Abstract
Intracerebral hemorrhage (ICH) is the most severe cerebrovascular disease, which represents a leading cause of death and disability in developed countries. However, therapeutic options are limited, so is mandatory to investigate repairing processes after stroke in order to develop new therapeutic strategies able to promote brain repair processes. Therapeutic angiogenesis and vasculogenesis hold promise to improve outcome of ICH patients. In this regard, circulating endothelial progenitor cells (EPCs) have recently been suggested to be a marker of vascular risk and endothelial function. Moreover, EPC levels have been associated with good neurological and functional outcome as well as reduced residual hematoma volume in ICH patients. Finally, experimental and clinical studies indicate that EPC might mediate endothelial cell regeneration and neovascularization. Therefore, EPC-based therapy could be an excellent therapeutic option in ICH. In this mini-review, we discuss the present status of knowledge about the possible therapeutic role of EPCs in ICH, molecular mechanisms, and the future perspectives and strategies for their use in clinical practice.
Keywords: cellular therapy, endothelial progenitor cells, growth factors, intracerebral hemorrhage, neurorepair, outcome
Intracerebral Hemorrhage (ICH) is a Devastating Disease and it Lacks Medical Treatment
ICH is the subtype of stroke with the highest morbimortality. It is characterized by a primary rupture of an intracerebral blood vessel, leading to blood accumulation within the brain parenchyma. Overall, ICH is a major cause of death and disability in developed countries, and its incidence is growing in parallel with the increment of elderly population. Surgical procedures have restricted indications and represent only a small clinically relevant survival advantage. Despite being the most severe cerebrovascular disorder, ICH has no specific pharmacological treatment. As in ischemic stroke, neuroprotective strategies have so far yielded repeated failure in clinical trials due to side effects or to lack of effectiveness. On the other hand, neurorepair approaches focused on the repairing of damaged vessels emerge as possible therapeutic targets. Bone marrow-derived progenitor cells (BMPCs) have evidenced beneficial effects in animal models of ICH, such as immature neuron formation, synaptogenesis, neuronal migration, reduced tissue loss and neurological improvement (Li et al., 2015). Circulating endothelial progenitor cells (EPCs), a subtype of BMPCs, have ample evidence supporting their important role in re-endothelization, angiogenesis and vasculogenesis. Indeed, it has been described that patients with ICH have increased levels of circulating EPCs (Paczkowska et al., 2013). Congruently, a recent research by our group has observed that EPC levels are associated with good neurological and functional outcome, as well as reduced residual volume in patients with acute ICH (Pias-Peleteiro et al., 2016). EPC supported angiogenesis would be an early and crucial step in neurorepair, as it is likely linked to subsequent neurogenesis (Zhang et al., 2009). Thus, an EPC-based therapy, based on exogenous supplementation or endogenous stimulation, may be a viable therapeutic option in ICH, acting primarily through angiogenesis and secondarily through neurogenic mechanisms.
EPCs are Associated with Good Prognosis and Reduced Residual Volume in ICH, although the Underlying Mechanisms Remain Largely Unknown
A recent study published by our group (Pias-Peleteiro et al., 2016) represents the first prospective analysis evaluating the association between circulating EPCs and functional outcome in patients with ICH. EPCs were defined as CD34+/CD133+/KDR+, which is widely accepted as an optimal characterization (Urbich and Dimmeler, 2004). In this study, not only circulating EPC levels at day 7 were independently associated with good functional outcome at 12 months, but also residual ICH volume at 6 months was reduced and patients suffered milder neurological deficits. The fact that these associations were found regarding “late” EPC levels at day 7, and not at admission, shoulders the hypothesis that EPCs can mediate processes of chronic vessel repair and neurorepair.
