Abstract
Familial dysautonomia (FD) is a rare children neurodegenerative disease caused due to a point mutation in the IKBKAP gene that results in decreased IKK complex-associated protein (IKAP) protein production. The disease affects mostly the dorsal root ganglion (DRG) and the sympathetic ganglion. Recently, we found that the molecular mechanisms underlying neurodegeneration in FD patients are defects in axonal transport of nerve growth factors and microtubule stability in the DRG. Neurons are highly polarized cells with very long axons. In order to survive and maintain proper function, neurons depend on transport of proteins and other cellular components from the neuronal body along the axons. We further demonstrated that IKAP is necessary for axon maintenance and showed that phosphatidylserine acts as an HDAC6 inhibitor to rescue neuronal function in FD cells. In this review, we will highlight our latest research findings.
Keywords: axonal transport, neurodegeneration, microtubule, familial dysautonomia, phosphatidylserine, HDAC6
Introduction
Familial dysautonomia (FD) is a rare autosomal recessive congenital neurodegenerative neuropathy, which occurs almost exclusively in children of the Ashkenazi Jewish population with remarkably high carrier frequencies of 1 in 32 overall and of 1 in 18 in those of Polish descent (Lehavi et al., 2003). Individuals with FD suffer from a variety of symptoms including vomiting crises, pneumonia, ataxia, difficulty swallowing, gastrointestinal and cardiovascular dysfunction, and short life spans (Riley et al., 1949; Mahloudji et al., 1970; Axelrod et al., 2002; Wan et al., 2011; Palma et al., 2014). FD patients exhibit abnormal development and progressive depletion of unmyelinated sensory and autonomic neurons (Fogelson et al., 1967; Pearson and Pytel, 1978a, b; Pearson et al., 1978; Axelrod et al., 1995) and reductions in sizes and numbers of dorsal root ganglion (DRG) and sympathetic ganglion (SG) neurons (Pearson et al., 1975, 1978; Abashidze et al., 2014; Jackson et al., 2014). The genetic cause of FD is a point mutation in the IKBKAP gene, which encodes the IκB kinase complex-associated protein (IKAP). The mutation alters the splicing pattern of the IKBKAP gene in a tissue-specific manner, leading to lower than normal levels of IKAP in the nervous systems. The exact role of IKAP in neurons and why neurons lacking IKAP degenerate are not entirely understood.
As neurons are highly polarized cells with very long axons that can be more than a meter long in adult humans, these cells are uniquely dependent on efficient intracellular transport to maintain spatiotemporal signaling, structural integrity, and function. Proper microtubule polymerization and stabilization is also essential to regulate axonal transport. Microtubules are formed from the dynamic polymerization of αβ-tubulin dimmers required for the normal outgrowth of the axon and growth cone. Impairment of axonal transport appears to be one of the major pathogenic mechanisms that result in neurodegeneration in patients with diseases such as amyotrophic lateral sclerosis (ALS), Huntington's, Alzheimer's, Parkinson's, and Charcot-Marie-Tooth diseases (Perlson et al., 2010; Hinckelmann et al., 2013; Millecamps and Julien, 2013). Therefore, we speculated that axonal transport and microtubule stability are defective in FD patients.
Transport along DRG Axons is Impaired in Neurons that Lack IKAP
Several FD models indicate that there are alterations in microtubule acetylation (Gardiner et al., 2007; Creppe et al., 2009), a process that regulates axonal transport, in cells deficient in IKAP. Tubulin acetylation is a reversible post-translational modification that occurs at lysine 40 in the N-terminal region of α-tubulin. Acetylated tubulin levels impact the degree of protein trafficking along microtubules (Reed et al., 2006; Li et al., 2011; Jakovcevski and Akbarian, 2012) and stability of the microtubule backbone (Rosenbaum, 2000; Westermann and Weber, 2003), and defects in tubulin acetylation are associated with Alzheimer's, Huntington's, and ALS diseases (Hempen and Brion, 1996; Dompierre et al., 2007).
Histone deacetylase 6 (HDAC6) is a key regulator of axonal α-tubulin acetylation (Hubbert et al., 2002). HDAC6 expression increases following neuronal injury (Rivieccio et al., 2009), and treatment with an HDAC inhibitor promotes neuronal outgrowth (Gaub et al., 2010). Also inhibition of HDAC6 expression or use of the deacetylase inhibitor trichostatin A (TSA) elevates axonal transport rates by enhancing acetylated α-tubulin levels (Dompierre et al., 2007; d’Ydewalle et al., 2011; Godena et al., 2014). Thus, HDAC inhibitors have been evaluated pre-clinically and clinically for treatment of neurodegenerative diseases and neuropathologic events; however, side effects and toxicity limit their utility (Dietz and Casaccia, 2010).
