Abstract
The endocannabinoid system plays a regulatory role in a number of physiological processes and has been found altered in different pathological conditions, including movement disorders. The interactions between cannabinoids and dopamine in the basal ganglia are remarkably complex and involve both the modulation of other neurotransmitters (γ-aminobutyric acid, glutamate, opioids, peptides) and the activation of different receptors subtypes (cannabinoid receptor type 1 and 2). In the last years, experimental studies contributed to enrich this scenario reporting interactions between cannabinoids and other receptor systems (transient receptor potential vanilloid type 1 cation channel, adenosine receptors, 5-hydroxytryptamine receptors). The improved knowledge, adding new interpretation on the biochemical interaction between cannabinoids and other signaling pathways, may contribute to develop new pharmacological strategies. A number of preclinical studies in different experimental Parkinson's disease (PD) models demonstrated that modulating the cannabinoid system may be useful to treat some motor symptoms. Despite new cannabinoid-based medicines have been proposed for motor and nonmotor symptoms of PD, so far, results from clinical studies are controversial and inconclusive. Further clinical studies involving larger samples of patients, appropriate molecular targets, and specific clinical outcome measures are needed to clarify the effectiveness of cannabinoid-based therapies.
Keywords: : basal ganglia, cannabinoids, dopamine, levodopa-induced dyskinesia, Parkinson's disease
Introduction
The endocannabinoid system (ECS) modulates a huge range of physiological functions, including mood, cognition, motor control, feeding behavior, and pain.1–5 In recent years, a number of studies explored the role of cannabinoids (CBs) in different pathological conditions.
Approximately 105 CBs have been extracted so far from cannabis.6 These phytocannabinoids include Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD).7 Several CB-based medicines are currently approved for clinical indications, including pain, anorexia, spasticity, chemotherapy-induced nausea, and severe refractory epileptogenic encephalopathies of the childhood.5,8
The ECS is highly represented in the basal ganglia and has been found altered in several movement disorders, including Parkinson's disease (PD).9–11 Preclinical research suggests that modulating CB signaling could improve motor symptoms.12,13 Among motor symptoms, levodopa-induced dyskinesias (LIDs) dramatically complicate long-term pharmacological treatment in PD patients. LIDs are thought to arise from pulsatile stimulation of dopamine (DA) receptors with progressive sensitization of DA receptor-associated striatal signaling.14,15 So far, despite an increased knowledge of CBs–DA interactions at molecular level, the clinical relevance of CB-based therapies on PD motor symptoms and LIDs has been poorly detailed. The aim of this minireview is to provide an overview of the biochemical interactions between CBs and DA. Furthermore, results from preclinical and clinical studies involving CB-based therapies in PD will be discussed.
Endocannabinoid System and Dopamine
The ECS is constituted by endocannabinoids (eCBs), biosynthesizing (N-arachidonoyl-phosphatidylethanolamine [NAPE]-specific phospholipase D and diacylglycerol [DAG] lipase-a) and degrading (fatty acid amide hydrolysis [FAAH] and monoacylglycerol lipase [MAGL]) enzymes, and CB receptors (CBRs).
The best characterized eCBs (N-arachidonoylethanolamine [AEA] or anandamide and 2-arachidonoylglycerol [2-AG]) interact with the two main CBRs subtypes (CB1R and CB2R) and also with other receptors, including the transient receptor potential vanilloid type 1 (TRPV1) cation channel,16 the GTP-binding protein-coupled receptor GPR55,17 the abnormal-CBD receptor,18 and the peroxisome proliferator-activated receptor (PPAR).19
eCBs regulate synaptic transmission producing a physiological feedback mechanism aimed to prevent an excess of excitation or inhibition.20 This “retrograde signaling”21 results in depolarization-induced suppression of inhibition (DSI) at γ-aminobutyric acid (GABA)ergic synapses and in depolarization-induced suppression of excitation (DSE) at glutamatergic synapses.22–24 The presynaptic location of CB1R, also allows eCBs to directly modulate other neurotransmitters, including opioid peptides, acetylcholine, and 5-hydroxytryptamine (5-HT).25,26
Although nigrostriatal dopaminergic neurons seem not to express CB1R,27,28 they are significantly affected by either the activation or the blockade of the ECS.29,30 These effects are likely mediated by CB1R located in other neuronal subpopulations (i.e., GABAergic, glutamatergic, and opioidergic neurons) located near to and connected with dopaminergic neurons.10,31,32 Indeed, it should be reminded that dopaminergic neurons may, in turn, produce eCBs from their somata and dendrites,33,34 thus facilitating the retrograde signaling at excitatory and inhibitory synapses.35
Additional direct mechanisms have been proposed to explain the modulation of eCBs on DA transmission. Some eCBs, including AEA, have been found to interact with TRPV1 receptors,36 which are expressed in dopaminergic neurons.37 CB1R can form heteromers with other metabotropic receptors, including the dopamine D1 and D2 receptor.38 Finally, CB2R have been identified in human nigrostriatal dopaminergic neurons,12 this may support a direct role of eCBs in modulating dopaminergic transmission.
