Regeneration in the central nervous system (CNS) is limited, and CNS damage often leads to cognitive impairment or permanent functional motor and sensory loss. Impaired regenerative capacity is multifactorial and includes inflammation, loss of the blood-brain barrier, and alteration in the extracellular matrix (ECM). One of the main problems is the formation of a glial scar and the production of inhibitory ECM, such as proteoglycans, that generates a physical and mechanical barrier, impeding axonal regrowth (Figure 1A). However, in vivo studies of axons from injured spinal cords reveal that they initially enter an acute fragmentation period following lesion formation, which is followed by proximal axonal end regrowth over several weeks. At this point, it is possible to see the axonal tip advancing and branching with an erratic growth pattern (Kerschensteiner et al., 2005). The authors conclude that the impaired reinnervation is due not only to the presence of inhibitory ECM, but also to the absence of directional guiding to the synaptic counterpart. Similar axonal misguidance occurs during optic nerve regeneration, where injured axons can grow in the presence of neurotrophic factors, including ciliary neurotrophic factor (CNTF), although they follow irregular pathways (Pernet and Schwab, 2014).
Figure 1.
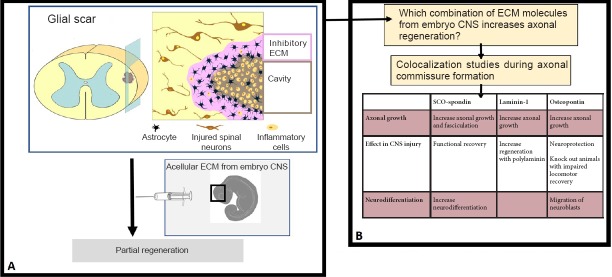
Schematic view of the approach proposed.
(A) The injection of acellular extracellular matrix (ECM) form embryo central nervous system (CNS) in the injured spinal cord results in partial regeneration. (B) Laminin, osteopontin and SCO-spondin are expressed together during axonal commissure formation, and all of them have properties in axonal growth, neurodifferentiation and CNS injury. Their combination in CNS injury therapies is a promissory approach.
More promising strategies to improve CNS regeneration include the combination of several approaches, such as reducing the inflammatory processes generated in response to the injury, addition of growth factors, incorporation of stem cells, and modification of the ECM. One of the approaches to induce matrix remodeling is to neutralize the intrinsic inhibitory matrix (e.g., enzymatic digestion of proteoglycans with chondroitinase ABC) and generate a permissive matrix where the axons can grow. With recent rapid advances in nanotechnology, the use of tissue-engineered scaffolds has allowed some advances in the reconstruction of injured tissues and reconnection of neuronal processes. These matrices are based on particular ECM molecules (e.g., laminin) as well as natural or synthetic polymers (e.g., chitosan or polyhydroxy acids) and decellularized tissue (review in Ricks et al., 2014).
The embryo as a model for regeneration: The poor regenerative capacity of the adult CNS contrasts with the remarkable plasticity displayed by embryonic and perinatal CNS. One reason for this difference is the change in the ECM components, which promote neuronal circuit formation and plasticity during the embryonic period but consolidate neuronal circuitry and inhibit regeneration in adults. Thus, one less explored possibility to improve regenerative capacity is to emulate the embryonic environment to which axons were exposed during development, permitting them to grow in a satisfactory way. Information from developmental biology studies has been used to recreate the environment for use in regeneration therapies in several organs, such as the kidney (Little et al., 2016). However, few studies have linked CNS regeneration with development. Of the few that have examined this possibility, they have principally consisted of the transplantation of immature cells into the injured CNS, including the use of immature astrocytes to promote axonal regeneration or oligodendrocyte precursors to improve myelination (Li and Leung, 2015). Transplantation of fetal neural stem cell in combination with a growth factor cocktail into injured spinal cords has been also analyzed (Steward et al., 2014). The implanted cells expanded and filled the space generated in the region of injury. Although these studies hold promise as future therapeutic tools, an essential problem with this approach is the occurrence of ectopic colonies derived from these cells at long distances from the transplant site (Steward et al., 2014).
In relation to the embryonic ECM, there are several in vivo and in vitro studies that have analyzed the individual effect of one ECM component on axonal growth and migration. However, the fetal ECM is a complex medium composed of several molecules with a high degree of interaction. One of the proposed approaches includes implanting an ECM bioscaffold from porcine or bovine tissues (Figure 1A). Preliminary results have shown that the effect of this technology is dependent on the age of the transplanted tissue, with fetal tissue being the best option as compared to adult-derived tissue, and the nervous system versus others tissues (Ren et al., 2015). However, animal-derived biomaterial has the risk of pathogen transmission as well as eliciting an immune response. Synthetic scaffolds have emerged as an alternative, more controllable tool, which have been successfully used for the regeneration of skin, bone, and peripheral nerves (review in Ricks et al., 2014). Thus, recreation of the fetal ECM in a synthetic scaffold may represent a novel regenerative approach; however, multicomponent studies that shed some light about the matrisome of the CNS are required, especially on the matrisome that axons navigate in the developing nervous system (Figure 1B). In this respect, it is important to not only study the expression of ECM molecules by biochemical or genetic analyses (e.g., transcriptomic studies), but also analyze the localization of the different ECM components. This aspect is important because the ECM components are interrelated, having different effects alone as compared to in combination. Similar effects have been observed in regenerative scaffolds, where the use of more than one component seems to have an advantageous effect.
