords: Alzheimer's disease, F-18 fluorodeoxyglucose positron emission tomography, mini-mental state examination, posterior cingulate cortex
Introduction
Dementia is a group of clinical manifestation from several kinds of diseases. Clinical manifestation could be loss of memory, mental disturbance, and other cognitive impairment, but without any consciousness disorder.[1] Dementia becomes the main health problem in the elderly population, particularly in the age of 60 years or more. According to WHO report on 2001, it was estimated that 24.3 million people over the world were suffering from dementia. Sixty percent of them were in developing countries.[2]
The causes of dementia are classified into cerebro-organic (primary) and noncerebro-organic (secondary). Ninety percent of cerebro-organic dementia caused by neurodegenerative impairment, vascular impairment, or both of them. On the other hand, noncerebro-organic dementia caused by an exaggerate alcohol consumption and impairment in metabolic or encephalitis. Some abnormalities that lead to neurodegenerative dementia are Alzheimer disease (AD), frontotemporal degeneration, corticobasal degeneration, Lewy-Bodies Dementia, Parkinson disease, and Huntington disease. AD is in the first rank (around 60–80%). AD is a progressive neurodegenerative disease with cognitive and episodic memory impairment accompanied by neuropathology.[2,3]
It is difficult to distinguish between AD and other types of dementia as their symptoms overlap each other. There are three common clinical approaches conducted to diagnose AD. Those are based on the criteria of The Diagnostic and Statistical Manual of Mental Disorders 4th edition (DSM-IV-TR), The International Classification of Disease 10th revision (ICD-10), and The National Institute of Neurological and Communicative Disorders and Stroke-AD and Related Disorders Association.[4,5] However, the result of those clinical diagnosis is not definite yet to confine the probability of whether dementia or not. The definite diagnosis of AD is histopathology examination, but this procedure is a high-risk. Sometimes, it diagnosed in postmortem examination (autopsy).[6,7]
Clinicians usually use the mini-mental state examination (MMSE) score to determine the severity of cognitive impairment. This test is quite simple. It consists of eleven questions and only need 5–15 min to complete the test.[8] Nevertheless, MMSE has some limitations such as more subjective, influenced by age, educational degree, and local culture.[8,9]
Noninvasive functional imaging to diagnose AD with sensitivity and accuracy are closed enough to autopsy is positron emission tomography (PET) imaging using F-18 fluorodeoxyglucose (F-18 FDG).[6,7,10,11] The principle of this imaging technique is identify the existence of hypometabolic area in the brain, mainly on posterior cingulate cortex (PCC). Hypometabolic region can be observed too in temporoparietal and frontal cortex. Determination of hypometabolic region can be done either qualitatively or quantitatively.[10,11,12,13,14]
The aim of this study was to determine the correlation between F-18 FDG metabolic activity in PCC area and MMSE score in predicting the severity of dementia in a patient with AD.[15,16]
Subjects and Methods
A cross-sectional study was done on following approved by the local Research Ethics Committee of Health. This study was performed on October 2014 until May 2015. Subjects were patients with suspected of having AD based on ICD-10 and DSM IV. F-18 FDG PET brain imaging was done at our Nuclear Medicine Department and PET Center.
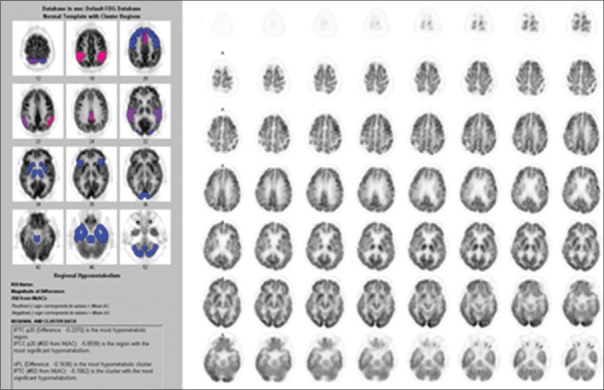
The diagnosis of AD based on F-18 FDG PET brain imaging was made when hypometabolic activity was found in PCC area with or without hypometabolic activity in temporoparietal or frontal cortices. Subjects with hypertension, history of other brain disorder such as stroke, head trauma, and malignancy were excluded from this study. The analysis of F-18 FDG PET brain imaging was done by nuclear medicine physician. Quantitative analysis region of interest on PCC area is done automatically using NeuroQ software version 3.0 (Philips Medical Systems, Ohio, USA) [Figure 1]. Cutoff for metabolic activity was more than − 1.65 standard deviation.[17]
Figure 1.