CB–DA Interactions in the Basal Ganglia
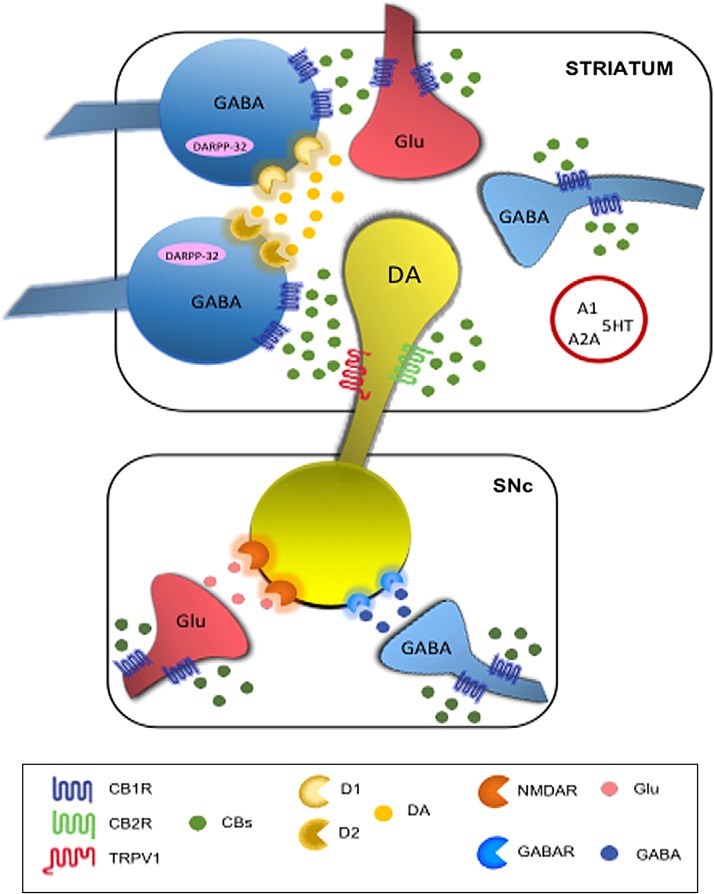
Activation of the ECS has been associated with motor inhibition and reduced dopaminergic activity. Classically, in hyperkinetic conditions reduced eCB tone accompany increased dopaminergic activity, whereas in hypokinetic movement disorders, the opposite pattern is observed.29 In experimental models of PD, eCBs can enhance the hypokinetic effects of DA-depleting agents and reduce the effects of drugs producing hyperstimulation of DA receptors.27,29 In particular, it has been proposed that motor inhibition produced by CB1R stimulation is mediated by the regulation of the phosphorylation state of a critical mediator of DA action in striatal neurons, DA- and cAMP-regulated phosphoprotein of 32 kDa39 (DARPP-32).
At cellular level, CB–DA interactions seem to be much more complex. First, dopaminergic transmission can influence the eCBs levels in the striatum as shown by the increase of AEA levels after D2-like receptor stimulation.40,41 This effect depends on both stimulation of its synthesis and inhibition of its degradation, as suggested by the ability of D2-like receptor agonists to modulate the activity of NAPE-phospholipase D and FAAH. Such DA-stimulated eCB activity can counter the action of D2 receptor activation in the striatum, suggesting an inhibitory feedback mechanism aimed at limiting the hyperkinetic effect of DA. To add more complexity, a cooperative action of CB1 and D2 receptors has also been proposed by the findings that AEA produced by DA stimulation can enhance the effects of D2 receptor activation.42–44 Indeed, inhibition of GABA transmission via D2-like receptors can be partly prevented by CBR blockade suggesting that eCBs may act as downstream effectors of D2 receptors.45 Accordingly, both D2 and CB1 receptors are expressed on GABA terminals of the striatum.46–48
The complex interaction between DA and eCBs (Fig. 1) well explains the reorganization of these systems in both idiopathic and experimental PD. Previous studies in experimental PD showed enhanced eCB activity in the basal ganglia, including increased CB1 mRNA levels, CB1 activity, AEA levels, and decreased CB clearance.9,10,27,49–52 Accordingly, increased level of AEA has been shown in the cerebrospinal fluid of untreated PD patients.11 Also, increased expression of CB1 receptors in the basal ganglia has been reported.51 These changes are associated with movement suppression and may be reversed by chronic levodopa treatment.9,51,53
FIG. 1.
Schematic mechanisms explaining the interactions between cannabinoid system and dopaminergic transmission at basal ganglia level. In the red circle are depicted additional receptors involved in cannabinoid signaling. A1, adenosine A1 receptor; A2A, adenosine A2A receptor; CB1R, cannabinoid receptor type 1; CB2R, cannabinoid receptor type 1; CBs, cannabinoids; D1, dopamine receptor type 1; D2, dopamine receptor type 2; DA, dopamine; DARPP-32, DA- and cAMP regulated phosphoprotein of 32 kDa; Glu, glutamate; GABAR, γ-aminobutyric acid receptor; GABA, γ-aminobutyric acid; 5HT, 5-hydroxytryptamine receptor; NMDAR, N-methyl-d-aspartate receptor; TRPV1, transient receptor vanilloid type 1 cation channel.
