Abstract
The complex anatomy and function of the foot and ankle can make it difficult to determine the cause of symptoms in patients with foot and ankle pathology. Following initial clinical and radiographic assessment, additional imaging with magnetic resonance imaging may be required, which is often seen as the modality of choice. Although sensitive to pathological changes in bone metabolism and vascularity, technetium-99m (Tc-99m) bone scintigraphy often lacks the specificity and resolution required to evaluate the structures of the foot and ankle. Tc-99m methylene diphosphonate single-photon emission computed tomography/computed tomography (SPECT/CT) combines this sensitivity with the superior anatomical detail of CT, enabling better localization of pathological uptake and evaluation of associated structural changes. As a result, SPECT/CT has been growing in popularity for the assessment of patients with foot and ankle pathology where it can provide additional information that may change the initial diagnosis and subsequent management plan. Studies have reported modification of the surgical approach and site of intra-articular local anesthetic injections following SPECT/CT with good results. Interpretation of SPECT/CT studies requires an understanding of the pathological changes that result in increased tracer accumulation in addition to the CT changes that may be seen. This review aims to highlight the advantages of SPECT/CT, potential applications and explain the imaging appearances of common pathologies that may be observed.
Keywords: Ankle, foot, single-photon emission computed tomography/computed tomography
Introduction
Identifying the underlying cause of foot and ankle pain can be challenging due to the complex anatomy and function of the foot and ankle.[1] Conventional imaging including plain radiography, ultrasound, computed tomography (CT), and magnetic resonance imaging (MRI) is frequently required as an adjunct to clinical assessment to guide further management. Following initial radiographic assessment, MRI is often favored due to its superior soft-tissue contrast and ability to demonstrate osseous abnormalities.[2,3] Earlier studies attempted to use technetium-99m methylene diphosphonate (Tc-99m MDP) bone scintigraphy to identify pathological changes in bone metabolism related to foot and ankle pain. Although sensitive, the low spatial resolution made it difficult to accurately localize the areas of abnormal uptake and correlate findings with radiological studies.[1] Tc-99m MDP single-photon emission computed tomography/CT (SPECT/CT) overcomes this problem, allowing accurate localization of abnormal bone metabolism by fusing these data with corresponding CT images. A number of potential applications have been described in the foot and ankle, including imaging of osteoarthritis (OA), stress fractures, tarsal coalition, sesamoiditis, osteochondral lesions (OCLs), tendonitis, plantar fasciitis, and impingement syndromes.[4,5,6] As a result, there has been growing interest in Tc-99m MDP SPECT/CT with a number of studies evaluating the impact of SPECT/CT on the investigation and management of foot and ankle pain.[7,8,9,10,11,12] The purpose of this review is to highlight the advantages of this modality and describe the appearances of common pathologies that may be encountered when performing Tc-99m MDP SPECT/CT in patients with foot and ankle pain.
Added Value Of Single-photon Emission Computed Tomography/Computed Tomography
Many studies have reported the advantages of SPECT/CT over planar or SPECT imaging with either more accurate localization or additional sites of uptake identified [Table 1].[7,8,10] In a small group of 25 patients, Mohan et al. found that the SPECT/CT study provided additional information compared to planar scintigraphy in 20/25 (80%) patients, with more accurate pathology localization in 16/25 (64%) and new abnormalities in 10/25 (40%) patients.[7] Similarly, Gnanasegaran et al. reported additional information from the SPECT/CT study in 25/31 (81%) patients with foot and ankle pathology.[8] In a cohort of patients with impingement syndromes and soft-tissue pathology, improvement in localization and characterization of uptake with SPECT/CT was reported in 76% (31/43) patients when compared to clinical assessment and conventional two-phase bone scan.[10]
Table 1.
Added value of single-photon emission computed tomography/computed tomography
Additional information derived from SPECT/CT may lead to revision of the initial diagnosis and subsequent change to the management plan [Table 1].[6,7,8,9,10,11,12,16,17] Mohan et al. reported that SPECT/CT influenced management in 10/19 (53%) patients in whom follow-up data were available.[7] Similarly, Gnanasegaran et al. reported a change in management in 62% of patients based on additional diagnostic information provided by SPECT/CT.[8] More recently, a study of 52 patients with degenerative joint disease of the foot and ankle suggested a difference in the site responsible for the patient's symptoms in 23 (44%) patients following SPECT/CT when compared to initial assessment based on history, examination, and radiographs. Following input from orthopedic surgeons, the site of intra-articular injection was modified in 19/23 cases based on the SPECT/CT findings.[16] A larger discrepancy between SPECT/CT and the initial diagnosis based on clinical examination and plain radiography was reported by Singh et al. in 39/50 (78%) cases. Although the cohort was limited to patients where there was continued diagnostic uncertainty following initial evaluation, the study still highlights the added value of SPECT/CT as a problem-solving tool in these patients. Most of the cases where there was disagreement between initial assessment and SPECT/CT were related to difficulties identifying osteoarthritic joints within the mid-foot on initial evaluation.[11] This has also been reported by other authors.[9,12] Kretzschmar et al. reported disagreement between clinical assessment and SPECT/CT findings in 16/30 (53%) cases. Of these cases, disagreement was recorded in all 9 patients with mid-food symptoms compared to only 7/21 (33%) patients with hindfoot pathology.[9] Similarly, Claassen et al. found that changes in diagnoses and symptomatic improvement following SPECT/CT-guided treatment were more frequent in the more complex Chopart and Lisfranc joints when a subgroup analysis was performed for different regions of the foot and ankle.[12]
Single-photon Emission Computed Tomography/Computed Tomography Guided Local Anesthetic Injections
The complex anatomy of the foot and ankle can make it challenging to localize the source of symptoms.[18] Local anesthetic injections have long been used as a diagnostic tool to help identify the source of symptoms in patients with foot and ankle pain and differentiate between pain arising from an articulation and the adjacent soft tissues. Accurate localization of symptoms is important to help guide subsequent management including arthrodesis of the symptomatic arthritic joint.[18,19,20,21] Poor correlation between radiographic and CT findings, and response to local anesthetic injection, has been reported. This emphasizes the difficultly in determining the source of symptoms when relying on radiological changes alone.[18,19]
A number of studies have demonstrated significant symptomatic improvement when local anesthetic injection has been directed by SPECT-CT findings.[9,11,16] In a study of thirty patients with chronic foot pain, 27 (90%) reported reduction in pain intensity of 50% or more immediately post-CT-guided local anesthetic injection by targeting the most active SPECT/CT structures. Interestingly, when cases were subdivided, there were higher response rates in 25/26 (96%) cases where uptake and injections were centered on an articulation compared to 2/4 cases where uptake was centered on bone and the periosseous soft tissues were infiltrated. Both the patients who failed to respond to periosseous injection demonstrated bone-centered uptake after calcaneal osteotomies. The authors suggested that the osseous uptake may have been an asymptomatic postsurgical finding, which could have explained the poor response to treatment.[9] A similar improvement in symptoms was also reported in a subset of 25/26 (96%) patients from a larger study of fifty patients who received SPECT/CT-guided local anesthetic and steroid intra-articular injection.[11] More recently, a study consisting of patients with degenerative joint disease also described symptomatic improvement, with an improvement in visual analog score (VAS) of at least 50% in 43/48 (90%) patients where injection was performed for the most avid joints depicted by SPECT/CT.[16] In four cases where the clinician disagreed with the SPECT/CT findings, local anesthetic injection was injected into a joint, different to that indicated by SPECT/CT. Three of these four patients also responded to the injection.[16]
Fluoroscopic guidance is commonly used to guide intra-articular local anesthetic and steroid injections. However, this can prove difficult when there is significant anatomical distortion following previous fracture or OA.[19,22,23] CT guidance provides a useful alternative in these difficult cases with a high success rate described.[23] SPECT-CT offers the advantage of providing a road map of the joint to be injected, highlighting any potential difficulty which may favor the use of CT over fluoroscopic guidance. False-positive results may occur from communications between joints of the foot[24] which could lead to spreading of intra-articular steroid and local anesthetic to other joints. Therefore, addition of a contrast medium may help identify the spread of the injectate and visualize communication between joints which may affect results.
The reported response rates for SPECT/CT-guided local anesthetic injection were higher (90%–96%) when compared to a previous study (57%) where CT-guided injections were performed for patients referred from a foot and ankle clinic presumably following initial clinical and radiographic assessment before consideration of arthrodesis.[23] Based on a successful response to SPECT/CT-guided treatment in 96% of patients, Singh et al. discussed the potential use of SPECT/CT as a replacement for arthrographic injection. However, the authors highlighted the therapeutic benefit in 15/26 patients who had improvement in symptoms following the local anesthetic and steroid injection without a need for further intervention. The total length of follow-up was not specified for this group of patients.[11]
Technetium-99m-labeled Diphosphonate Uptake Mechanism
Bone is composed of cells held within a matrix comprising organic and inorganic components. Osteoblasts secrete osteoid which forms the organic matrix that is later mineralized by hydroxyapatite crystals.[25] Following intravenous administration, Tc-99m-labeled diphosphonates bind to hydroxyapatite crystals by chemisorption which depends on regional blood flow and osteoblastic activity.[26,27] Tc-99m-labeled diphosphonate uptake, therefore, serves as a marker of pathological changes in vascularity and osteoblastic activity.