This pattern is most consistent with metabolically middle-stage Alzheimer's-type changes occurring in this patient's brain. Region of interest on posterior cingulate cortex area is done automatically using NeuroQ software version 3.0
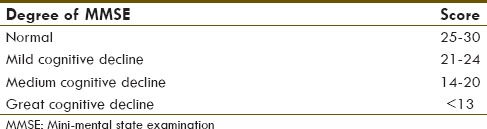
All of the subjects that fulfilled the inclusion criteria were proceed to MMSE examination by psychiatrist using MMSE 1–30 method.[18] MMSE score was divided into four categories [Table 1]. Statistical analysis was done to examine the correlation between F-18 FDG PET brain and MMSE score. The test was considered to be significant if the P ≤ 0.05.
Table 1.
Subject categories based on mini-mental state examination score
Results
Thirty subjects with average age 72.1 ± 8.1-year-old were included in this study. Characteristics of the subjects were shown in Table 2. The subject is mostly male with a comparison of male to female were 2:1. Subjects with high school degree were 76.7% and university degree were 23.3%.
Table 2.
Characteristics of subject (n=30)

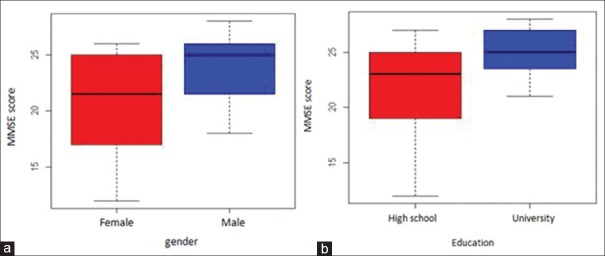
Further analysis on MMSE score based on gender revealed that male's score was higher than those of female's [Figure 2a], median MMSE score of subject with high school degree were lower than university degree [Figure 2b], and result of correlation study between MMSE score and age revealed that MMSE score prone to decline along with increase age [Figure 3]. Regarding that gender, education degree, and age are thus will be considered as a predictor variable in the next analysis.
Figure 2.
Mini-mental state examination score distribution based on gender (a) and education degree (b)
Figure 3.
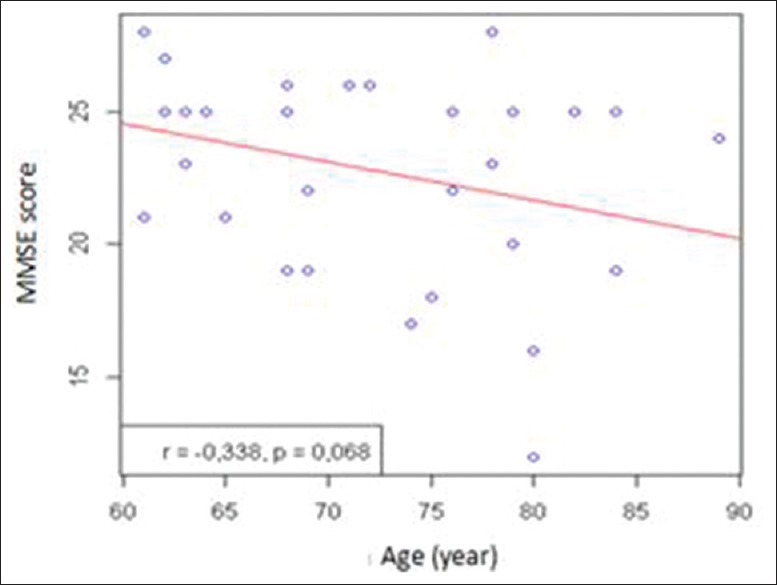
Correlation between mini-mental state examination score and age
It was also observed that metabolic activity in PCC area tends to increase along with the increase of MMSE score (rs = 0 411, P = 0.024) [Figure 4].