Whereas some of these alterations may reflect endogenous compensatory mechanisms aimed at limiting the effects of DA loss in the basal ganglia, others probably contribute in generating the typical parkinsonian motor symptoms.52
Basal Ganglia Plasticity in LID
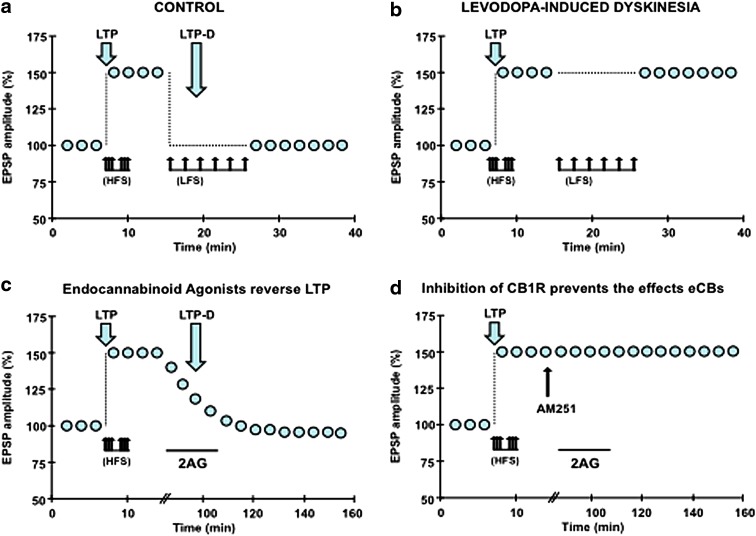
DA plays a pivotal role in producing two opposite forms of corticostriatal synaptic plasticity: long-term depression (LTD) and long-term potentiation (LTP). LTD makes glutamatergic synapses less excitable to future stimulation, LTP strengthens the connections between cortical and striatal neurons (Fig. 2a). The reversal of LTP is termed depotentiation (LTP-D) and operates to reset synaptic transmission to the naive state.54,55 Although both depotentiation and LTD reduce the strength of synaptic transmission, depotentiation is unable per se to depress nonpotentiated synapses15 and requires N-methyl-d-aspartate (NMDA) receptors activation.15,56 In experimental PD, LIDs are associated with aberrant corticostriatal plasticity (Fig. 2b), in particular, corticostriatal LTP is favored over LTD15,56 and is also abnormally stable and refractory to depotentiation.57
FIG. 2.
Synaptic plasticity in levodopa-induced dyskinesia and role of endocannabinoids in synaptic depotentiation. (a) In normal conditions, HFS induces LTP of the amplitude of EPSPs. LFS delivered after LTP induction reset synapses to naïve state. (b) In levodopa-induced dyskinesia, HFS produced LTP as in control condition, but LFS failed to induce LTP-D. (c) Perfusion of 20 μM 2AG (black bar), an endocannabinoid agonist, reversed LTP induced by HFS. (d) The effects of 2AG on LTP were blocked by 5 μM AM251, an inhibitor of CB1 receptors. 2AG, 2-arachidonoylglycerol; EPSP, excitatory postsynaptic potential; HFS, high-frequency stimulation; LFS, low-frequency stimulation; LTP, long-term potentiation; LTP-D, depotentiation.
Depotentiation can follow different mechanism, homosynaptic LTP-D requiring the activation of the same pathways that triggered LTP58,59; conversely, heterosynaptic LTP-D involves inputs different from those engaged in LTP. Previous studies have shown that heterosynaptic LTP-D entails CB1, GABA-A, and adenosine A1 receptors, and ERK 1/2 and p38 MAPK signaling and also showed that eCBs play a complex role in both presynaptic and postsynaptic changes60 (Fig. 2c, d). It is worth noting that activation of adenosine A1 receptors is also involved in other forms of LTD and depotentiation.61–64
Preclinical Studies
Preclinical studies using different models of experimental PD have investigated the effects of both agonists and antagonists of the CBR, used alone or as coadjuvants.13,29,52,65
CB1 agonists inhibit basal ganglia DA release and are therefore expected to be ineffective in alleviating PD motor symptoms. CB1 agonists exacerbated bradykinesia in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primates.66 However, different CB1 agonists have also been reported to improve motor impairment, possibly through nondopaminergic mechanisms, including interactions with adenosine A2A and 5-HT receptors.67–72
Studies of CB1 antagonists more consistently showed improvement of motor symptoms.73–77 Blockade of CB1R with rimonabant or other antagonists reduced akinesia and motor impairment in experimental models of PD,73,75,77,78 although a few other studies showed conflicting results.9,66 Moreover, rimonabant was more effective when used at low doses,75,77 and in very advanced phases of the disease characterized by extreme nigral damage.73 These effects appear to involve nondopaminergic mechanisms, including enhanced striatal glutamate release.9,73,75
The ECS might be involved in LIDs, although the results are controversial. Although this system is modulated in different experimental models of PD and in response to chronic levodopa treatment,51,79 it is not known whether these changes are compensatory or causal.80 Preclinical studies showed that both CB1R agonists and antagonists represent potentially useful antidyskinetic agents.69,74,81
The antidyskinetic effects of CBR agonists81–84 are mediated by a normalization of cAMP/PKA signaling and are associated to an increased DARPP-32 phosphorylation.84 However, as higher doses of CB1 agonists may impair motor function, it has been suggested that the effects on LIDs may be related to a global motor inhibition.85 In one study, FAAH inhibitors failed to reproduce the beneficial effects of CB agonists when given alone. As FAAH inhibitors showed antidyskinetic properties only when combined with a TRPV1 receptor antagonist, it is conceivable that CB1 and TRPV1 receptors operate in opposite directions to control LIDs.83 A recent study added more complexity by suggesting that certain CBs (e.g., AEA) may reduce LIDs by activating PPAR-γ.86 Beneficial effects were also reported for the PPAR-α receptor endogenous lipid ligand oleoylethanolamide, although the antidyskinetic effect was attributed to the blockade of TRPV1 receptors rather than the activation of PPAR-α receptors.87
Clinical Studies
Observational studies suggest that CBs may improve some motor and nonmotor symptoms associated to PD (Table 1). In two published surveys of PD patients, smoked cannabis was reported to produce some benefit on motor and nonmotor symptoms, although these studies present several limitations that could have influenced the results.88,89 A small case series showed no benefit for tremor following a single administration of smoked cannabis.90 In contrast, a small open-label study assessing motor exam 30 min after smoking cannabis reported improvement in tremor, rigidity, bradykinesia, pain, and sleep.91 Regarding nonmotor symptoms, a small 4-week open-label study of CBD for psychosis in PD found improvement on the Brief Psychiatric Rating Scale and Parkinson Psychosis Questionnaire,92 and another small case series reported benefits for rapid eye movement sleep behavior disorder.93
Table 1.