Osteoarthritis
Previous trauma is the most common cause of ankle OA, affecting a younger subgroup of patients compared to primary ankle OA.[28] Radiographic changes of OA are well documented and comprise four key features: loss of joint space, subchondral cysts, osteophytes, and subchondral sclerosis.[29] CT enables tomographic assessment which can aid visualization of these changes.[30]
Cartilage thickness and therefore joint space reduces with age. This occurs due to wear of collagen fibers within the cartilage and abnormal hydrophilic proteoglycan molecule formation by chondrocytes, which prevents retention of water molecules within cartilage with subsequent loss of chondral thickness. When the articular cartilage is disrupted, fissures can form which may permit passage of joint fluid into subchondral bone under pressure and result in subchondral cysts evident on imaging.[31] Osteophytes result from endochondral ossification of cartilaginous proliferation at the bone – articular cartilage interface of a joint in response to joint instability.[31,32] Radiologically evident subchondral sclerosis may be present due to bone remodeling in response to changes in mechanical stress as described by Wolff's law.[33] It has also been suggested that increased bone formation may be an important feature in the pathophysiology of OA based on the association between increased bone mineral density and osteophytosis.[15,34]
Remodeling of cartilage and bone at the cellular level with osteoblast activation are some of the earliest features of OA.[32] Subchondral osteoblasts have an important role in the progression of OA[32,34] with increased uptake reported to be predictive of joint space and therefore cartilage loss.[35] Increased levels of technetium-labeled diphosphonate uptake have also been shown to correlate with the severity of radiographic changes.[36]
More recently, Paul et al. correlated the SPECT/CT and histologic findings in patients with end-stage ankle OA.[37] Abnormal bone remodeling in OA leads to an abundance of unmineralized osteoid and collagen.[34,38] These pathological changes in subchondral bone were found in areas of increased Tc-99m 3,3-diphosphono-1,2-propanodicarboxylicacid (DPD) uptake on SPECT/CT, in which active osteoblasts and increased randomly organized collagen fibers were observed.[37]
SPECT/CT is increasingly used to assess degenerative changes in the foot and ankle given the added anatomical information and improved uptake localization.
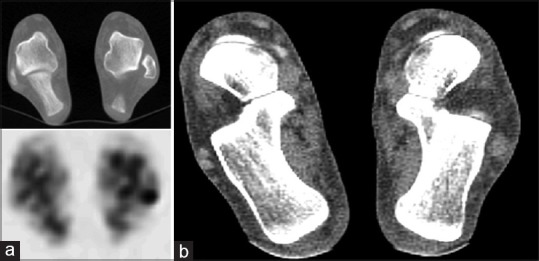
Pagenstert et al. found that mean interobserver and intraobserver reliability was significantly higher for the assessment of degenerative joint disease with SPECT/CT than CT alone, bone scintigraphy, or CT and bone scintigraphy in combination. When subgrouped according to anatomical regions of the foot, a significant improvement in interobserver reliability was only described for SPECT/CT when compared to the combined use of CT and bone scintigraphy for the naviculocuneiform and tarsometatarsal joints, supporting its use for more complex joints where anatomical localization may prove difficult [Figure 1]. The authors also evaluated differences in intraobserver reliability for different levels of training, including a musculoskeletal radiologist, orthopedic consultants, and residents. In contrast to the other methods of uptake localization, a significant difference in intraobserver reliability was not present between radiologists and orthopedic residents for SPECT/CT, suggesting the modality lends itself well to less experienced readers.[13]
Figure 1.
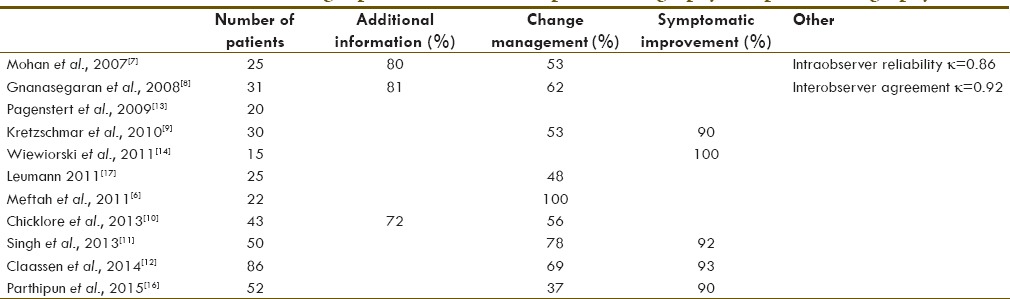
Moderate degenerative change of the right naviculocuneiform joint in a 66-year-old female. Axial computed tomography and fused technetium-99m methylene diphosphonate single-photon emission computed tomography/computed tomography images show subchondral sclerosis with mild irregularity of the joint margins and corresponding increased uptake of tracer at the articulation between the navicular and intermediate cuneiform
The correlation between pain and increased uptake of 99mTc-labeled diphosphonates has long been described.[39] Kim et al. observed a correlation between uptake of 99mTc-MDP and knee symptoms, as measured by VAS and Western Ontario and McMaster Universities Arthritis Index score in a small group of thirty patients with knee OA.[40] An association between pain and pattern of uptake was also described by McCrae et al. in a group of 100 patients with knee OA with a generalized pattern of uptake found to correlate with pain (odds ratio 45.1).[36]
More recently, SPECT/CT has been used to guide intra-articular injections in the foot and ankle with improvement in VAS reported in 90%–96% of patients.[9,11,16]
Alignment
SPECT/CT has also been used to investigate the relationship between Tc-99m DPD uptake and hindfoot alignment. Although the degree of malalignment did not correlate with uptake on conventional planar scintigraphy, Knupp et al observed a relationship between alignment and ankle zone uptake measured by SPECT-CT. Significantly increased medial zone uptake and lateral zone uptake was evident in varus and valgus deformities, respectively, suggesting the ability of SPECT-CT to identify pathologically increased metabolism in response to eccentric loading of the malaligned ankle.[41]
Osteochondral Lesions
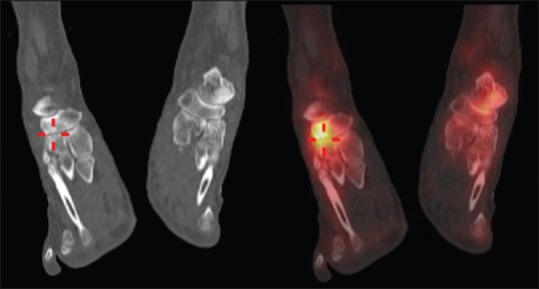
In the ankle, the term OCL is used to describe lesions involving articular cartilage and the subchondral bone of the talus or tibial plafond.[42] Although several etiologies have been suggested, a previous history of trauma is often present.[43] Two main lesion patterns have been described involving the anterosuperior aspect of the lateral talar dome and the posterosuperior aspect of the medial talar dome.[43] Based on the radiographic appearances, Berndt and Hardy initially described a four-stage classification of OCLs: (1) compression injury, (2) avulsion of an undisplaced chip of bone, (3) detached but undisplaced lesion, and (4) detached and displaced fragment.[44] Although useful for initial assessment, OCLs can be radiographically occult with only 50% of OCLs identified in a previous study.[45] Early studies have emphasized the added value of bone scintigraphy to improve sensitivity and CT to improve localization and evaluation of OCLs.[45,46] When assessing OCLs with CT, Loomer et al. found a radiolucent fibrous lesion in 77% of cases which led them to propose an additional 5th grade to be incorporated into the initial classification described by Berndt and Harty.[45] MRI is often favored for the assessment of OCLs with the added advantage of assessing the integrity of the overlying cartilage and stability of the lesion based on the presence of circumferential high T2 signal.[47,48] More recently, studies have compared SPECT/CT and MRI for the assessment of OCLs. Meftah et al. reviewed MRI and SPECT/CT imaging in 22 patients with OCLs to determine the added value of SPECT/CT. Although MRI confirmed the presence of OCLs, SPECT/CT was felt to aid the decision to proceed to surgery and have a significant impact on the surgical approach. In particular, SPECT/CT improved preoperative planning by establishing the most active foci to be targeted in large or multiple lesions. In addition, SPECT/CT was felt to demonstrate the depth of lesions more accurately than the bone marrow signal changes present on the corresponding MR studies. Ten patients were managed conservatively as SPECT/CT demonstrated minimal or absent OCL uptake, or a separate focus of uptake despite the presence of OCLs or subchondral edema on MRI. In the four cases where a separate focus of uptake was evident on SPECT/CT, the MRI demonstrated no corresponding abnormality. The authors also described the benefits of SPECT/CT for evaluating early OCLs where pronounced SPECT/CT activity was observed in the presence of minimal subchondral edema on MRI.[6] The impact of SPECT/CT and MRI on the management of chronic OCLs has also been evaluated. When compared to MRI alone, SPECT/CT resulted in a change to the treatment plan in 48% of cases. This increased to 52% when MRI and SPECT/CT findings were evaluated together compared to MRI alone. Interestingly, the only good correlation between modalities was for the assessment of articular cartilage. Interpretation of other features of the OCL including the subchondral bone plate demonstrated substantial variation between modalities.[17] Similarly, discrepancies were also noted between the area of increased tracer uptake and bone marrow edema, a finding also described by other authors [Figure 2a and b].[6,49] The authors suggested that the poor intraobserver correlation highlighted the differences in information provided by both modalities, supported by the change in treatment decision when interpreting both studies in combination.