Recently, we developed a novel conditional knockout (CKO) mouse model of FD using Cre-loxP system to extract IKBKAP exon 20 in the nervous system and DRGs; these mice demonstrated downregulate of IKAP levels in the DRGs and have many FD symptoms. Using this model we identified the underlying causes of degeneration in FD. We demonstrated using live imaging assays in microfluidic chambers that there is a significant decrease of nerve growth factor (NGF) retrograde transport along DRG axons derived from the FD mice compared to controls. We further found using several models, including FD patient cells, that this axonal transport inhibition is accompanied by lower levels of acetylated α-tubulin and by an increase in HDAC6 levels. These findings demonstrate the urgent need to find an effective, non-toxic HDAC6 inhibitor that can be used to treat FD patients.
Phosphatidylserine Enhances NGF Axonal Transport by Inhibiting HDAC6 Levels
Phosphatidylserine (PS), a food supplement with no reported side effects, was previously shown to have potential as an FD therapy (Keren et al., 2010; Bochner et al., 2013; Salani et al., 2013; Donyo et al., 2016). PS promotes cell survival (Maragno et al., 2015), reduces pro-inflammatory signals (Monastra and Bruni, 1992), activates the MAP/ERK kinase pathway (Donyo et al., 2016), and inactivates JNK and p38 signaling after lipopolysaccharide treatment (Nolan et al., 2004). In FD mouse models, PS increases NGF axonal transport, downregulates HDAC6 levels, and elevates acetylated α-tubulin levels (Naftelberg et al., 2016). When treated with PS, cultured DRG neurons deficient in IKAP have more NGF tracks per axon and transport occurs at higher velocity than in untreated neurons (Naftelberg et al., 2016). Interestingly, phosphatidylserine also significantly improves axonal transport in normal healthy DRGs compared to vehicle-treated controls, which indicates that the beneficial effects of PS are not limited to FD. The effect of PS in other neuropathological disease models and neuron types should be tested in the near future. Our analysis suggests that PS inhibits HDAC, elevating acetylated α-tubulin levels and impacting dynamics, stability, and growth of axons (Naftelberg et al., 2016).
Conclusions
In our work to elucidate the neurodegenerative pathway in familial dysautonomia disease, we demonstrated that the mechanism involves an increase in HDAC6 expression, which results in aberrant NGF axonal transport and decreased DRG neuron survival with attenuated outgrowth axons (Figure 1). We discovered that PS is a safe, potent regenerative therapy that acts as a HDAC inhibitor. Pharmacological inhibition of HDAC6 activity by PS treatment will likely enhance neuronal survival in FD patients and could be effective for treatment of patients with other neurodegenerative disorders that have molecular features similar to FD such as elevated HDAC6 levels, reduced acetylatedtubulin levels, and alterations in axonal transport (Figure 1).
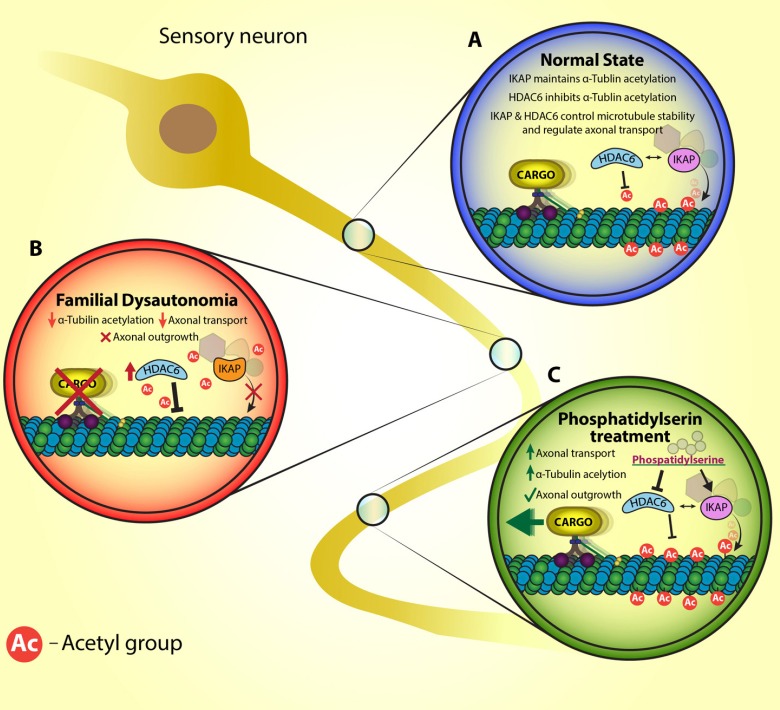
Figure 1.