These findings are in line with a previous study observing an independent association between higher increments of generic bone marrow CD34+ progenitor cells and reduced residual volume and better functional outcome at three months in patients with ICH (Sobrino et al., 2011). Nevertheless, the mechanisms underlying these benefits remain unclear. We hypothesize four complementary actions that may be involved: 1) EPC-mediated re-endothelization of damaged vessels would be the first mechanism, an EPC repairing action amply demonstrated in both animal and human models (Melchiorri et al., 2016); 2) A second mechanism would be EPC mediated vasculogenesis, a replacement of vessels too damaged to be simply re-endothelized. This process involves EPC recruitment from the bone marrow to the neovascularization areas, where they differentiate into mature endothelial cells (Grant et al., 2002); 3) Simultaneously to this direct formation of new blood vessels, EPCs can exert a paracrine action which would indirectly promote angiogenesis, being a possible third mechanism (Grant et al., 2002); 4) Finally, EPCs may play an early role in protecting the blood-brain barrier (BBB) in the acute phase of ICH (Borlongan, 2011). BBB disruption in ICH is caused both by a mechanic disruption of blood vessels due to the high pressure exerted by the blood accumulation and to uncontrolled inflammation. In a vicious circle, BBB disruption further aggravates inflammation favoring the leakage of more proinflammatory factors, which is associated with hematoma growth and subsequent poor outcome. This possible fourth mechanism is coherent with current findings from our group that show how impaired flow mediated dilation (a marker of endothelial function) is negatively correlated with EPC counts and positively associated with increased hematoma growth.
Angiogenesis links to neurogenesis, enhancing neurorepair processes: Angiogenesis is an early step in neurorepair that provides nutritive blood flow for subsequent neurogenesis. In addition, EPCs exert paracrine actions as they secrete factors that create a supportive microenvironment for neural regeneration and survival, such as VEGF and SDF-1. Thus, neuroblasts preferentially migrate towards the proximity of developing microvessels. Congruently, angiogenesis suppression markedly reduces migration of neuroblasts from the subventricular zone to the ischemic region (Zhang et al., 2009).
EPC-Based Cellular Therapy for ICH
Exogenous administration or endogenous stimulation? Resident pools of adult stem cells, such as EPCs, may be applied through two different strategies. The first one is exogenous administration, which implies isolating, harvesting and growing EPCs by means of in vitro procedures and subsequently administering them locally in the affected region or systemically in the blood circulation. In the case of allogeneic EPC transplantation, it is also debatable whether to obtain EPCs from stroke patients or from healthy subjects. Proteomic studies have analyzed differences in protein expression of early outgrowth colony forming unit-endothelial cell (CFU-EC) from ischemic stroke patients and healthy subjects (Brea et al., 2011). These investigations have concluded that EPCs from stroke patients, with a higher expression of elongation factor 2 (eF2) and peroxiredoxin 1 (PRDX1) may be in a more advanced differentiation state than EPCs isolated from control subjects. On the other hand, CdC-42 and ERp29 were found to be up-regulated in EPCs from healthy subjects, hinting that these EPCs may have a greater capacity of proliferation. It is also debatable whether to use EPCS obtained from patients in the acute, subacute or chronic phases of stroke. Overall, coadministration of different types of progenitor/stem cells may constitute the best therapeutic strategy.
With regard to the optimal therapeutic window for EPC administration in ICH, based on our previous study, we consider that a continuous administration until day 7 represents the best protocol because this is the time-frame when the highest concentrations of EPCs were found (Pias-Peleteiro et al., 2016). Nevertheless, a potential beneficial effect of EPCs beyond day 7 cannot be discarded. This possibility is supported by a recent study regarding EPC response after a left ventricular assist device implantation, extending a possible EPC contribution to a 6 month frame after surgery (Ivak et al., 2016).
Lastly, intravenous infusion of EPCs is probably the optimal administration route. It evades the direct damage of the intracerebral route and gets round possible adverse effects of intra-arterial infusion such as embolisms.
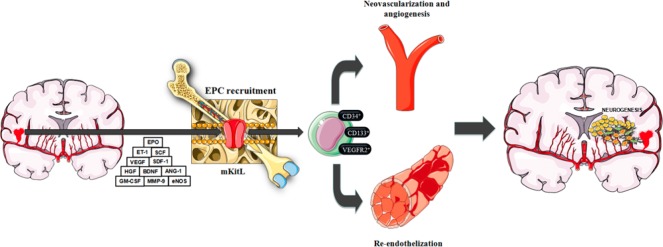
A feasible alternative to exogenous administration is endogenous stimulation of EPCs. The incorporation of EPCs from the bone marrow to neovascularization areas involves a sequential process including mobilization, chemoattraction, adhesion, migration, tissue invasion and in situ differentiation. Many molecular and physiological-pathological factors are involved in these processes (Sobrino et al., 2011; Paczkowska et al., 2013). EPC plasma levels in ICH patients hold a positive association with a higher expression of vascular endothelial growth factor (VEGF), stromal cell-derived factor 1 (SDF-1), hepatocyte growth factor (HGF) and endothelin 1 (ET-1). Angiopoietin 1 (ANG-1) and brain-derived neurotrophic factor (BDNF) may also play a role. The activity of matrix metalloproteinase 9 (MMP-9), which causes a massive release of stem cell factor (SCF) and activation of membrane bound Kit ligand (mKitL) also favors EPC recruitment and mobilization. Moreover, EPC release and mobilization is regulated by erythropoietin (EPO), endothelial nitric oxide synthase (eNOS), exercise and estrogens (Figure 1).