Figure 2.
Lateral talar dome osteochondral lesion in a 32-year-old male. (a) Coronal fused technetium-99m methylene diphosphonate single-photon emission computed tomography/computed tomography image demonstrates a subchondral lucency in keeping with Type 5 lesion as described by Loomer et al. with surrounding increased uptake of tracer. The subchondral bone plate appears intact. (b) Coronal short tau inversion recovery image demonstrates a subchondral abnormality which correlates with the site of the lucency seen on the corresponding single-photon emission computed tomography/computed tomography image. Note the discrepancy between the area of increased uptake on (a) single-photon emission computed tomography/computed tomography and high signal marrow edema on the (b) magnetic resonance imaging
SPECT/CT has also been used to evaluate patients following osteochondral autologous transplantation and revision procedures for the treatment of OCLs.[50,51] The authors described the use of SPECT/CT to assess the disruption of the subchondral plate, cyst formation, osseous bridging, and uptake surrounding the graft. Increased uptake was observed due to increased remodeling related to failed osseous integration[14] or in the setting of subchondral bone plate instability.[50]
More recently, SPECT/CT has been used to direct CT-guided local anesthetic injections in symptomatic patients demonstrating OCL uptake with all patients experiencing immediate improvement in symptoms (≥50% reduction in VAS) postprocedurely.[14]
Stress Fractures
Stress fractures occur more commonly in the lower extremity in seemingly normal bone subjected to unaccustomed stress and physical activity.[52]
Many classifications for stress fractures have been described. Wilson and Katz described 4 radiographic patterns: Type I, only fracture line demonstrable; Type II, focal sclerosis with endosteal callus; Type III, periosteal reaction and external callus; and Type IV, mixed combinations of the above.[52]99mTc bone scintigraphy allows visualization of early physiological changes enabling stress fractures to be identified weeks before radiographic changes.[53] Initially, when the fracture is 2–4 weeks old, increased uptake on the early and blood pool phases may also be seen which is likely to represent acute inflammation and angiogenesis related to trauma and subsequent healing.[54] Tracer accumulation has also been demonstrated in areas of increased stress and bone remodeling in the absence of a fracture.[53] In a recent study comparing imaging appearances of early tibial stress injuries, Gaeta et al. found MRI (88%) to be more sensitive than CT (42%) and bone scintigraphy (74%). However, CT was able to better evaluate cortical osteopenia related to early cortical bone fatigue.[55] The combination of metabolic information in addition to the structural detail to depict features such as cortical osteopenia may make SPECT/CT a useful modality to evaluate early tibial stress injury. Although sensitive, planar scintigraphy lacks specificity[56] and has demonstrated false-positive uptake in the lower extremities of asymptomatic young athletes.[57] This may be less problematic for SPECT/CT with the CT component, allowing assessment of any coexisting structural abnormality and improving uptake localization.
As Wolff's law describes, changes to internal architecture and configuration occur in response to changes in mechanical stress. The histologic changes in response to bone stress in an animal model have been described in detail by Li et al.[58] Initial changes include congestion and dilatation of the Haversian canal vasculature followed by osteoclastic resorption and cavity formation, findings which correlate with the early cortical changes on CT and MRI described by Gaeta et al.[55]
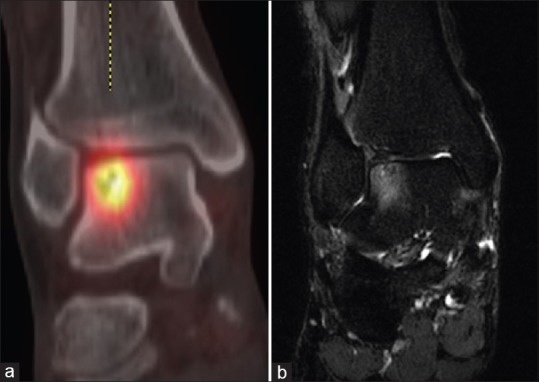
Small numbers of osteoblasts are present within the cavities, which are slowly filled with lamellar bone in a reparative response. During this time, the periosteum thickens with an abundance of capillaries, subperiosteal osteoblastic activity, and new woven bone formation.[58] Microscopic fractures of the cancellous bone appear which, despite osteoblastic new bone formation, may propagate into a stress fracture in the presence of continued stress.[58,59,60] The increased vascularity and osteoblastic remodeling explain the increased uptake seen in early tibial stress injury and stress fractures [Figure 3].
Figure 3.
Distal tibial stress fracture in a 48-year-old female. Coronal computed tomography image demonstrates a cortical lucency through the medial aspect of the distal tibia with a subtle periosteal reaction (arrow). The fused technetium-99m methylene diphosphonate single-photon emission computed tomography/computed tomography image shows associated increased technetium-99m methylene diphosphonate uptake in keeping with osseous remodeling
Coalition
Tarsal coalition describes the fusion of two or more tarsal bones, which may be osseous (synostosis), fibrous (syndesmosis), or cartilaginous (synchondrosis).[61]
Although acquired coalition may occur secondary to trauma, inflammatory arthropathies, joint degeneration, and infection, congenital tarsal coalition remains the most common form. Calcaneonavicular and talocalcaneal coalition involving the middle facet of the joint have been reported as the most common types of congenital tarsal coalition.[62] Coalition occurs from failure of segmentation of the primitive mesenchyme, a finding supported by previous fetal studies.[63,64,65]
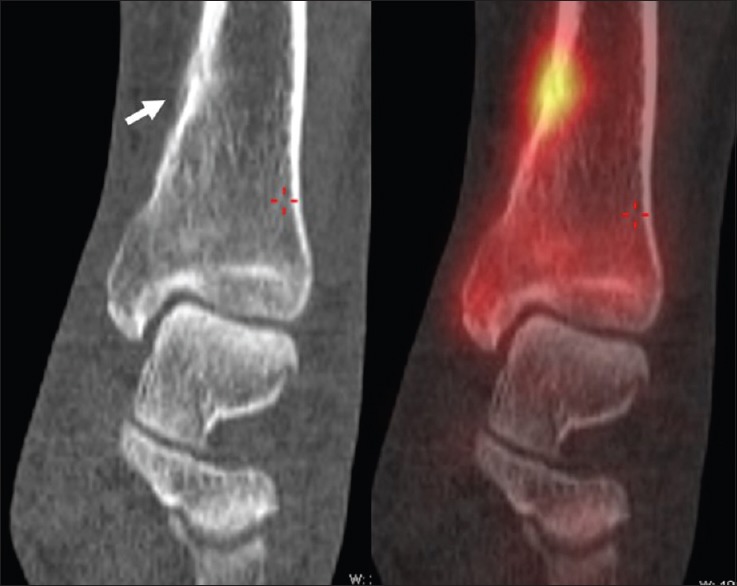
The radiographic features of tarsal coalition are well described in the radiology literature.[66,67] In addition to visualizing these changes, CT may also provide valuable information regarding the extent of the coalition, associated degenerative changes, and regional anatomy, useful for surgical planning.[68,69] Osseous coalition would be apparent on CT as an osseous bar communicating between the tarsal bones with bridging trabeculae and cortex. Fibrous or cartilaginous calcaneonavicular coalition may appear as approximation of the calcaneal and navicular cortices with sclerosis and irregularity of the margins. In addition, axial CT images may demonstrate broadening of the medial aspect of the anterior and dorsal calcaneus adjacent to the navicular [Figure 4].[66,69] Similar findings are also described in fibrous or cartilaginous talocalcaneal coalition.[67] However, in a study of a small group of patients correlating imaging findings with surgical specimens, CT was found to underestimate the presence of fibrous coalition when compared to MRI. Although CT can demonstrate detailed bony anatomy, it lacks the contrast resolution required to appreciate the fibrous component of a coalition,[70] when compared to MR where the signal characteristics of the coalition help discriminate between fibrous and cartilaginous coalition.[71] Abnormal subtalar motion related to talocalcaneal coalition may also result in additional imaging findings. A bony projection, known as a talar beak, has been described in the region of the talar ridge, the site of insertion of the talocrural joint capsule and talonavicular ligament.[72] It has been suggested that abnormal subtalar joint motion in talocalcaneal coalition may result in excessive traction at the talonavicular ligament insertion on the talus leading to periosteal elevation and new bone formation.[66] The importance of the coronal plane has been stressed to best evaluate talocalcaneal coalition.[71] In particular, the coronal plane allows evaluation of the position of the articulation of the middle facet and the inferior margin of the sustentaculum tali which has a more horizontal orientation in patients with coalition than the superomedial angulation that is normally seen. This, in addition to the valgus angulation of the calcaneus in cases of talocalcaneal coalition, results in the C sign seen on the lateral radiograph described by Lateur et al.[73]
Figure 4.