Clinical Studies Examining Whether Cannabinoids Improve Motor and Nonmotor Symptoms in Parkinson's Disease
| Study design | Number of patients | Cannabinoids | Results | Authors |
|---|---|---|---|---|
| Patient survey | 84 | Smoked cannabis | Forty-six percent of patients described some benefit; 31% reported improvement of rest tremor, 45% of bradykinesia and 14% of LID | Venderová et al.88 |
| Patient survey | 9 | Cannabis | Seven patients (78%) reported improvement of mood and sleep, two patients reported improved motor symptoms, not specifically dyskinesias | Finseth et al.89 |
| Case series | 5 | Smoked cannabis, 1 g cannabis (2–9% THC) | No benefit for tremor following single administration | Frankel et al.90 |
| Open-label | 22 | Smoked cannabis, 0.5 g cannabis | Thirty minutes after smoking cannabis, patients reported improvement in tremor, rigidity, bradykinesia, pain, and sleep | Lotan et al.91 |
| Four-week open-label | 6 | CBD up to 400 mg/day | Improvements on the Brief Psychiatric Rating Scale and Parkinson Psychosis Questionnaire | Zuardi et al.92 |
| Case series | 4 | CBD 75 or 300 mg/day | Benefits for rapid eye movement sleep behavior disorder | Chagas et al.93 |
| Randomized, double-blind, placebo-controlled crossover | 5 | Nabilone | Significant reduction of the Rush Dyskinesia Disability Scale and total LID time; two patients reported improvement in painful off-dystonia | Sieradzan et al.94 |
| Four-week randomized, double-blind, placebo-controlled crossover | 17 | Cannador (1.25 mg CBD and 2.5 mg THC) | No improvement of LIDs on multiple outcomes. | Carroll et al.95 |
| No significant changes for motor symptoms (UPDRS-III), quality of life (PDQ-39) or sleep | ||||
| Randomized, double-blind, placebo-controlled | 8 | Rimonabant | No effect on motor symptoms or LID (UPDRS and standardized videotape) | Mesnage et al.96 |
| Randomized, double-blind, placebo-controlled | 21 | CBD 75 or 300 mg/day | No changes for total UPDRS or any subscales. | Chagas et al.97 |
| Improvement for total PDQ-39 score and activities of daily living subscores for the CBD 300 mg/day group |
CBD, cannabidiol; LID, levodopa-induced dyskinesia; PDQ-39, Parkinson's Disease Questionnaire-39; THC, tetrahydrocannabinol; UPDRS, Unified Parkinson's Disease Rating Scale.
Few controlled clinical studies explored the effects of CBs on motor and nonmotor symptoms in PD patients.94–97 A small randomized, double-blind, placebo-controlled crossover trial (Class III) assessing the efficacy on LIDs of nabilone (CB1 and CB2 agonist) showed reduction of the Rush Dyskinesia Disability Scale and of total LID time.94 A small 4-week randomized double-blind crossover study (Class I) explored the effect of Cannador (oral cannabis extract: 1.25 mg CBD and 2.5 mg THC) on LIDs.95 Cannador failed to improve LIDs. Moreover, no significant changes were observed for other secondary outcomes, including motor symptoms (Unified Parkinson's Disease Rating Scale [UPDRS-III]), quality of life (Parkinson's Disease Questionnaire-39 [PDQ-39]), or sleep. However, it should be again considered that some issues compromised the results (i.e., 71% correct identification of treatment). Most recently, 21 PD patients were randomized to placebo, CBD 75 mg/day, or CBD 300 mg/day for a 6-week trial.97 Although no significant changes were found for the total UPDRS, some improvement was noted in the CBD 300 mg/day group for the quality of life (total PDQ-39 score and activities of daily living subscores).
For the purposes of this minireview, it should be mentioned another small 16-day randomized placebo-controlled trial assessing the efficacy of 20 mg daily oral rimonabant (CB1 antagonist)s, which showed no effect on parkinsonian motor symptoms or LIDs as measured by the UPDRS and a standardized videotape procedure.96
Despite the low sample size and quality of these studies, the data suggest that some motor symptoms in PD, in particular LIDs, may respond to cannabis-based therapies.98 Indeed, several factors (i.e., disease stage and levodopa treatment, lack of standardized methods) may explain the conflicting findings. While no serious adverse events were reported, side effects included hypotension, vertigo, visual hallucinations, dizziness, and somnolence. Further studies are warranted using different doses, formulations or target symptoms (e.g., dystonia, psychosis, sleep).
Conclusions
Cannabis is a psychoactive compound widely used along history for recreational and therapeutic purposes. Although many open questions remain, cannabis-based therapies have become increasingly common raising considerable interest in politics as well as in general public for legalization of medical cannabis.