Figure 4.
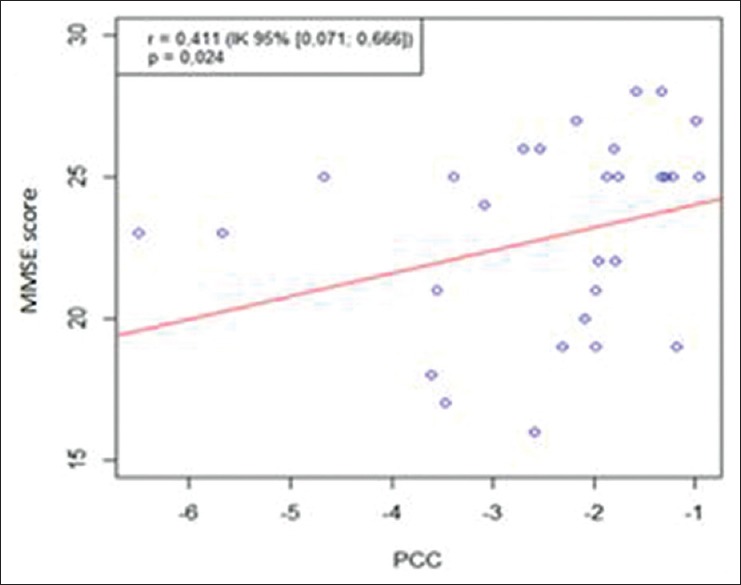
Correlation between fluorodeoxyglucose positron emission tomography with mini-mental state examination score in posterior cingulate cortex area
From Figure 4, it is revealed that even the PCC activity score and MMSE score have a correlation, but there are several parameters that might be confound it. They are gender, education degree, and age factor. Hence, we had done further analysis using multiple linear regression.
Result from multiple linear regression analysis in which the predictor variable consist of brain FDG-PET metabolic activity in PCC, gender, education degree, age, and interaction between brain FDG-PET metabolic activity and gender are known be able to decrease MMSE score which square variability up to 51.9% [Table 3].
Table 3.
Estimation of parameter and error standard of multiple linear regression
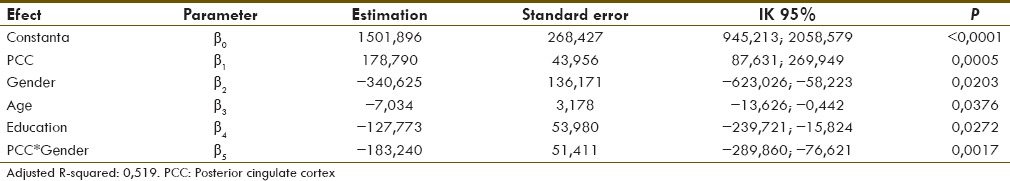
Based on multiple linear regression model above, the following formula was obtained:
MMSE2 = 1,501,896 + 178,790 × PCC – 340,625 × Gender – 7034 × Age – 127,773 × Education – 183,240 × PCC × Gender
Discussion
This study showed a correlation between glucose metabolic activity in PCC and MMSE score. The score of metabolic activity in PCC area tends to increase along with the increase of MMSE score. Decreased metabolism rate in PCC may result in clinically declined cognitive function measured by MMSE. Cognitive function decrease that we can measured by MMSE score, seems to be the result of metabolic decrease in PCC. Previous study showed that modality of functional imaging such as PET had potential in identifying any changes in the pathological pathway of AD compared to structural imaging (computed tomography or magnetic resonance imaging). PET imaging used to assess metabolic activity has been developed since 1970. PET provides breakthrough in understanding brain metabolism and the function of neurotransmitter both in health and disease including in AD. FDG is the most often used radiopharmaceuticals for PET brain imaging since it can reflect the degree of glucose metabolism in brain.[10,11,12,19,20]
The result also revealed that metabolic activity decrease in PCC area was much higher than those in temporoparietal and frontal cortices area. This result was coherent with some previous studies which mentioned that PCC area is a region that endures a declining in patient suffered from early stadium of AD. The decreased metabolic activity in PCC area was significantly much greater compared to other area of brain cortices.[12,21,22,23]
The severity of cognitive declining that measured by MMSE score could be proven by the decreased metabolic activity in brain. The scoring system of that cognitive function itself becomes one of the parameters to withstand AD diagnosis. Folstein et al., on 1973, used MMSE as a helpful tool in examining the cognitive function, but not directly helpful in cases of diagnosed AD.[8,21]