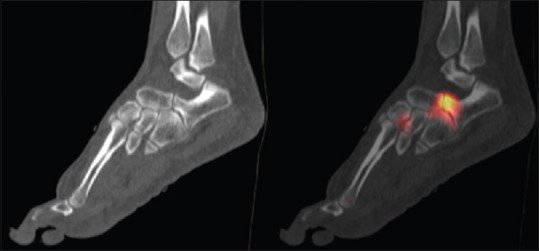
Calcaneonavicular coalition in a 37-year-old male. Sagittal computed tomography and fused technetium-99m methylene diphosphonate single-photon emission computed tomography/computed tomography images demonstrate approximation of the calcaneal and navicular cortices with broadening of the anterior process of the calcaneus in keeping with fibrocartilaginous coalition. Increased uptake is seen in the region of the coalition on the fused images in keeping with mechanical stress and bone remodeling at the bone-coalition interface
Impingement between the lateral process of the talusand adjacent calcaneus, with broadening orrounding of the lateral process of the talus related to calcaneal valgus angulation has also been described.[66,67] Degenerative changes with narrowing of the posteriortalocalcaneal facet may also be present.[66,67,74]
Accumulation of 99mTc MDP has been described in patients with coalition and foot and ankle pain.[75,76,77] De Lima and Mishkin suggested that increased uptake in the region of the coalition may be related to increased bone remodeling related to abnormal force vectors, whereas Goldman et al. suggested that the lack of motion at the site of the coalition would cause a lack of bone turnover and the increased uptake seen was likely to be explained by abnormal forces at the articular surfaces of the joints adjacent to the site of fusion. However, histological studies have demonstrated features of repetitive mechanical stress at the bone-coalition interface, with microfractures, osteoblastic activity, bone remodeling, and degenerative changes, in patients with nonosseous coalition,[78] which is likely to result in tracer accumulation at the coalition site [Figure 4]. Goldman et al. also identified increased uptake in the region of the superior talus and talonavicular joint. This was attributed to the overriding of the navicular on the talus and the talar beak,[75] which is attributed to to relate to new bone formation relating to traction from the talonavicular ligament.
Sesamoids
The medial and lateral sesamoids are embedded in the tendons of the medial and lateral heads of the flexor hallucis brevis muscle. In addition, the medial sesamoid also receives attachments from the abductor hallucis and the lateral sesamoid from the oblique and transverse heads of adductor hallucis.[79] The sesamoids are believed to have a similar function to the patella, providing a mechanical advantage by increasing leverage and modifying forces from the adjacent musculature.[80] The sesamoids are susceptible to acute trauma, chronic weight-bearing stresses, and can be affected by a range of other pathologies which include intra-articular disease processes.[79,81]
Acute fractures are rare and typically involve direct trauma or forced dorsiflexion, with the medial sesamoid more frequently involved.[79,82] The failure of ossification centers to fuse can result in partite sesamoids, again more commonly seen involving the medial sesamoid, which may be confused with fractures.[79,80] However, features have been described which favor the diagnosis of a fracture including the degree of separation and irregularity of the margins.[82,83] Skeletal scintigraphy has been used to localize abnormal bone metabolism and aid in the diagnosis of sesamoid pathology.[84] Previous studies have demonstrated increased uptake in acute sesamoid fractures later confirmed at surgery, in contrast to normal bipartite sesamoids which typically do not accumulate tracer.[1] CT is a useful modality to provide further detail regarding fracture fragments, bony alignment, and malunion.[81]
Sesamoiditis has been used to describe a painful inflammatory process with edematous marrow and soft-tissue changes on MRI and increased uptake of radiotracer.[85,86] However, the definition varies in the literature, and more recently, has been described as generic term encompassing several pathological processes including chronic repetitive stress, avascular necrosis, chondrosis, and joint degeneration.[84,87] Bone scintigraphy has proved to be useful in this group of disorders,[84,87,88,89,90] helping to localize pathological uptake to the sesamoids before radiographic changes.[84,91] Although the sensitivity may be superior to other modalities, uptake alone does not reliably differentiate between pathologies[90] and has been identified in asymptomatic patients.[92] In these cases, the CT component of the SPECT/CT study may add value to help demonstrate pathological changes in the appearances of the sesamoids.[90] Stress fractures of the sesamoids have been described following strenuous exercise in the absence of acute trauma[80] which can be identified by increased uptake on bone scintigraphy.[93] Like stress fractures elsewhere in the foot and ankle, SPECT/CT may be a useful modality to demonstrate changes in structure and bone remodeling [Figure 5].
Figure 5.
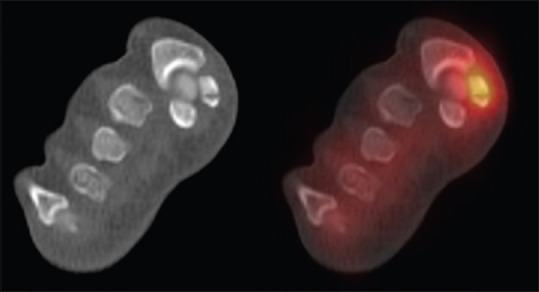
Medial sesamoid stress fracture in a 31-year-old female. The coronal oblique computed tomography and fused technetium-99m methylene diphosphonate single-photon emission computed tomography/computed tomography images demonstrate a well-defined lucency through the center of the medial sesamoid with associated increased uptake in keeping with increased osteoblastic activity
Increased Tc-99m MDP uptake in the presence of sclerosis and fragmentation on plain radiography has been described in histologically confirmed sesamoid osteonecrosis. Specimens demonstrated new bone formation related to necrotic trabeculae and degenerative changes of the articular cartilage,[91,94] both of which could contribute to increased uptake of tracer. The superior localization of SPECT/CT may help differentiate between new bone formation within the necrotic sesamoid and degenerative uptake at the articular margins.
Given that the hallux sesamoids form part of a synovial articulation, they can also be involved in degenerative and inflammatory arthropathies. Erosions have been described in rheumatoid, crystal, and seronegative arthropathies.[95] Periostitis and enthesopathy of the sesamoids in seronegative arthropathies have also been described with periosteal and entheseal new bone formation causing a whiskering appearance of the sesamoid margins.[85,95,96]
Accessory Ossicles
Accessory ossicles arise from unfused accessory ossification centers and appear as small, mature ossific foci adjacent to the main parent bone.[97,98] Although often asymptomatic and noted as an incidental finding, accessory ossicles may be mistaken for fractures and can become symptomatic.[97] Several accessory ossicles have been described in the foot. The accessory navicular and os trigonum are among the most common[90] and may be subject to degenerative and stress-related changes at the synchondrosis.[99] Three types of accessory naviculars have been described: Type 1 is a sesamoid bone located within the distal posterior tibial tendon; Type II is an accessory ossification center, connected to the navicular by a cartilaginous synchondrosis; and Type III is an enlarged medial tuberosity of the navicular, also described as a cornuate navicular.[100] The Type II accessory navicular may become symptomatic due to stress across the synchondrosis.[101] Histological analysis of surgically resected specimens from patients with symptomatic accessory naviculars has demonstrated chondro-osseous changes at the synchondrosis in keeping with chronic trauma and stress reaction. The proliferation of vascular mesenchymal tissue and cartilage, with bone remodeling, increased osteoblastic and osteoclastic activity has been described in these patients who reported symptomatic relief following surgical resection.[100,101] This bone remodeling is likely to explain the increased uptake on planar bone scintigraphy reported in patients with symptomatic accessory naviculars.[100,101,102,103] Although uptake has been reported to be side specific in patients with bilateral accessory naviculars and unilateral symptoms,[101] more recently, Chiu et al. found that increased uptake was also present in the contralateral asymptomatic Type II accessory navicular in a small group of patients with bilateral accessory naviculars.[103]
The os trigonum represents an unfused posterolateral talar tubercle secondary ossification center.[104] Tc 99m MDP bone scintigraphy has also been used to identify the presence of a symptomatic os trigonum in patients with ankle pain.[99,104,105,106] Like the type II accessory navicular, histological evidence of chronic chondro-osseous disruption has also been described following resection of the symptomatic os trigonum.[99] Bony impingement of the os trigonum between the posterior tibia and calcaneus has been described as a cause of ankle pain and is associated with extreme or forced plantar flexion, for example, during the en pointe position in ballet, while playing football or running downhill.[104,107,108] The synchondrosis may also be acutely disrupted by a single-plantar flexion injury or subject to chronic injury following repetitive stress. Similarly, the posterolateral talar process may be acutely fractured or subject to chronic stress injury.[105,108] In some cases, differentiation between a previous fracture and an os trigonum can prove difficult.[106] With an overlap of imaging features and symptoms, many terms have been used to describe the presence of pain in patients with pathology of the posterolateral talar process or os trigonum with os trigonum syndrome favored by some authors.[105,106] CT may be able to differentiate acute fractures from chronic chondro-osseous disruption between the os trigonum and talus, with cystic and sclerotic changes observed adjacent to the synchondrosis in the latter.[105]
Soft tissue
Chicklore et al. investigated the added value of Tc-99m MDP SPECT/CT in patients with impingement syndrome and soft-tissue pathology compared to clinical assessment and conventional two-phase bone scan. Common pathologies encountered included impingement syndromes, plantar fasciitis, and tendonitis [Figure 6a and b].[10] In over half of the cases (24/43), the pathology demonstrated by SPECT/CT was not suspected by the clinician. Although pathological uptake was also apparent on planar scintigraphy in all but one case (42/43) the improved localization and characterization of SPECT/CT provided additional information to the planar study in 76% (31/43) of cases. Despite this, the authors still favored the initial use of MRI or ultrasound in the assessment of impingement syndromes and soft-tissue pathology in patients with foot and ankle pain following a normal radiograph. The authors suggested reserving SPECT/CT for cases where soft-tissue pathology was suspected and there was a contraindication to MRI (e.g., metallic implant, claustrophobia) or where an osseous pathology was suspected despite a normal radiograph. Although MRI is often preferred because of its superior soft-tissue contrast and high sensitivity,[2,3,109] a recent study has demonstrated comparable sensitivities for SPECT/CT and MRI for symptomatic osseous, ligamentous/tendinous, and joint pathology of the foot and ankle with SPECT/CT demonstrating significantly higher specificity.[109]
Figure 6.