Figure 1.
Recruitment, re-endothelization and neurorepair processes mediated by EPCs.
Vascular trauma in the setting of ICH induces the expression of several cytoquines and trophic factors such as VEGF, SDF-1α, MMP-9, ANG-1, HGF, BDNF, SCF, EPO and eNOS. These molecular factors will in turn boost EPC recruitment from the bone marrow. EPCs will primarily engage in re-endothelization and neovascularization processes, which may lead to secondary further neurorepair processes. ICH patients with higher EPC levels will ultimately benefit from a better functional recovery. ANG-1: Angiopoietin 1; BDNF: brain-derived neurotrophic factor; eNOS: endothelial nitric oxide synthase; EPC: endothelial progenitor cell; EPO: erythropoietin; ET-1: endothelin 1; GM-CSF: granulocyte-macrophage colony-stimulating factor; HGF: hepatocyte growth factor; ICH: intracerebral hemorrhage; mKitL: membrane bound Kit ligand; MMP-9: matrix metalloproteinase 9; SCF: stem cell factor; SDF-1α: stromal cell-derived factor.
Moreover, several drugs, such as statins, EPO, metformin, G-CSF, angiotensin II type 1 receptors blocker, angiotensin-converting enzyme inhibitors, berberine, citicoline, recombinant tissue plasminogen activator (r-tPA) and PPAR-γ agonist have also been shown to increase the number, behavior and functional activity of EPCs in vitro and in vivo. On the other hand, the same factors that promote EPC mobilization, such as VEGF and SDF-1, as well as several drugs including statins and erythropoietin, play an important role in EPC migration, survival and differentiation.
Future Challenges regarding Cellular Therapy with EPCs in ICH
A notable obstacle for cell therapy is the small proportion of cells actually reaching the target areas. The development of new vectorization strategies such as the use of superparamagnetic iron oxide nanoparticles (SPION)-loaded EPCs (which may be magnetically guided to the areas in need of neurorepair) may improve this proportion (Carenza et al., 2014).
Safety is another major concern regarding cellular therapy with EPCs, as pathological angiogenesis within tumors depends on hematopoietic stem cells, including EPCs. Clinical trials with EPCs have so far demonstrated safety (D’Avola, 2016), but larger trials are needed in order to assess this important issue.
An ample evaluation of neuroplasticity in animal models of ICH following EPC treatment, including not only angiogenesis but also neurogenesis, sinaptogenesis and white matter remodeling, is another pending issue.
Induced pluripotent stem cells (iPSCs) technology represents a promising strategy for cellular-based therapies for ICH, including but not limited to EPCs. The therapeutic capability of human iPSC-derived EPCs (hiPSC-EPCs) has already been evidenced in animal models of hind-limb ischemia (Lai et al., 2013).
Conclusions
Both animal and human studies sustain that EPCs are recruited from bone marrow to participate in re-endothelization and vasculogenic processes following ICH damage. EPCs probably favor a more extensive neurorepair, as higher numbers of these cells are associated with reduced residual volume and a better clinical outcome. Endogenous stimulation of EPCs may represent the most straightforward strategy to boost circulating EPC numbers. Cellular therapy with EPCs represents a hopeful novel strategy for ICH, a disabling when not lethal disease currently lacking medical treatment.
Footnotes
Funding: This study has been partially supported by grants from the Spanish Ministry of Economy and Competitiveness (SAF2014-56336), the Instituto de Salud Carlos III (PI13/00292 & PI14/01879), the Spanish Research Network on Cerebrovascular Diseases (RETICS INVICTUS; RD12/0014), the Xunta de Galicia (Department of Education, GRC2014/027), and the European Union program FEDER. Furthermore, F. Campos (CP14/00154) and TS (CP12/03121) are recipients of a research contract from Miguel Servet Program of Instituto de Salud Carlos III. The funders had no role in the review design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest: None declared.