In recent years, a growing body of literature addressed the role of CBs in physiological and pathological conditions. In movement disorders, preclinical studies strongly contributed to increase knowledge on the interaction between CBs, DA, and other signaling pathways, adding novel insight on pathophysiology and contributing to identify new pharmacological targets.
Results from available clinical studies are controversial and inconclusive due to several limitations, including small sample size, lack of standardized outcome measures, and expectancy bias. Well-designed studies involving larger sample of patients, appropriate molecular targets, objective biological measures (i.e., CBs blood level), and specific clinical outcome measures are needed to clarify the effectiveness of CB-based therapies. In addition, health concerns associated with medical cannabis use have to be carefully addressed by preclinical safety studies evaluating acute and long-term effects on motor functions as well on mood and cognition.
In this view, ongoing research and public policy should help to clarify these issues reducing the incongruence between approved and actual use of medical cannabis.
Abbreviations Used
- AEA
N-arachidonoylethanolamine
- 2-AG
2-arachidonoylglycerol
- CB
cannabinoid
- CBD
cannabidiol
- CBRs
CB receptors
- DA
dopamine
- DARPP-32
DA- and cAMP-regulated phosphoprotein of 32 kDa
- DAG
diacylglycerol
- DSE
depolarization-induced suppression of excitation
- DSI
depolarization-induced suppression of inhibition
- eCBs
endocannabinoids
- ECS
endocannabinoid system
- FAAH
fatty acid amide hydrolysis
- GABA
γ-aminobutyric acid
- 5-HT
5-hydroxytryptamine
- LIDs
levodopa-induced dyskinesias
- LTD
long-term depression
- LTP
long-term potentiation
- LTP-D
depotentiation
- MAGL
monoacylglycerol lipase
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NAPE
N-arachidonoyl-phosphatidylethanolamine
- NMDA
N-methyl-D-aspartate
- PD
Parkinson's disease
- PPAR
peroxisome-proliferator-activated receptor
- THC
tetrahydrocannabinol
- TRPV1
transient receptor potential vanilloid type 1
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Di Marzo V, Melck D, Bisogno T, et al. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528 [DOI] [PubMed] [Google Scholar]
- 2.Howlett AC, Barth F, Bonner TI, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202 [DOI] [PubMed] [Google Scholar]
- 3.Di Marzo V. The endocannabinoid system: its general strategy of action, tools for its pharmacological manipulation and potential therapeutic exploitation. Pharmacol Res. 2009;60:77–84 [DOI] [PubMed] [Google Scholar]
- 4.Castillo PE, Younts TJ, Chavez AE, et al. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pacher P, Kunos G. Modulating the endocannabinoid system in human health and disease—successes and failures. FEBS J. 2013;280:1918–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Husni AS, McCurdy CR, Radwan MM, et al. Evaluation of phytocannabinoids from high potency Cannabis sativa using in vitro bioassays to determine structure-activity relationships for cannabinoid receptor 1 and cannabinoid receptor 2. Med Chem Res. 2014;23:4295–4300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4:1770–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devinsky O, Cilio MR, Cross H, et al. Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55:791–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Marzo V, Hill MP, Bisogno T, et al. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson's disease. FASEB J. 2000;14:1432–1438 [DOI] [PubMed] [Google Scholar]
- 10.Gubellini P, Picconi B, Bari M, et al. Experimental parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission. J Neurosci. 2002;22:6900–6907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisani A, Fezza F, Galati S, et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinson's disease patients. Ann Neurol. 2005;57:777–779 [DOI] [PubMed] [Google Scholar]
- 12.García MC, Cinquina V, Palomo-Garo C, et al. Identification of CB2 receptors in human nigral neurons that degenerate in Parkinson's disease. Neurosci Lett. 2015;587:1–4 [DOI] [PubMed] [Google Scholar]
- 13.Kluger B, Triolo P, Jones W, et al. The therapeutic potential of cannabinoids for movement disorders. Mov Disord. 2015;30:313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obeso JA, Rodriguez-Oroz MC, Rodriguez M, et al. Pathophysiology of levodopa-induced dyskinesias in Parkinson's disease: problems with the current model. Ann Neurol. 2000;47:S22–S32 [PubMed] [Google Scholar]
- 15.Picconi B, Centonze D, Håkansson K, et al. Loss of bidirectional striatal synaptic plasticity in l-DOPA-induced dyskinesia. Nat Neurosci. 2003;6:501–506 [DOI] [PubMed] [Google Scholar]
- 16.Toth A, Blumberg PM, Boczan J. Anandamide and the vanilloid receptor (TRPV1). Vitam Horm. 2009;81:389–419 [DOI] [PubMed] [Google Scholar]
- 17.Ross RA. The enigmatic pharmacology of GPR55. Trends Pharmacol Sci. 2009;30:156–163 [DOI] [PubMed] [Google Scholar]
- 18.McHugh D, Hu SS, Rimmerman N, et al. N-arachidonoyl glycine, an abundant endogenous lipid, potently drives directed cellular migration through GPR18, the putative abnormal cannabidiol receptor. BMC Neurosci. 2010;11:4–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Sullivan SE. Cannabinoids go nuclear: evidence for activation of peroxisome proliferator-activated receptors. Br J Pharmacol. 2007;152:576–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008;184:435–477 [DOI] [PubMed] [Google Scholar]