A suggested model for the impaired axonal transport in familial dysautonomia (FD) and the effect of phosphatidylserine.
A scheme representation of IKK complex-associated protein (IKAP) involvement in axonal transport in sensory neuron at (A) normal state, (B) Familial dysautonomia patients and (C) after phosphatidylserine treatment. (A) In normal state IKAP is part of a complex that acetylates α-tubulin and histone deacetylase 6 (HDAC6) is the major α-tubulin deacetylates. Acetylated α-tubulin is essential for dynein movement and polymerization of microtubules. (B) In FD, lower levels of IKAP are indicated and influence HDAC6 levels. As a result of the imbalance interplay in IKAP and HDAC6 levels, microtubule stabilization and axonal transport affected. (C) Phosphatidylserine elevates IKAP levels and downregulates HDAC6 levels and thus facilitates axonal transport and microtubule stabilization.
Acknowledgments
We are grateful to Enzymotec for supplying the PS. We are also grateful for the support of the Dysautonomia Foundation and the Israeli National Network of Excellence from Teva Pharmaceutical Industries Ltd. We are grateful to Keren Avraham, Avraham Yaron, Illana Gozes and Miguel Weil labs for reagents and useful discussions. We also thank Ariel Ionescu for creating the figure.
Footnotes
Funding: Funding for this work was provided by grants from the Dysautonomia Foundation. Israel Science Foundation (ISF) [142/13, 1439/14], and by Teva Pharmaceutical Industries Ltd as part of the Israeli National Network of Excellence in Neuroscience (NNE) [1234944]. E.P. was supported by grants from the Israel Science Foundation (ISF) [561/11]; and the European Research Council (ERC) [309377]. S.N. was supported by grants from Teva Pharmaceutical Industries Ltd. under the Israeli National Network of Excellence in Neuroscience.
Conflicts of interest: None declared.
References
- Abashidze A, Gold V, Anavi Y, Greenspan H, Weil M. Involvement of IKAP in peripheral target innervation and in specific JNK and NGF signaling in developing PNS neurons. PLoS One. 2014;9:e113428. doi: 10.1371/journal.pone.0113428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod FB, Goldberg JD, Ye XY, Maayan C. Survival in familial dysautonomia: Impact of early intervention. J Pediatr. 2002;141:518–523. doi: 10.1067/mpd.2002.127088. [DOI] [PubMed] [Google Scholar]
- Axelrod FB, Krey L, Glickstein JS, Allison JW, Friedman D. Preliminary observations on the use of midodrine in treating orthostatic hypotension in familial dysautonomia. J Auton Nerv Syst. 1995;55:29–35. doi: 10.1016/0165-1838(95)00023-q. [DOI] [PubMed] [Google Scholar]
- Bochner R, Ziv Y, Zeevi D, Donyo M, Abraham L, Ashery-Padan R, Ast G. Phosphatidylserine increases IKBKAP levels in a humanized knock-in IKBKAP mouse model. Hum Mol Genet. 2013;22:2785–2794. doi: 10.1093/hmg/ddt126. [DOI] [PubMed] [Google Scholar]
- Creppe C, Malinouskaya L, Volvert ML, Gillard M, Close P, Malaise O, Laguesse S, Cornez I, Rahmouni S, Ormenese S, Belachew S, Malgrange B, Chapelle JP, Siebenlist U, Moonen G, Chariot A, Nguyen L. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell. 2009;136:551–564. doi: 10.1016/j.cell.2008.11.043. [DOI] [PubMed] [Google Scholar]
- d’Ydewalle C, Krishnan J, Chiheb DM, Van Damme P, Irobi J, Kozikowski AP, Vanden Berghe P, Timmerman V, Robberecht W, Van Den Bosch L. HDAC6 inhibitors reverse axonal loss in a mouse model of mutant HSPB1-induced Charcot-Marie-Tooth disease. Nat Med. 2011;17:968–974. doi: 10.1038/nm.2396. [DOI] [PubMed] [Google Scholar]