Left peroneal tendinosis in a 48-year-old male. (a) Axial unfused technetium-99m methylene diphosphonate single-photon emission computed tomography/computed tomography images demonstrate increased uptake at the posterior aspect of the left lateral malleolus which demonstrates no associated bony abnormality on the computed tomography component of the study. (b) Axial image from the computed tomography component (soft tissue windows) at a more inferior slice demonstrates thickening and poor definition of the left peroneal tendons when compared to the normal right side
Plantar Fascia
The plantar fascia is a strong fibrous structure within the sole of the foot[110] which has an important role in supporting the longitudinal arch.[111] The plantar fascia arises from the posteromedial calcaneal tuberosity and divides into central, lateral, and medial bands. The central component is the largest and functionally most important component.[110,112] The term plantar fasciitis has been used to describe changes in the proximal fascia close to its calcaneal insertion associated with heel pain.[113,114] Bone scintigraphy typically demonstrates increased uptake in the region of the calcaneus and along the plantar fascia on blood pool imaging suggesting hyperemia and increased vascularity, with more focal calcaneal uptake on delayed imaging.[115] Although histological specimens demonstrate thin-walled capillaries and increased vascularity in areas of plantar fascia degeneration, only degenerative changes of collagen tissue have been described without evidence of inflammation.[116,117,118] The authors have therefore proposed using the terms plantar fasciosis or fasciopathy[116,119] to describe this process which involves microtrauma, degeneration, and inadequate reparative attempts.[117,118] MRI and ultrasound demonstrate significant thickening of the plantar fascia in patients with plantar fasciitis [Figure 7a],120,121] a finding that can also been observed on the CT component of the SPECT/CT study.[122,123] As with the plantar fascia, specimens have also demonstrated increased vascularization of the calcaneal bone marrow[116] which is likely to explain The presence of calcaneal uptake on the dynamic and early blood pool phases. Calcaneal spurs, or enthesophytes, in the region of the plantar fascia origin may also be evident on the CT component of the SPECT/CT study. Plantar calcaneal enthesophytes can vary in location relative to the plantar fascia[124,125] and have been reported in symptomatic and asymptomatic subjects.[120,126,127] Formation of plantar enthesophytes is believed to be related to tractional forces from plantar muscles and the plantar fascia which interdigitate with each other and insert on the calcaneus.[124] Associated periosteal inflammation and periosteal new bone formation has been reported[116,124,128] which is likely to account for localized calcaneal uptake on delayed scintigraphy in patients with plantar fasciitis/fasciosis [Figure 7b]. Similar patterns of uptake have also been described in patients with underlying seronegative arthropathy.[129] It has been suggested that periosteal inflammation of the os calcis may be responsible for the associated heel pain[129] although others suggest symptoms may arise from the plantar fascia itself.[130]
Figure 7.
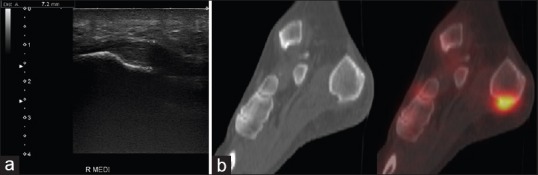
Plantar fasciitis in a 64-year-old female. (a) Longitudinal ultrasound image shows fusiform thickening of the plantar fascia with loss of the normal reflectivity and fibrillar pattern. (b) Sagittal fused technetium-99m methylene diphosphonate single-photon emission computed tomography/computed tomography images of the same patient demonstrate increased entheseal uptake related to the plantar fascia origin at the os calcis
The combination of increased metabolic activity related to the plantar fascial calcaneal insertion and plantar fascial thickening on SPECT/CT has therefore been used to identify patients with plantar fasciitis or plantar fasciosis.[122,123]
Conclusion
Hybrid Tc-99m MDP SPECT/CT is a useful tool for the assessment of a number of pathologies seen in the foot and ankle. The fusion of functional information with high resolution CT enables accurate localization of sites of abnormal bone metabolism and evaluation of coexisting structural changes. This review summarizes recent studies evaluating the use of Tc-99m MDP SPECT/CT for the assessment of foot and ankle pathology, many of which have emphasized the added value of this modality, particularly for the small joints of the mid-foot. The studies have also highlighted the subsequent impact on patient management with revision of initial diagnoses, altered sites of local anesthetic injection, and modified surgical approaches.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Maurice HD, Newman JH, Watt I. Bone scanning of the foot for unexplained pain. J Bone Joint Surg Br. 1987;69:448–52. doi: 10.1302/0301-620X.69B3.3108262. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg ZS, Beltran J, Bencardino JT. From the RSNA refresher courses. Radiological Society of North America. MR imaging of the ankle and foot. Radiographics. 2000;20:S153–79. doi: 10.1148/radiographics.20.suppl_1.g00oc26s153. [DOI] [PubMed] [Google Scholar]
- 3.Luchs JS, Flug JA, Weissman BN, Kransdorf MJ, Appel M, Arnold E, et al. ACR appropriateness criteria chronic ankle pain. Am Coll Radiol. 2012 [Google Scholar]
- 4.Nathan M, Mohan H, Vijayanathan S, Fogelman I, Gnanasegaran G. The role of 99mTc-diphosphonate bone SPECT/CT in the ankle and foot. Nucl Med Commun. 2012;33:799–807. doi: 10.1097/MNM.0b013e328355880b. [DOI] [PubMed] [Google Scholar]
- 5.Huellner MW, Strobel K. Clinical applications of SPECT/CT in imaging the extremities. Eur J Nucl Med Mol Imaging. 2014;41(Suppl 1):S50–8. doi: 10.1007/s00259-013-2533-5. [DOI] [PubMed] [Google Scholar]
- 6.Meftah M, Katchis SD, Scharf SC, Mintz DN, Klein DA, Weiner LS. SPECT/CT in the management of osteochondral lesions of the talus. Foot Ankle Int. 2011;32:233–8. doi: 10.3113/FAI.2011.0233. [DOI] [PubMed] [Google Scholar]
- 7.Mohan H, Holker P, Gnanasegaran G, Vijayanathan S, Sharp D, Langroudi B, et al. The applicability of SPECT-CT in directing the management of bony ankle and foot pathology. Eur J Nucl Med Mol Imaging. 2007;34:S166. [Google Scholar]
- 8.Gnanasegaran G, Mohan HK, Sharp D, Adamson K, Greig J, Vijayanathan S, et al. Single photon emission computed tomography-computed tomography in the management of foot pathology. Nucl Med Commun. 2008;29:482. [Google Scholar]
- 9.Kretzschmar M, Wiewiorski M, Rasch H, Jacob AL, Bilecen D, Walter MA, et al. 99mTc-DPD-SPECT/CT predicts the outcome of imaging-guided diagnostic anaesthetic injections: A prospective cohort study. Eur J Radiol. 2011;80:e410–5. doi: 10.1016/j.ejrad.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Chicklore S, Gnanasegaran G, Vijayanathan S, Fogelman I. Potential role of multislice SPECT/CT in impingement syndrome and soft-tissue pathology of the ankle and foot. Nucl Med Commun. 2013;34:130–9. doi: 10.1097/MNM.0b013e32835c0964. [DOI] [PubMed] [Google Scholar]
- 11.Singh VK, Javed S, Parthipun A, Sott AH. The diagnostic value of single photon-emission computed tomography bone scans combined with CT (SPECT-CT) in diseases of the foot and ankle. Foot Ankle Surg. 2013;19:80–3. doi: 10.1016/j.fas.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Claassen L, Uden T, Ettinger M, Daniilidis K, Stukenborg-Colsman C, Plaass C. Influence on therapeutic decision making of SPECT-CT for different regions of the foot and ankle. Biomed Res Int. 2014;2014:927576. doi: 10.1155/2014/927576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagenstert GI, Barg A, Leumann AG, Rasch H, Müller-Brand J, Hintermann B, et al. SPECT-CT imaging in degenerative joint disease of the foot and ankle. J Bone Joint Surg Br. 2009;91:1191–6. doi: 10.1302/0301-620X.91B9.22570. [DOI] [PubMed] [Google Scholar]