- 21.Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296:678–682 [DOI] [PubMed] [Google Scholar]
- 22.Alger BE. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 68;2002:247–286 [DOI] [PubMed] [Google Scholar]
- 23.Heinbockel T, Brager DH, Reich CG, et al. Endocannabinoid signaling dynamics probed with optical tools. J Neurosci. 2005;25:9449–9459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Makara JK, Mor M, Fegley D, et al. Selective inhibition of 2-AG hydrolysis enhances endocannabinoid signaling in hippocampus. Nat Neurosci. 2005;8:1139–1141 [DOI] [PubMed] [Google Scholar]
- 25.Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kano M, Ohno-Shosaku T, Hashimotodani Y, et al. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–380 [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Ruiz J, Lastres-Becker I, Cabranes A, et al. Endocannabinoids and basal ganglia functionality. Prostaglandins Leukot Essent Fatty Acids. 2002;66:257–267 [DOI] [PubMed] [Google Scholar]
- 28.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884 [DOI] [PubMed] [Google Scholar]
- 29.Fernández-Ruiz J. The endocannabinoid system as a target for the treatment of motor dysfunction. Br J Pharmacol. 2009;156:1029–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fernández-Ruiz J, Hernández M, Ramos JA. Cannabinoid-dopamine interaction in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther. 2010;16:e72–e91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Stelt M, Di Marzo V. The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur J Pharmacol. 2003;480:133–150 [DOI] [PubMed] [Google Scholar]
- 32.Centonze D, Rossi S, Prosperetti C, et al. Endocannabinoids limit metabotropic glutamate 5 receptor-mediated synaptic inhibition of striatal principal neurons. Mol Cell Neurosci. 2007;35:302–310 [DOI] [PubMed] [Google Scholar]
- 33.Melis M, Pistis M, Perra S, et al. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seutin V. Dopaminergic neurones: much more than dopamine? Br J Pharmacol. 2005;146:167–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Starowicz K, Nigam S, Di Marzo V. Biochemistry and pharmacology of endovanilloids. Pharmacol Ther. 2007;114:13–33 [DOI] [PubMed] [Google Scholar]
- 37.Mezey E, Toth ZE, Cortright DN, et al. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc Natl Acad Sci U S A. 2000;97:3655–3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferré S, Goldberg SR, Lluis C, et al. Looking for the role of cannabinoid receptor heteromers in striatal function. Neuropharmacology. 2009;56:226–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersson M, Usiello A, Borgkvist A, et al. Cannabinoid action depends on phosphorylation of dopamine- and cAMP-regulated phosphoprotein of 32 kDa at the protein kinase A site in striatal projection neurons. J Neurosci. 2005;25:8432–8438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giuffrida A, Parsons LH, Kerr TM, et al. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neurosci. 1999;2:358–363 [DOI] [PubMed] [Google Scholar]
- 41.Beltramo M, de Fonseca FR, Navarro M, et al. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J Neurosci. 2000;20:3401–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alonso R, Voutsinos B, Fournier M, et al. Blockade of cannabinoid receptors by SR141716 selectively increases fos expression in rat mesocorticolimbic areas via reduced dopamine D2 function. Neuroscience. 91;1999:607–620 [DOI] [PubMed] [Google Scholar]
- 43.Meschler JP, Conley TJ, Howlett AC. Cannabinoid and dopamine interaction in rodent brain: effects on locomotor activity. Pharmacol Biochem Behav. 2000;67:567–573 [DOI] [PubMed] [Google Scholar]
- 44.Nava F, Carta G, Battasi AM, et al. D(2) dopamine receptors enable delta(9)-tetrahydrocannabinol induced memory impairment and reduction of hippocampal extracellular acetylcholine concentration. Br J Pharmacol. 2000;130:1201–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centonze D, Battista N, Rossi S, et al. A critical interaction between dopamine D2 receptors and endocannabinoids mediates the effects of cocaine on striatal GABAergic transmission. Neuropsychopharmacology. 2004;29:1488–1497 [DOI] [PubMed] [Google Scholar]
- 46.Delgado A, Sierra A, Querejeta E, et al. Inhibitory control of the GABAergic transmission in the rat neostriatum by D2 dopamine receptors. Neuroscience. 95;2000:1043–1048 [DOI] [PubMed] [Google Scholar]
- 47.Hermann H, Marsicano G, Lutz B. Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience. 2002;109:451–460 [DOI] [PubMed] [Google Scholar]
- 48.Iversen L. Cannabis and the brain. Brain 2003;126:1252–1270 [DOI] [PubMed] [Google Scholar]
- 49.Mailleux P, Vanderhaeghen JJ. Dopaminergic regulation of cannabinoid receptor mRNA levels in the rat caudate-putamen: an in situ hybridization study. J Neurochem. 1993;61:1705–1712 [DOI] [PubMed] [Google Scholar]
- 50.Romero J, Berrendero F, Perez-Rosado A, et al. Unilateral 6-hydroxydopamine lesions of nigrostriatal dopaminergic neurons increased CB1 receptor mRNA levels in the caudate-putamen. Life Sci. 2000;66:485–494 [DOI] [PubMed] [Google Scholar]