- Dietz KC, Casaccia P. HDAC inhibitors and neurodegeneration: at the edge between protection and damage. Pharmacol Res. 2010;62:11–17. doi: 10.1016/j.phrs.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dompierre JP, Godin JD, Charrin BC, Cordelieres FP, King SJ, Humbert S, Saudou F. Histone deacetylase 6 inhibition compensates for the transport deficit in Huntington's disease by increasing tubulin acetylation. J Neurosci. 2007;27:3571–3583. doi: 10.1523/JNEUROSCI.0037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donyo M, Hollander D, Abramovitch Z, Naftelberg S, Ast G. Phosphatidylserine enhances IKBKAP transcription by activating the MAPK/ERK signaling pathway. Hum Mol Genet. 2016;25:1307–1317. doi: 10.1093/hmg/ddw011. [DOI] [PubMed] [Google Scholar]
- Fogelson MH, Rorke LB, Kaye R. Spinal cord changes in familial dysautonomia. Arch Neurol. 1967;17:103–108. doi: 10.1001/archneur.1967.00470250107012. [DOI] [PubMed] [Google Scholar]
- Gardiner J, Barton D, Marc J, Overall R. Potential role of tubulin acetylation and microtubule-based protein trafficking in familial dysautonomia. Traffic. 2007;8:1145–1149. doi: 10.1111/j.1600-0854.2007.00605.x. [DOI] [PubMed] [Google Scholar]
- Gaub P, Tedeschi A, Puttagunta R, Nguyen T, Schmandke A, Di Giovanni S. HDAC inhibition promotes neuronal outgrowth and counteracts growth cone collapse through CBP/p300 and P/CAF-dependent p53 acetylation. Cell Death Differ. 2010;17:1392–1408. doi: 10.1038/cdd.2009.216. [DOI] [PubMed] [Google Scholar]
- Godena VK, Brookes-Hocking N, Moller A, Shaw G, Oswald M, Sancho RM, Miller CC, Whitworth AJ, De Vos KJ. Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nat Commun. 2014;5:5245. doi: 10.1038/ncomms6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempen B, Brion JP. Reduction of acetylated alpha-tubulin immunoreactivity in neurofibrillary tangle-bearing neurons in Alzheimer's disease. J Neuropathol Exp Neurol. 1996;55:964–972. doi: 10.1097/00005072-199609000-00003. [DOI] [PubMed] [Google Scholar]
- Hinckelmann MV, Zala D, Saudou F. Releasing the brake: restoring fast axonal transport in neurodegenerative disorders. Trends Cell Biol. 2013;23:634–643. doi: 10.1016/j.tcb.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP. HDAC6 is a microtubule-associated deacetylase. Nature. 2002;417:455–458. doi: 10.1038/417455a. [DOI] [PubMed] [Google Scholar]
- Jackson MZ, Gruner KA, Qin C, Tourtellotte WG. A neuron autonomous role for the familial dysautonomia gene ELP1 in sympathetic and sensory target tissue innervation. Development. 2014;141:2452–2461. doi: 10.1242/dev.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakovcevski M, Akbarian S. Epigenetic mechanisms in neurological disease. Nat Med. 2012;18:1194–1204. doi: 10.1038/nm.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren H, Donyo M, Zeevi D, Maayan C, Pupko T, Ast G. Phosphatidylserine increases IKBKAP levels in familial dysautonomia cells. PLoS One. 2010;5:e15884. doi: 10.1371/journal.pone.0015884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehavi O, Aizenstein O, Bercovich D, Pavzner D, Shomrat R, Orr-Urtreger A, Yaron Y. Screening for familial dysautonomia in Israel: evidence for higher carrier rate among Polish Ashkenazi Jews. Genet Test. 2003;7:139–142. doi: 10.1089/109065703322146830. [DOI] [PubMed] [Google Scholar]
- Li G, Jiang H, Chang M, Xie H, Hu L. HDAC6 alpha-tubulin deacetylase: a potential therapeutic target in neurodegenerative diseases. J Neurol Sci. 2011;304:1–8. doi: 10.1016/j.jns.2011.02.017. [DOI] [PubMed] [Google Scholar]
- Mahloudji M, Brunt PW, McKusick VA. Clinical neurological aspects of familial dysautonomia. J Neurol Sci. 1970;11:383–395. doi: 10.1016/0022-510x(70)90083-3. [DOI] [PubMed] [Google Scholar]
- Maragno H, Rodella P, Silva Freitas J, Fernando Takase L. The effects of acute and chronic administration of phosphatidylserine on cell proliferation and survival in the dentate gyrus of adult and middle-aged rats. Brain Res. 2015;1609:72–81. doi: 10.1016/j.brainres.2015.03.017. [DOI] [PubMed] [Google Scholar]
- Millecamps S, Julien JP. Axonal transport deficits and neurodegenerative diseases. Nat Rev Neurosci. 2013;14:161–176. doi: 10.1038/nrn3380. [DOI] [PubMed] [Google Scholar]