- 14.Wiewiorski M, Pagenstert G, Rasch H, Jacob AL, Valderrabano V. Pain in osteochondral lesions. Foot Ankle Spec. 2011;4:92–9. doi: 10.1177/1938640010395749. [DOI] [PubMed] [Google Scholar]
- 15.Hannan MT, Anderson JJ, Zhang Y, Levy D, Felson DT. Bone mineral density and knee osteoarthritis in elderly men and women. The Framingham Study. Arthritis Rheum. 1993;36:1671–80. doi: 10.1002/art.1780361205. [DOI] [PubMed] [Google Scholar]
- 16.Parthipun A, Moser J, Mok W, Paramithas A, Hamilton P, Sott AH. 99mTc-HDP SPECT-CT aids localization of joint injections in degenerative joint disease of the foot and ankle. Foot Ankle Int. 2015;36:928–35. doi: 10.1177/1071100715579263. [DOI] [PubMed] [Google Scholar]
- 17.Leumann A, Valderrabano V, Plaass C, Rasch H, Studler U, Hintermann B, et al. A novel imaging method for osteochondral lesions of the talus – comparison of SPECT-CT with MRI. Am J Sports Med. 2011;39:1095–101. doi: 10.1177/0363546510392709. [DOI] [PubMed] [Google Scholar]
- 18.Khoury NJ, el-Khoury GY, Saltzman CL, Brandser EA. Intraarticular foot and ankle injections to identify source of pain before arthrodesis. AJR Am J Roentgenol. 1996;167:669–73. doi: 10.2214/ajr.167.3.8751679. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell MJ, Bielecki D, Bergman AG, Kursunoglu-Brahme S, Sartoris DJ, Resnick D. Localization of specific joint causing hindfoot pain: Value of injecting local anesthetics into individual joints during arthrography. AJR Am J Roentgenol. 1995;164:1473–6. doi: 10.2214/ajr.164.6.7754895. [DOI] [PubMed] [Google Scholar]
- 20.Lucas PE, Hurwitz SR, Kaplan PA, Dussault RG, Maurer EJ. Fluoroscopically guided injections into the foot and ankle: Localization of the source of pain as a guide to treatment – prospective study. Radiology. 1997;204:411–5. doi: 10.1148/radiology.204.2.9240528. [DOI] [PubMed] [Google Scholar]
- 21.Chow S, Brandser E. Diagnostic and therapeutic foot and ankle injections. Semin Musculoskelet Radiol. 1998;2:421–32. doi: 10.1055/s-2008-1080122. [DOI] [PubMed] [Google Scholar]
- 22.Ruhoy MK, Newberg AH, Yodlowski ML, Mizel MS, Trepman E. Subtalar joint arthrography. Semin Musculoskelet Radiol. 1998;2:433–438. doi: 10.1055/s-2008-1080123. [DOI] [PubMed] [Google Scholar]
- 23.Saifuddin A, Abdus-Samee M, Mann C, Singh D, Angel JC. CT guided diagnostic foot injections. Clin Radiol. 2005;60:191–5. doi: 10.1016/j.crad.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Carmont MR, Tomlinson JE, Blundell C, Davies MB, Moore DJ. Variability of joint communications in the foot and ankle demonstrated by contrast-enhanced diagnostic injections. Foot Ankle Int. 2009;30:439–42. doi: 10.3113/FAI-2009-0439. [DOI] [PubMed] [Google Scholar]
- 25.Little N, Rogers B, Flannery M. Bone formation, remodelling and healing. Surg Oxf Int Ed. 2011;29:141–5. [Google Scholar]
- 26.Jones AG, Francis MD, Davis MA. Bone scanning: Radionuclidic reaction mechanisms. Semin Nucl Med. 1976;6:3–18. doi: 10.1016/s0001-2998(76)80032-3. [DOI] [PubMed] [Google Scholar]
- 27.Brenner AI, Koshy J, Morey J, Lin C, DiPoce J. The bone scan. Semin Nucl Med. 2012;42:11–26. doi: 10.1053/j.semnuclmed.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Valderrabano V, Horisberger M, Russell I, Dougall H, Hintermann B. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2009;467:1800–6. doi: 10.1007/s11999-008-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan WP, Lang P, Stevens MP, Sack K, Majumdar S, Stoller DW, et al. Osteoarthritis of the knee: Comparison of radiography, CT, and MR imaging to assess extent and severity. AJR Am J Roentgenol. 1991;157:799–806. doi: 10.2214/ajr.157.4.1892040. [DOI] [PubMed] [Google Scholar]
- 31.Gourtsoyiannis NC, Ros PR, editors. Radiologic-Pathologic Correlations from Head to Toe. Berlin, Heidelberg: Springer Publishing; 2005. [Google Scholar]
- 32.Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol. 2011;7:43–9. doi: 10.1038/nrrheum.2010.197. [DOI] [PubMed] [Google Scholar]
- 33.Thomas RH, Resnick D, Alazraki NP, Daniel D, Greenfield R. Compartmental evaluation of osteoarthritis of the knee. A comparative study of available diagnostic modalities. Radiology. 1975;116:585–94. doi: 10.1148/116.3.585. [DOI] [PubMed] [Google Scholar]
- 34.Lajeunesse D, Reboul P. Subchondral bone in osteoarthritis: A biologic link with articular cartilage leading to abnormal remodeling. Curr Opin Rheumatol. 2003;15:628–33. doi: 10.1097/00002281-200309000-00018. [DOI] [PubMed] [Google Scholar]
- 35.Dieppe P, Cushnaghan J, Young P, Kirwan J. Prediction of the progression of joint space narrowing in osteoarthritis of the knee by bone scintigraphy. Ann Rheum Dis. 1993;52:557–63. doi: 10.1136/ard.52.8.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCrae F, Shouls J, Dieppe P, Watt I. Scintigraphic assessment of osteoarthritis of the knee joint. Ann Rheum Dis. 1992;51:938–42. doi: 10.1136/ard.51.8.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul J, Barg A, Kretzschmar M, Pagenstert G, Studler U, Hügle T, et al. Increased osseous 99mTc-DPD uptake in end-stage ankle osteoarthritis: Correlation between SPECT-CT imaging and histologic findings. Foot Ankle Int. 2015;36:1438–47. doi: 10.1177/1071100715596745. [DOI] [PubMed] [Google Scholar]
- 38.Mansell JP, Bailey AJ. Abnormal cancellous bone collagen metabolism in osteoarthritis. J Clin Invest. 1998;101:1596–603. doi: 10.1172/JCI867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holder LE. Radionuclide bone-imaging in the evaluation of bone pain. J Bone Joint Surg Am. 1982;64:1391–6. [PubMed] [Google Scholar]
- 40.Kim HR, So Y, Moon SG, Lee IS, Lee SH. Clinical value of (99m)Tc-methylene diphosphonate (MDP) bone single photon emission computed tomography (SPECT) in patients with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16:212–8. doi: 10.1016/j.joca.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 41.Knupp M, Pagenstert GI, Barg A, Bolliger L, Easley ME, Hintermann B. SPECT-CT compared with conventional imaging modalities for the assessment of the varus and valgus malaligned hindfoot. J Orthop Res. 2009;27:1461–6. doi: 10.1002/jor.20922. [DOI] [PubMed] [Google Scholar]
- 42.O'Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38:392–404. doi: 10.1177/0363546509336336. [DOI] [PubMed] [Google Scholar]
- 43.Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus): Review of the literature and new surgical approach for medial dome lesions. Foot Ankle. 1985;5:165–85. doi: 10.1177/107110078500500403. [DOI] [PubMed] [Google Scholar]
- 44.Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. J Bone Joint Surg Am. 1959;41-A:988–1020. [PubMed] [Google Scholar]
- 45.Loomer R, Fisher C, Lloyd-Smith R, Sisler J, Cooney T. Osteochondral lesions of the talus. Am J Sports Med. 1993;21:13–9. doi: 10.1177/036354659302100103. [DOI] [PubMed] [Google Scholar]
- 46.Sisler J. Imaging assessment of posttraumatic tarsal pain. Clin J Sport Med. 1991;1:127–32. [Google Scholar]
- 47.Stroud CC, Marks RM. Imaging of osteochondral lesions of the talus. Foot Ankle Clin. 2000;5:119–33. [PubMed] [Google Scholar]
- 48.De Smet AA, Fisher DR, Burnstein MI, Graf BK, Lange RH. Value of MR imaging in staging osteochondral lesions of the talus (osteochondritis dissecans): Results in 14 patients. AJR Am J Roentgenol. 1990;154:555–8. doi: 10.2214/ajr.154.3.2106221. [DOI] [PubMed] [Google Scholar]
- 49.Verhagen RA, Maas M, Dijkgraaf MG, Tol JL, Krips R, van Dijk CN. Prospective study on diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br. 2005;87:41–6. [PubMed] [Google Scholar]