- 51.Lastres-Becker I, Cebeira M, de Ceballos ML, et al. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinson's syndrome and of MPTP-treated marmosets. Eur J Neurosci. 2001;14:1827–1832 [DOI] [PubMed] [Google Scholar]
- 52.Brotchie JM. CB1 cannabinoid receptor signalling in Parkinson's disease. Curr Opin Pharmacol. 2003;3:54–61 [DOI] [PubMed] [Google Scholar]
- 53.Maccarrone M, Gubellini P, Bari M, et al. Levodopa treatment reverses endocannabinoid system abnormalities in experimental parkinsonism. J Neurochem. 2003;85:1018–1025 [DOI] [PubMed] [Google Scholar]
- 54.Huang CC, Hsu KS. Progress in understanding the factors regulating reversibility of long-term potentiation. Rev Neurosci. 2001;12:51–68 [DOI] [PubMed] [Google Scholar]
- 55.Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the ‘long’ in long-term potentiation. Trends Neurosci. 2007;30:167–175 [DOI] [PubMed] [Google Scholar]
- 56.Calabresi P, Picconi B, Tozzi A, et al. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219 [DOI] [PubMed] [Google Scholar]
- 57.Centonze D, Bernardi G, Koch G. Mechanisms of disease: basic-research-driven investigations in humans—the case of hyperkinetic disorders. Nat Clin Pract Neurol. 2007;3:572–580 [DOI] [PubMed] [Google Scholar]
- 58.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci U S A. 1992;89:4363–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dudek SM, Bear MF. Bidirectional long-term modification of synaptic effectiveness in the adult and immature hippocampus. J Neurosci. 1993;13:2910–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Izumi Y, Zorumski CF. GABA and endocannabinoids mediate depotentiation of Schaffer collateral synapses induced by stimulation of temperoammonic inputs. PLoS One. 2016;11:e014903–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang CC, Liang YC, Hsu KS. A role for extracellular adenosine in time-dependent reversal of longterm potentiation by low-frequency stimulation at hippocampal CA1 synapses. J Neurosci. 1999;19:9728–9738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang Y-C, Huang C-C, Hsu K-S. A role of p38 mitogen-activated protein kinase in adenosine A1 receptor-mediated synaptic depotentiation in area CA1 of the rat hippocampus. Mol Brain. 2008;1:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chung HJ, Ge WP, Qian X, et al. G protein-acitvated inward rectifying potassium channels mediate depotentiation of long-term potentiation. Proc Natl Acad Sci U S A. 2009;106:635–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Z, Xiong C, Pancyr C, et al. Prolonged adenosine A1 receptor activation in hypoxia and pial vessel disruption focal cortical ischemia facilitates clathrin-mediated AMPA receptor endocytosis and long-lasting synaptic inhibition in rat hippocampal CA3-Ca1 synapses: differential regulation of GluA2 and GluA1 subunits by p38 MAPK and JNK. J Neurosci. 2014;34:9621–9643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.García-Arencibia M, García C, Fernández-Ruiz J. Cannabinoids and Parkinson's disease. CNS Neurol Disord Drug Targets. 2009;8:432–439 [DOI] [PubMed] [Google Scholar]
- 66.Meschler JP, Howlett AC, Madras BK. Cannabinoid receptor agonist and antagonist effects on motor function in normal and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP)-treated non-human primates. Psychopharmacology (Berl). 2001;156:79–85 [DOI] [PubMed] [Google Scholar]
- 67.Brotchie JM. Adjuncts to dopamine replacement: a pragmatic approach to reducing the problem of dyskinesia in Parkinson's disease. Mov Disord. 1998;13:871–876 [DOI] [PubMed] [Google Scholar]
- 68.Sanudo-Pena MC, Patrick SL, Khen S, et al. Cannabinoid effects in basal ganglia in a rat model of Parkinson's disease. Neurosci Lett. 1998;248:171–174 [DOI] [PubMed] [Google Scholar]
- 69.Segovia G, Mora F, Crossman AR, et al. Effects of CB1 cannabinoid receptor modulating compounds on the hyperkinesia induced by high-dose levodopa in the reserpine-treated rat model of Parkinson's disease. Mov Disord. 2003;18:138–149 [DOI] [PubMed] [Google Scholar]
- 70.Fernandez-Espejo E, Caraballo I, Rodriguez de Fonseca F, et al. Experimental parkinsonism alters anandamide precursor synthesis, and functional deficits are improved by AM404: a modulator of endocannabinoid function. Neuropsychopharmacology. 2004;29:1134–1142 [DOI] [PubMed] [Google Scholar]
- 71.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445:643–647 [DOI] [PubMed] [Google Scholar]
- 72.van Vliet SA, Vanwersch RA, Jongsma MJ, et al. Therapeutic effects of Delta9-THC and modafinil in a marmoset Parkinson model. Eur Neuropsychopharmacol. 2008;18:383–389 [DOI] [PubMed] [Google Scholar]
- 73.Fernandez-Espejo E, Caraballo I, de Fonseca FR, et al. Cannabinoid CB1 antagonists possess antiparkinsonian efficacy only in rats with very severe nigral lesion in experimental parkinsonism. Neurobiol Dis. 2005;18:591–601 [DOI] [PubMed] [Google Scholar]