- Monastra G, Bruni A. Decreased serum level of tumor necrosis factor in animals treated with lipopolysaccharide and liposomes containing phosphatidylserine. Lymphokine Cytokine Res. 1992;11:39–43. [PubMed] [Google Scholar]
- Naftelberg S, Abramovitch Z, Gluska S, Yannai S, Joshi Y, Donyo M, Ben-Yaakov K, Gradus T, Zonszain J, Farhy C, Ashery-Padan R, Perlson E, Ast G. Phosphatidylserine ameliorates neurodegenerative symptoms and enhances axonal transport in a mouse model of familial dysautonomia. PLoS Genet. 2016;12:e1006486. doi: 10.1371/journal.pgen.1006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan Y, Martin D, Campbell VA, Lynch MA. Evidence of a protective effect of phosphatidylserine-containing liposomes on lipopolysaccharide-induced impairment of long-term potentiation in the rat hippocampus. J Neuroimmunol. 2004;151:12–23. doi: 10.1016/j.jneuroim.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Palma JA, Norcliffe-Kaufmann L, Fuente-Mora C, Percival L, Mendoza-Santiesteban C, Kaufmann H. Current treatments in familial dysautonomia. Expert Opin Pharmacother. 2014;15:2653–2671. doi: 10.1517/14656566.2014.970530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J, Pytel BA. Quantitative studies of sympathetic ganglia and spinal cord intermedio-lateral gray columns in familial dysautonomia. J Neurol Sci. 1978a;39:47–59. doi: 10.1016/0022-510x(78)90187-9. [DOI] [PubMed] [Google Scholar]
- Pearson J, Pytel B. Quantitative studies of ciliary and sphenopalatine ganglia in familial dysautonomia. J Neurol Sci. 1978b;39:123–130. doi: 10.1016/0022-510x(78)90193-4. [DOI] [PubMed] [Google Scholar]
- Pearson J, Dancis J, Axelrod F, Grover N. The sural nerve in familial dysautonomia. J Neuropathol Exp Neurol. 1975;34:413–424. doi: 10.1097/00005072-197509000-00004. [DOI] [PubMed] [Google Scholar]
- Pearson J, Pytel BA, Grover-Johnson N, Axelrod F, Dancis J. Quantitative studies of dorsal root ganglia and neuropathologic observations on spinal cords in familial dysautonomia. J Neurol Sci. 1978;35:77–92. doi: 10.1016/0022-510x(78)90103-x. [DOI] [PubMed] [Google Scholar]
- Perlson E, Maday S, Fu MM, Moughamian AJ, Holzbaur EL. Retrograde axonal transport: pathways to cell death? Trends Neurosci. 2010;33:335–344. doi: 10.1016/j.tins.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed NA, Cai D, Blasius TL, Jih GT, Meyhofer E, Gaertig J, Verhey KJ. Microtubule acetylation promotes kinesin-1 binding and transport. Curr Biol. 2006;16:2166–2172. doi: 10.1016/j.cub.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Riley CM, Day RL, Greeley DM, Langford W. Central autonomic dysfunction with defective lacrimation; report of five cases. Pediatrics. 1949;3:468–478. [PubMed] [Google Scholar]
- Rivieccio MA, Brochier C, Willis DE, Walker BA, D’Annibale MA, McLaughlin K, Siddiq A, Kozikowski AP, Jaffrey SR, Twiss JL, Ratan RR, Langley B. HDAC6 is a target for protection and regeneration following injury in the nervous system. P Proc Natl Acad Sci U S A. 2009;106:19599–19604. doi: 10.1073/pnas.0907935106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum J. Cytoskeleton: functions for tubulin modifications at last. Curr Biol. 2000;10:R801–803. doi: 10.1016/s0960-9822(00)00767-3. [DOI] [PubMed] [Google Scholar]
- Salani M, Norcliffe-Kaufmann L, Martinez J, Morini E, Axelrod F, Slaugenhaupt S. Phosphatidylserine: A potential gene modifying therapy for Familial Dysautonomia? American Society of Human Genetics Boston USA. 2013 [Google Scholar]
- Wan DW, Levy J, Ginsburg HB, Kaufmann H, Axelrod FB. Complicated peptic ulcer disease in three patients with familial dysautonomia. J Clin Gastroenterol. 2011;45:611–613. doi: 10.1097/MCG.0b013e3181e5e8ed. [DOI] [PubMed] [Google Scholar]
- Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol. 2003;4:938–947. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]