- 50.Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med. 2009;37(Suppl 1):105S–11S. doi: 10.1177/0363546509351481. [DOI] [PubMed] [Google Scholar]
- 51.Wiewiorski M, Miska M, Nicolas G, Valderrabano V. Revision of failed osteochondral autologous transplantation procedure for chronic talus osteochondral lesion with iliac crest graft and autologous matrix-induced chondrogenesis: A case report. Foot Ankle Spec. 2012;5:115–20. doi: 10.1177/1938640011434046. [DOI] [PubMed] [Google Scholar]
- 52.Wilson ES, Jr, Katz FN. Stress fractures. An analysis of 250 consecutive cases. Radiology. 1969;92:481–6. doi: 10.1148/92.3.481. [DOI] [PubMed] [Google Scholar]
- 53.Geslien GE, Thrall JH, Espinosa JL, Older RA. Early detection of stress fractures using 99mTc-polyphosphate. Radiology. 1976;121(3 Pt 1):683–7. doi: 10.1148/121.3.683. [DOI] [PubMed] [Google Scholar]
- 54.Rupani HD, Holder LE, Espinola DA, Engin SI. Three-phase radionuclide bone imaging in sports medicine. Radiology. 1985;156:187–96. doi: 10.1148/radiology.156.1.2860698. [DOI] [PubMed] [Google Scholar]
- 55.Gaeta M, Minutoli F, Scribano E, Ascenti G, Vinci S, Bruschetta D, et al. CT and MR imaging findings in athletes with early tibial stress injuries: Comparison with bone scintigraphy findings and emphasis on cortical abnormalities. Radiology. 2005;235:553–61. doi: 10.1148/radiol.2352040406. [DOI] [PubMed] [Google Scholar]
- 56.Ammann W. Radionuclide bone imaging in the detection of stress fractures. Clin J Sports Med. 1991;1:115–22. [Google Scholar]
- 57.Drubach LA, Connolly LP, D'Hemecourt PA, Treves ST. Assessment of the clinical significance of asymptomatic lower extremity uptake abnormality in young athletes. J Nucl Med. 2001;42:209–12. [PubMed] [Google Scholar]
- 58.Li GP, Zhang SD, Chen G, Chen H, Wang AM. Radiographic and histologic analyses of stress fracture in rabbit tibias. Am J Sports Med. 1985;13:285–94. doi: 10.1177/036354658501300501. [DOI] [PubMed] [Google Scholar]
- 59.Savoca CJ. Stress fractures. A classification of the earliest radiographic signs. Radiology. 1971;100:519–24. doi: 10.1148/100.3.519. [DOI] [PubMed] [Google Scholar]
- 60.Anderson MW, Greenspan A. Stress fractures. Radiology. 1996;199:1–12. doi: 10.1148/radiology.199.1.8633129. [DOI] [PubMed] [Google Scholar]
- 61.Lemley F, Berlet G, Hill K, Philbin T, Isaac B, Lee T. Current concepts review: Tarsal coalition. Foot Ankle Int. 2006;27:1163–9. doi: 10.1177/107110070602701229. [DOI] [PubMed] [Google Scholar]
- 62.Stormont DM, Peterson HA. The relative incidence of tarsal coalition. Clin Orthop Relat Res. 1983;181:28–36. [PubMed] [Google Scholar]
- 63.Harris B. Anomalous structure in the developing human foot. Anat Rec. 1955;121:399. [Google Scholar]
- 64.Leonard MA. The inheritance of tarsal coalition and its relationship to spastic flat foot. J Bone Joint Surg Br. 1974;56B:520–6. [PubMed] [Google Scholar]
- 65.Kawashima T, Uhthoff HK. Prenatal development around the sustentaculum tali and its relation to talocalcaneal coalitions. J Pediatr Orthop. 1990;10:238–43. [PubMed] [Google Scholar]
- 66.Conway JJ, Cowell HR. Tarsal coalition: Clinical significance and roentgenographic demonstration. Radiology. 1969;92:799–811. doi: 10.1148/92.4.799. [DOI] [PubMed] [Google Scholar]
- 67.Beckly DE, Anderson PW, Pedegana LR. The radiology of the subtalar joint with special reference to talo-calcaneal coalition. Clin Radiol. 1975;26:333–41. doi: 10.1016/s0009-9260(75)80072-9. [DOI] [PubMed] [Google Scholar]
- 68.Pineda C, Resnick D, Greenway G. Diagnosis of tarsal coalition with computed tomography. Clin Orthop Relat Res. 1986;208:282–8. [PubMed] [Google Scholar]
- 69.Newman JS, Newberg AH. Congenital tarsal coalition: Multimodality evaluation with emphasis on CT and MR imaging. Radiographics. 2000;20:321–32. doi: 10.1148/radiographics.20.2.g00mc03321. [DOI] [PubMed] [Google Scholar]
- 70.Linklater J, Hayter CL, Vu D, Tse K. Anatomy of the subtalar joint and imaging of talo-calcaneal coalition. Skeletal Radiol. 2009;38:437–49. doi: 10.1007/s00256-008-0615-4. [DOI] [PubMed] [Google Scholar]
- 71.Wechsler RJ, Karasick D, Schweitzer ME. Computed tomography of talocalcaneal coalition: Imaging techniques. Skeletal Radiol. 1992;21:353–8. doi: 10.1007/BF00241812. [DOI] [PubMed] [Google Scholar]
- 72.Resnick D. Talar ridges, osteophytes, and beaks: A radiologic commentary. Radiology. 1984;151:329–32. doi: 10.1148/radiology.151.2.6709899. [DOI] [PubMed] [Google Scholar]
- 73.Lateur LM, Van Hoe LR, Van Ghillewe KV, Gryspeerdt SS, Baert AL, Dereymaeker GE. Subtalar coalition: Diagnosis with the C sign on lateral radiographs of the ankle. Radiology. 1994;193:847–51. doi: 10.1148/radiology.193.3.7972836. [DOI] [PubMed] [Google Scholar]
- 74.Sartoris DJ, Resnick DL. Tarsal coalition. Arthritis Rheum. 1985;28:331–8. doi: 10.1002/art.1780280314. [DOI] [PubMed] [Google Scholar]
- 75.Goldman AB, Pavlov H, Schneider R. Radionuclide bone scanning in subtalar coalitions: Differential considerations. AJR Am J Roentgenol. 1982;138:427–32. doi: 10.2214/ajr.138.3.427. [DOI] [PubMed] [Google Scholar]
- 76.Deutsch AL, Resnick D, Campbell G. Computed tomography and bone scintigraphy in the evaluation of tarsal coalition. Radiology. 1982;144:137–40. doi: 10.1148/radiology.144.1.6211690. [DOI] [PubMed] [Google Scholar]
- 77.de Lima RT, Mishkin FS. The bone scan in tarsal coalition: A case report. Pediatr Radiol. 1996;26:754–6. doi: 10.1007/BF01383400. [DOI] [PubMed] [Google Scholar]
- 78.Kumai T, Takakura Y, Akiyama K, Higashiyama I, Tamai S. Histopathological study of nonosseous tarsal coalition. Foot Ankle Int. 1998;19:525–31. doi: 10.1177/107110079801900804. [DOI] [PubMed] [Google Scholar]
- 79.Jahss MH. The sesamoids of the hallux. Clin Orthop Relat Res. 1981;157:88–97. [PubMed] [Google Scholar]
- 80.Parra G. Stress fractures of the sesamoids of the foot. Clin Orthop. 1960;18:281–5. [Google Scholar]
- 81.Potter HG, Pavlov H, Abrahams TG. The hallux sesamoids revisited. Skeletal Radiol. 1992;21:437–44. doi: 10.1007/BF00190986. [DOI] [PubMed] [Google Scholar]
- 82.Bizarro AH. On the traumatology of the sesamoid structures. Ann Surg. 1921;74:783–91. doi: 10.1097/00000658-192112000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feldman F, Pochaczevsky R, Hecht H. The case of the wandering sesamoid and other sesamoid afflictions. Radiology. 1970;96:275–83. doi: 10.1148/96.2.275. [DOI] [PubMed] [Google Scholar]
- 84.Cohen BE. Hallux sesamoid disorders. Foot Ankle Clin. 2009;14:91–104. doi: 10.1016/j.fcl.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 85.Taylor JA, Sartoris DJ, Huang GS, Resnick DL. Painful conditions affecting the first metatarsal sesamoid bones. Radiographics. 1993;13:817–30. doi: 10.1148/radiographics.13.4.8356270. [DOI] [PubMed] [Google Scholar]
- 86.Karasick D, Schweitzer ME. Disorders of the hallux sesamoid complex: MR features. Skeletal Radiol. 1998;27:411–8. doi: 10.1007/s002560050410. [DOI] [PubMed] [Google Scholar]
- 87.Schein AJ, Skalski MR, Patel DB, White EA, Lundquist R, Gottsegen CJ, et al. Turf toe and sesamoiditis: What the radiologist needs to know. Clin Imaging. 2015;39:380–9. doi: 10.1016/j.clinimag.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 88.Yang RH, Chu YK. Hallucal sesamoiditis manifested on bone scan. Clin Nucl Med. 2013;38:1019–21. doi: 10.1097/RLU.0000000000000283. [DOI] [PubMed] [Google Scholar]
- 89.Srinivasan R. The hallucal-sesamoid complex: Normal anatomy, imaging, and pathology. Semin Musculoskelet Radiol. 2016;20:224–32. doi: 10.1055/s-0036-1581121. [DOI] [PubMed] [Google Scholar]