References
- Borlongan CV. Bone marrow stem cell mobilization in stroke: A ‘bonehead’ may be good after all! Leukemia. 2011;25:1674–1686. doi: 10.1038/leu.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brea D, Rodriguez-Gonzalez R, Sobrino T, Rodriguez-Yanez M, Blanco M, Castillo J. Proteomic analysis shows differential protein expression in endothelial progenitor cells between healthy subjects and ischemic stroke patients. Neurol Res. 2011;33:1057–1063. doi: 10.1179/1743132811Y.0000000038. [DOI] [PubMed] [Google Scholar]
- Carenza E, Barcelo V, Morancho A, Levander L, Boada C, Laromaine A, Roig A, Montaner J, Rosell A. In vitro angiogenic performance and in vivo brain targeting of magnetized endothelial progenitor cells for neurorepair therapies. Nanomedicine. 2014;10:225–234. doi: 10.1016/j.nano.2013.06.005. [DOI] [PubMed] [Google Scholar]
- D’Avola D, Fernández-Ruiz V, Carmona-Torre F, Mendez M, Pérez-Calvo J, Prósper F, Andreu E, Herrero JI, Iñarrairaegui M, Fuertes C, Bilbao JI, Sangro B, Prieto J, Quiroga J. Phase 1-2 pilot clinical trial in patients with decompensated liver cirrhosis treated with bone marrow-derived endothelial progenitor cells. Transl Res. 2016 doi: 10.1016/j.trsl.2016.02.009. pii:S1931-5244(16)00063-3. [DOI] [PubMed] [Google Scholar]
- Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–612. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- Ivak P, Pitha J, Wohlfahrt P, Kralova Lesna I, Stavek P, Melenovsky V, Dorazilova Z, Hegarova M, Stepankova J, Maly J, Sekerkova A, Turcani D, Netuka I. Biphasic response in number of stem cells and endothelial progenitor cells after left ventricular assist device implantation: A 6 month follow-up. Int J Cardiol. 2016;218:98–103. doi: 10.1016/j.ijcard.2016.05.063. [DOI] [PubMed] [Google Scholar]
- Lai WH, Ho JC, Chan YC, Ng JH, Au KW, Wong LY, Siu CW, Tse HF. Attenuation of hind-limb ischemia in mice with endothelial-like cells derived from different sources of human stem cells. PLoS One. 2013;8:e57876. doi: 10.1371/journal.pone.0057876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Bai W, Sun P, Zhou B, Hu B, Ying J. The effect of cxcl12 on endothelial progenitor cells: Potential target for angiogenesis in intracerebral hemorrhage. J Interferon Cytokine Res. 2015;35:23–31. doi: 10.1089/jir.2014.0004. [DOI] [PubMed] [Google Scholar]
- Melchiorri AJ, Bracaglia LG, Kimerer LK, Hibino N, Fisher JP. In vitro endothelialization of biodegradable vascular grafts via endothelial progenitor cell seeding and maturation in a tubular perfusion system bioreactor. Tissue Eng Part C Methods. 2016;22:663–670. doi: 10.1089/ten.tec.2015.0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paczkowska E, Golab-Janowska M, Bajer-Czajkowska A, Machalińska A, Ustianowski P, Rybicka M, Kłos P, Dziedziejko V, Safranow K, Nowacki P, Machaliński B. Increased circulating endothelial progenitor cells in patients with haemorrhagic and ischaemic stroke: The role of endothelin-1. J Neurol Sci. 2013;325:90–99. doi: 10.1016/j.jns.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Pias-Peleteiro J, Perez-Mato M, Lopez-Arias E, Rodríguez-Yáñez M, Blanco M, Campos F, Castillo J, Sobrino T. Increased endothelial progenitor cell levels are associated with good outcome in intracerebral hemorrhage. Sci Rep. 2016;6:28724. doi: 10.1038/srep28724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino T, Arias S, Perez-Mato M, Agulla J, Brea D, Rodríguez-Yáñez M, Castillo J. CD34+ progenitor cells likely are involved in the good functional recovery after intracerebral hemorrhage in humans. J Neurosci Res. 2011;89:979–985. doi: 10.1002/jnr.22627. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. Neurorestorative therapies for stroke: Underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]