- 74.van der Stelt M, Fox SH, Hill M, et al. A role for endocannabinoids in the generation of parkinsonism and levodopa-induced dyskinesia in MPTP-lesioned non-human primate models of Parkinson's disease. FASEB J. 2005;19:1140–1142 [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez S, Scorticati C, Garcia-Arencibia M, et al. Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinson's disease. Brain Res. 2006;1073–1074:209–219 [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Arencibia M, Ferraro L, Tanganelli S, et al. Enhanced striatal glutamate release after the administration of rimonabant to 6-hydroxydopamine-lesioned rats. Neurosci Lett. 2008;438:10–13 [DOI] [PubMed] [Google Scholar]
- 77.Kelsey JE, Harris O, Cassin J. The CB(1) antagonist rimonabant is adjunctively therapeutic as well as monotherapeutic in an animal model of Parkinson's disease. Behav Brain Res. 2009;203:304–307 [DOI] [PubMed] [Google Scholar]
- 78.Garcia C, Palomo-Garo C, Garcia-Arencibia M, et al. Symptom-relieving and neuroprotective effects of the phytocannabinoid 9-THCV in animal models of Parkinson's disease. Br J Pharmacol. 2011;163:1495–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeng BY, Dass B, Owen A, et al. Chronic L-DOPA treatment increases striatal cannabinoid CB1 receptor mRNA expression in 6-hydroxydopamine-lesioned rats. Neurosci Lett. 1999;276:71–74 [DOI] [PubMed] [Google Scholar]
- 80.Sierra S, Luquin N, Rico AJ, et al. Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain Struct Funct. 2014;220:2721–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fox SH, Henry B, Hill M, et al. Stimulation of cannabinoid receptors reduces levodopa-induced dyskinesia in the MPTP-lesioned nonhuman primate model of Parkinson's disease. Mov Disord. 2002;17:1180–1187 [DOI] [PubMed] [Google Scholar]
- 82.Ferrer B, Asbrock N, Kathuria S, et al. Effects of levodopa on endocannabinoid levels in rat basal ganglia: implications for the treatment of levodopa-induced dyskinesias. Eur J Neurosci. 2003;18:1607–1614 [DOI] [PubMed] [Google Scholar]
- 83.Morgese MG, Cassano T, Cuomo V, et al. Anti-dyskinetic effects of cannabinoids in a rat model of Parkinson's disease: role of CB(1) and TRPV1 receptors. Exp Neurol. 2007;208:110–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martinez A, Macheda T, Morgese MG, et al. The cannabinoid agonist WIN55212-2 decreases L-DOPA-induced PKA activation and dyskinetic behavior in 6-OHDA-treated rats. Neurosci Res. 2012;72:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Walsh S, Gorman AM, Finn DP, et al. The effects of cannabinoid drugs on abnormal involuntary movements in dyskinetic and non-dyskinetic 6-hydroxydopamine lesioned rats. Brain Res. 2010;1363:40–48 [DOI] [PubMed] [Google Scholar]
- 86.Martinez AA, Morgese MG, Pisanu A, et al. Activation of PPAR-γ receptors reduces levodopa-induced dyskinesias in 6-OHDA-lesioned rats. Neurobiol Dis. 2015;74:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.González-Aparicio R, Moratalla R. Oleoylethanolamide reduces L-DOPA-induced dyskinesia via TRPV1 receptor in a mouse model of Parkinson's disease. Neurobiol Dis. 2014;62:416–425 [DOI] [PubMed] [Google Scholar]
- 88.Venderová K, Ruzicka E, Vorísek V, et al. Survey on cannabis use in Parkinson's disease: subjective improvement of motor symptoms. Mov Disord. 2004;19:1102–1106 [DOI] [PubMed] [Google Scholar]
- 89.Finseth TA, Hedeman JL, Brown RP 2nd, et al. Self-reported efficacy of cannabis and other complementary medicine modalities by Parkinson's disease patients in Colorado. Evid Based Complement Alternat Med. 2015;2015:87484–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frankel JP, Hughes A, Lees AJ, et al. Marijuana for parkinsonian tremor. J Neurol Neurosurg Psychiatry. 1990;53:43–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lotan I, Treves TA, Roditi Y, et al. Cannabis (medical marijuana) treatment for motor and non-motor symptoms of Parkinson disease: an open-label observational study. Clin Neuropharmacol 2014;37:41–44 [DOI] [PubMed] [Google Scholar]
- 92.Zuardi AW, Crippa JA, Hallak JE, et al. Cannabidiol for the treatment of psychosis in Parkinson's disease. J Psychopharmacol. 2009;23:979–983 [DOI] [PubMed] [Google Scholar]
- 93.Chagas MH, Eckeli AL, Zuardi AW, et al. Cannabidiol can improve complex sleep-related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J Clin Pharm Ther. 2014;39:564–566 [DOI] [PubMed] [Google Scholar]
- 94.Sieradzan KA, Fox SH, Hill M, et al. Cannabinoids reduce levodopa-induced dyskinesia in Parkinson's disease: a pilot study. Neurology 2001;57:2108–2111 [DOI] [PubMed] [Google Scholar]
- 95.Carroll CB, Bain PG, Teare L, et al. Cannabis for dyskinesia in Parkinson disease: a randomized double-blind crossover study. Neurology. 2004;63:1245–1250 [DOI] [PubMed] [Google Scholar]
- 96.Mesnage V, Houeto JL, Bonnet AM, et al. Neurokinin B, neurotensin, and cannabinoid receptor antagonists and Parkinson's disease. Clin Neuropharmacol. 2004;27:108–110 [DOI] [PubMed] [Google Scholar]
- 97.Chagas MH, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double-blind trial. J Psychopharmacol. 2014;28:1088–1098 [DOI] [PubMed] [Google Scholar]
- 98.Koppel BS. Cannabis in the treatment of dystonia, dyskinesias, and tics. Neurotherapeutics. 2015;12:788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
References
Cite this article as: Stampanoni Bassi M, Sancesario A, Morace R, Centonze D, Iezzi E (2017) Cannabinoids in Parkinson's disease, Cannabis and Cannabinoid Research 2:1, 21–29, DOI: 10.1089/can.2017.0002.