- 90.Nwawka OK, Hayashi D, Diaz LE, Goud AR, Arndt WF, 3rd, Roemer FW, et al. Sesamoids and accessory ossicles of the foot: Anatomical variability and related pathology. Insights Imaging. 2013;4:581–93. doi: 10.1007/s13244-013-0277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kliman ME, Gross AE, Pritzker KP, Greyson ND. Osteochondritis of the hallux sesamoid bones. Foot Ankle. 1983;3:220–3. doi: 10.1177/107110078300300408. [DOI] [PubMed] [Google Scholar]
- 92.Williams T, Cullen N, Goldberg A, Singh D. SPECT-CT imaging of obscure foot and ankle pain. Foot Ankle Surg. 2012;18:30–3. doi: 10.1016/j.fas.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 93.Georgoulias P, Georgiadis I, Dimakopoulos N, Mortzos G. Scintigraphy of stress fractures of the sesamoid bones. Clin Nucl Med. 2001;26:944–5. doi: 10.1097/00003072-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 94.Ogata K, Sugioka Y, Urano Y, Chikama H. Idiopathic osteonecrosis of the first metatarsal sesamoid. Skeletal Radiol. 1986;15:141–5. doi: 10.1007/BF00350208. [DOI] [PubMed] [Google Scholar]
- 95.Resnick D, Niwayama G, Feingold ML. The sesamoid bones of the hands and feet: Participators in arthritis. Radiology. 1977;123:57–62. doi: 10.1148/123.1.57. [DOI] [PubMed] [Google Scholar]
- 96.Stadalnik RC, Dublin AB. Sesamoid periostitis in the thumb in Reiter's syndrome. Case report. J Bone Joint Surg Am. 1975;57:279. [PubMed] [Google Scholar]
- 97.Miller TT. Painful accessory bones of the foot. Semin Musculoskelet Radiol. 2002;6:153–61. doi: 10.1055/s-2002-32361. [DOI] [PubMed] [Google Scholar]
- 98.Sarrafian SK. Anatomy of the Foot and Ankle: Descriptive, Topographic, Functional. Philadelphia: Lippincott Williams and Wilkins; 1993. p. 648. [Google Scholar]
- 99.Lawson JP. Symptomatic radiographic variants in extremities. Radiology. 1985;157:625–31. doi: 10.1148/radiology.157.3.4059550. [DOI] [PubMed] [Google Scholar]
- 100.Sella EJ, Lawson JP, Ogden JA. The accessory navicular synchondrosis. Clin Orthop Relat Res. 1986;209:280–5. [PubMed] [Google Scholar]
- 101.Lawson JP, Ogden JA, Sella E, Barwick KW. The painful accessory navicular. Skeletal Radiol. 1984;12:250–62. doi: 10.1007/BF00349506. [DOI] [PubMed] [Google Scholar]
- 102.Romanowski CA, Barrington NA. The accessory navicular – An important cause of medial foot pain. Clin Radiol. 1992;46:261–4. doi: 10.1016/s0009-9260(05)80167-9. [DOI] [PubMed] [Google Scholar]
- 103.Chiu NT, Jou IM, Lee BF, Yao WJ, Tu DG, Wu PS. Symptomatic and asymptomatic accessory navicular bones: Findings of Tc-99m MDP bone scintigraphy. Clin Radiol. 2000;55:353–5. doi: 10.1053/crad.2000.0436. [DOI] [PubMed] [Google Scholar]
- 104.Marotta JJ, Micheli LJ. Os trigonum impingement in dancers. Am J Sports Med. 1992;20:533–6. doi: 10.1177/036354659202000508. [DOI] [PubMed] [Google Scholar]
- 105.Karasick D, Schweitzer ME. The os trigonum syndrome: Imaging features. AJR Am J Roentgenol. 1996;166:125–9. doi: 10.2214/ajr.166.1.8571860. [DOI] [PubMed] [Google Scholar]
- 106.Johnson RP, Collier BD, Carrera GF. The os trigonum syndrome: Use of bone scan in the diagnosis. J Trauma. 1984;24:761–4. [PubMed] [Google Scholar]
- 107.Hedrick MR, McBryde AM. Posterior ankle impingement. Foot Ankle Int. 1994;15:2–8. doi: 10.1177/107110079401500102. [DOI] [PubMed] [Google Scholar]
- 108.Giannini S, Buda R, Mosca M, Parma A, Di Caprio F. Posterior ankle impingement. Foot Ankle Int. 2013;34:459–65. doi: 10.1177/1071100713477609. [DOI] [PubMed] [Google Scholar]
- 109.Ha S, Hong SH, Paeng JC, Lee DY, Cheon GJ, Arya A, et al. Comparison of SPECT/CT and MRI in diagnosing symptomatic lesions in ankle and foot pain patients: Diagnostic performance and relation to lesion type. PLoS One. 2015;10:e0117583. doi: 10.1371/journal.pone.0117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hedrick MR. The plantar aponeurosis. Foot Ankle Int. 1996;17:646–9. doi: 10.1177/107110079601701012. [DOI] [PubMed] [Google Scholar]
- 111.Thordarson DB, Schmotzer H, Chon J, Peters J. Dynamic support of the human longitudinal arch. A biomechanical evaluation. Clin Orthop Relat Res. 1995;316:165–72. [PubMed] [Google Scholar]
- 112.Schepsis AA, Leach RE, Gorzyca J. Plantar fasciitis. Etiology, treatment, surgical results, and review of the literature. Clin Orthop Relat Res. 1991;266:185–96. [PubMed] [Google Scholar]
- 113.League AC. Current concepts review: Plantar fasciitis. Foot Ankle Int. 2008;29:358–66. doi: 10.3113/FAI.2008.0358. [DOI] [PubMed] [Google Scholar]
- 114.Karr SD. Subcalcaneal heel pain. Orthop Clin North Am. 1994;25:161–75. [PubMed] [Google Scholar]
- 115.Intenzo CM, Wapner KL, Park CH, Kim SM. Evaluation of plantar fasciitis by three-phase bone scintigraphy. Clin Nucl Med. 1991;16:325–8. doi: 10.1097/00003072-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 116.Lemont H, Ammirati KM, Usen N. Plantar fasciitis: A degenerative process (fasciosis) without inflammation. J Am Podiatr Med Assoc. 2003;93:234–7. doi: 10.7547/87507315-93-3-234. [DOI] [PubMed] [Google Scholar]
- 117.Snider MP, Clancy WG, McBeath AA. Plantar fascia release for chronic plantar fasciitis in runners. Am J Sports Med. 1983;11:215–9. doi: 10.1177/036354658301100406. [DOI] [PubMed] [Google Scholar]
- 118.Tountas AA, Fornasier VL. Operative treatment of subcalcaneal pain. Clin Orthop Relat Res. 1996;332:170–8. doi: 10.1097/00003086-199611000-00023. [DOI] [PubMed] [Google Scholar]
- 119.Beeson P. Plantar fasciopathy: Revisiting the risk factors. Foot Ankle Surg. 2014;20:160–5. doi: 10.1016/j.fas.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 120.Berkowitz JF, Kier R, Rudicel S. Plantar fasciitis: MR imaging. Radiology. 1991;179:665–7. doi: 10.1148/radiology.179.3.2027971. [DOI] [PubMed] [Google Scholar]
- 121.Cardinal E, Chhem RK, Beauregard CG, Aubin B, Pelletier M. Plantar fasciitis: Sonographic evaluation. Radiology. 1996;201:257–9. doi: 10.1148/radiology.201.1.8816554. [DOI] [PubMed] [Google Scholar]
- 122.Mohan HK, Gnanasegaran G, Vijayanathan S, Fogelman I. SPECT/CT in imaging foot and ankle pathology-the demise of other coregistration techniques. Semin Nucl Med. 2010;40:41–51. doi: 10.1053/j.semnuclmed.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 123.Breunung N, Barwick T, Fernando R, Gnanasegaran G, Vijayanathan S, Hosahalli M, et al. Additional benefit of SPECT-CT in investigating heel pain. Clin Nucl Med. 2008;33:705–6. doi: 10.1097/RLU.0b013e318184b987. [DOI] [PubMed] [Google Scholar]
- 124.Abreu MR, Chung CB, Mendes L, Mohana-Borges A, Trudell D, Resnick D. Plantar calcaneal enthesophytes: New observations regarding sites of origin based on radiographic, MR imaging, anatomic, and paleopathologic analysis. Skeletal Radiol. 2003;32:13–21. doi: 10.1007/s00256-002-0585-x. [DOI] [PubMed] [Google Scholar]
- 125.Barrett SL, Day SV, Pignetti TT, Egly BR. Endoscopic heel anatomy: Analysis of 200 fresh frozen specimens. J Foot Ankle Surg. 1995;34:51–6. doi: 10.1016/S1067-2516(09)80101-4. [DOI] [PubMed] [Google Scholar]
- 126.Ehrmann C, Maier M, Mengiardi B, Pfirrmann CW, Sutter R. Calcaneal attachment of the plantar fascia: MR findings in asymptomatic volunteers. Radiology. 2014;272:807–14. doi: 10.1148/radiol.14131410. [DOI] [PubMed] [Google Scholar]
- 127.Tanz SS. Heel pain. Clin Orthop Relat Res. 1963;28:169–78. [PubMed] [Google Scholar]
- 128.Chang CC, Miltner LJ. Periostitis of the os calcis. J Bone Joint Surg Am. 1934;16:355–64. [Google Scholar]
- 129.Sewell JR, Black CM, Chapman AH, Statham J, Hughes GR, Lavender JP. Quantitative scintigraphy in diagnosis and management of plantar fasciitis (calcaneal periostitis): Concise communication. J Nucl Med. 1980;21:633–6. [PubMed] [Google Scholar]
- 130.Leach RE, Seavey MS, Salter DK. Results of surgery in athletes with plantar fasciitis. Foot Ankle. 1986;7:156–61. doi: 10.1177/107110078600700305. [DOI] [PubMed] [Google Scholar]