Moreover, this study showed that the MMSE score results were not only influenced by the decreased metabolism in PCC but also by the variation of age, gender, and education degree. It was matched with several studies' results conveying that MMSE score on Alzheimer patients was not only influenced by age and education but also by gender. Female gender has been known to be a risk factor in dementia including AD. Previous studies showed that MMSE score declines along with the increase of age. The reason of that is somehow because declining cognitive function comes along with increasing age. On the other hand, the influence of educational degree is probably because of that direct interviews between clinicians and the patients are often subjective and will surely be influenced by the communication skill of both sides. The influence of gender is suspected emerging from the difference of brain cortex structure between both gender, in which the size of brain, the number, and density of neurons are greater in men compared to those in female. Consequently, men are more susceptible to neurodegeneration. According to the study conducted by Pradier et al., the life expectancy of women is higher than of men. This fact would possibly explain why the incidence of AD is higher in female.[9,24,25,26,27]
Linear regression analysis was conducted to observe how far a formula to predict the MMSE score based on FDG activity in PCC area could be created considering the age, education degree, and gender. From this analysis, the total square of MMSE score variability can be pressed up to 51.9%. It meant that brain F-18 FDG-PET could be used for measuring F-18 FDG uptake in PCC area, as well as to predict the severity of AD.
In this study, subjects with lower than high school education level and those who younger than 60 years of age were excluded to avoid variability in MMSE score. On the other side, these methods can be limiting age and education range that can be measured by this study.
Conclusion
There is a significant correlation observed between FDG uptake in PCC area with MMSE score. FDG brain PET can be used as a tool for predicting the severity of AD.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. American Psychiatric Association; pp. 123–55. [Google Scholar]
- 2.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: A Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Camicioli R. Distinguishing different dementias. The Canadian Alzheimer Disease Review. 2004;1:1–11. [Google Scholar]
- 4.Diagnostic and Statistical Manual of Mental Disorders (IV-TR) 4th ed. Washington, DC: American Psychiatric Association; 2000. American Psychiatric Association; pp. 147–71. [Google Scholar]
- 5.Khann G, David D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer's disease report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer's disease. Neurology. 1984;34:1–8. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 6.Jellinger KA. Clinicopathological analysis of dementia disorders in the elderly – An update. J Alzheimers Dis. 2006;9(3 Suppl):61–70. doi: 10.3233/jad-2006-9s308. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman JM, Welsh-Bohmer KA, Hanson M, Crain B, Hulette C, Earl N, et al. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med. 2000;41:1920–8. [PubMed] [Google Scholar]
- 8.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”.A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 9.Pradier C, Sakarovitch C, Le Duff F, Anthony S, Layese R, Anthony S, et al. The mini mental state examination at the time of Alzheimer's disease. Vol. 09. France: Nice University Hospital; 2014. pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merissa NZ, Garrett M, Peter JH. Dementia using nuclear medicine imaging modalities. In: Gholamrezanezhad A, editor. Chapters on Nuclear Medicine. 1st ed. Rijeka Croatia: InTech; 2011. pp. 199–222. [Google Scholar]
- 11.Herholz K, Herscovitch P, Heiss WD. Neuro PET-Positron Emission Tomography in Neuroscience and Clinical Neurology Originally. Heidelberg, New York: Springer Verlag; 2004. pp. 7–31. [Google Scholar]
- 12.Minoshima S, Giordani B, Berent S, Frey KA, Foster NL, Kuhl DE. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer's disease. Ann Neurol. 1997;42:85–94. doi: 10.1002/ana.410420114. [DOI] [PubMed] [Google Scholar]
- 13.Silverman D. PET in the Evaluation of Alzheimer's Disease and Related Disorders-Springer. 2009:1–208. [Google Scholar]
- 14.Chew J, BS, Silverman D. FDG-PET in Early AD Diagnosis. Med Clin N Am. 2013;97:485–94. doi: 10.1016/j.mcna.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Valla J, Berndt JD, Gonzalez-Lima F. Energy hypometabolism in posterior cingulate cortex of Alzheimer's patients: Superficial laminar cytochrome oxidase associated with disease duration. J Neurosci. 2001;21:4923–30. doi: 10.1523/JNEUROSCI.21-13-04923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leech R, Sharp DJ. The role of the posterior cingulate cortex in cognition and disease. Brain. 2014;137(Pt 1):12–32. doi: 10.1093/brain/awt162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Syntermed, NeuroQ™, Quantitative Analysis for Neuroimaging Technology Display and Analysis Program Ver. 3.0 Inc. United States of America: Philips Medical Systems; 2009. pp. 1–64. [Google Scholar]
- 18.Reisberg B, Jamil I, Khan S, Monteiro I, Torossian C, Ferris S, et al. Staging Dementia. In: Mohammed T, editor. Principles and Practice of Geriatric Psychiatry. 3rd ed. USA: John Wiley & Sons; 2011. pp. 162–9. [Google Scholar]
- 19.Minoshima S, Foster NL, Kuhl DE. Posterior cingulate cortex in Alzheimer's disease. Lancet. 1994;344:895. doi: 10.1016/s0140-6736(94)92871-1. [DOI] [PubMed] [Google Scholar]
- 20.Herholz K. PET studies in dementia. Ann Nucl Med. 2003;17:79–89. doi: 10.1007/BF02988444. [DOI] [PubMed] [Google Scholar]
- 21.Rush J. Psychiatric Measures. Washington. DC: American Psychiatric Association; 2000. [Google Scholar]
- 22.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of Alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334:752–8. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 23.Grand J, Feldman H. Historical concepts of Alzheimer's disease and dementia. In: Feldman HH, editor. Atlas of Alzheimer's Disease and Dementia. London: Informa Healthcare; 2007. pp. 19–20. [Google Scholar]
- 24.Hendrie HC. Epidemiology of dementia and Alzheimer's disease. Am J Geriatr Psychiatry. 1998;6(2 Suppl 1):S3–18. doi: 10.1097/00019442-199821001-00002. [DOI] [PubMed] [Google Scholar]
- 25.Lin RT, Lai CL, Tai CT, Liu CK, Yen YY, Howng SL. Prevalence and subtypes of dementia in southern Taiwan: Impact of age, sex, education, and urbanization. J Neurol Sci. 1998;160:67–75. doi: 10.1016/s0022-510x(98)00225-1. [DOI] [PubMed] [Google Scholar]
- 26.Stern Y, Gurland B, Tatemichi TK, Tang MX, Wilder D, Mayeux R. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA. 1994;271:1004–10. [PubMed] [Google Scholar]
- 27.Katzman R. Education and the prevalence of dementia and Alzheimer's disease. Neurology. 1993;43:13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]