The matrisome during CNS development: Although ECM components during CNS development have primarily been analyzed individually, there are a few studies that have examined the localization of several ECM components simultaneously. Recently, our group has performed a spatiotemporal analysis of eight ECM molecules during the development of the posterior commissure, an axonal tract located in the dorsal region of the caudal diencephalon and developed at early stages (Stanic et al., 2016). Some of these proteins, including osteopontin, are not detectable or at least not reported in adults; however, they reappear after trauma, although the reason for its expression is not totally understood. In the days that precede commissure development, no specific expression pattern of the proteins analyzed was identified with the exception of external basal membrane proteins. However, during maximus posterior commissure development, most of the proteins followed three expression patterns: 1) in the external limiting membrane (decorine, perlecan, and fibronectin); 2) in the forming corridor walls that delimit the region of axonal growth (tenascin and trisaccharide human natural killer-1 [HNK1]); or 3) inside the forming corridors, providing a permissive substrate that facilitates axonal advance (laminin and osteopontin) (Stanic et al., 2016). The colocalization of laminin and osteopontin can be important in axonal development, since in vitro studies show a synergistic effect on neurons plated on a mixture of both laminin and osteopontin as compared to when they are used separately (67% axonal growth vs. 41% and 15%, respectively).
In addition to osteopontin and laminin in the most dorsal region where the axons are highly fasciculated, a third protein, SCO-spondin, is added to the ECM. Because all three proteins act through β1-integrin receptors, it would be interesting to analyze how they compete or collaborate in order to bind these receptors. The possible effect of these proteins on regeneration has been studied separately. In the case of laminin, a positive effect on axonal growth has been shown using in vitro studies, and polylaminin, a polymerized form of laminin, promotes regeneration after spinal cord injury. In the case of SCO-spondin, effects on neurodifferentiation, axonal growth, and fasciculation in vivo and in vitro during CNS development have been reported (Stanic et al., 2010; Vera et al., 2014). In addition, a peptide derived from its sequence has been used in regeneration studies after spinal cord injury. Specifically, in two different models of spinal cord injury, this SCO-spondin peptide promotes axonal growth and functional recovery (Sakka et al., 2014). Similarly, osteopontin function is not only related with axonal growth, but also has been related with neuroprotection in stroke events, and migration of neuroblasts after cerebral ischemia or in Parkinson's disease. The pro-regenerative effects of osteopontin have also been shown in a spinal cord injury model as osteopontin-null animals experience greater tissue damage and impaired locomotor recovery as compared to wild-type animals (Hashimoto et al., 2007).
Future directions: The relationship between developmental biology and tissue regeneration is widely accepted, and in several organs, the focus of diverse therapies consists of the emulation of embryonic conditions. As for CNS, this option has been poorly explored, although some studies suggest that it is a promising approach. One of these studies reveals that transplantation of a cellular ECM from an embryonic nervous system is capable of repairing optic nerve injury better than that observed using adult acellular ECM (Ren et al., 2015). The questions that arise include the following. What does this acellular ECM contain? Which of these molecules are important for the regeneration observed? In this context, it would be interesting to study the matrisome during CNS development, and generate a scaffold comprised of these molecules. The recent study of the ECM during cerebral commissure development reveals the presence of SCO-spondin, laminin, and osteopontin in the ECM that surrounds growing axons. It is an interesting combination, since the three molecules have been individually used in promising regenerative therapies, showing that they not only promote axonal growth, but also have neuroprotective, neurodifferentiative, and anti-inflammatory proprieties. The use of a scaffold with these molecules in combination with other approaches, such as injection of growth factors or neural stem cells, may represent a new alternative to improve CNS regeneration.
References
- Hashimoto M, Sun D, Rittling SR, Denhardt DT, Young W. Osteopontin-deficient mice exhibit less inflammation, greater tissue damage, and impaired locomotor recovery from spinal cord injury compared with wild-type controls. J Neurosci. 2007;27:3603–3611. doi: 10.1523/JNEUROSCI.4805-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschensteiner M, Schwab ME, Lichtman JW, Misgeld T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat Med. 2005;11:572–577. doi: 10.1038/nm1229. [DOI] [PubMed] [Google Scholar]
- Li N, Leung GK. Oligodendrocyte precursor cells in spinal cord injury: A review and update. Biomed Res Int. 2015;2015:235195. doi: 10.1155/2015/235195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little MH, Combes AN, Takasato M. Understanding kidney morphogenesis to guide renal tissue regeneration. Nat Rev Nephrol. 2016;12:624–635. doi: 10.1038/nrneph.2016.126. [DOI] [PubMed] [Google Scholar]
- McCreedy DA, Sakiyama-Elbert SE. Combination therapies in the CNS: engineering the environment. Neurosci Lett. 2012;519:115–121. doi: 10.1016/j.neulet.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernet V, Schwab ME. Lost in the jungle: new hurdles for optic nerve axon regeneration. Trends Neurosci. 2014;37:381–387. doi: 10.1016/j.tins.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Ren T, van der Merwe Y, Steketee MB. Developing extracellular matrix technology to treat retinal or optic nerve injury(1,2,3) eNeuro 2:ENEURO.0077-15.2015. 2015 doi: 10.1523/ENEURO.0077-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricks CB, Shin SS, Becker C, Grandhi R. Extracellular matrices, artificial neural scaffolds and the promise of neural regeneration. Neural Regen Res. 2014;9:1573–1577. doi: 10.4103/1673-5374.141778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakka L, Delétage N, Lalloué F, Duval A, Chazal J, Lemaire JJ, Meiniel A, Monnerie H, Gobron S. SCO-spondin derived peptide NX210 induces neuroprotection in vitro and promotes fiber regrowth and functional recovery after spinal cord injury. PLoS One. 2014;9:e93179. doi: 10.1371/journal.pone.0093179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanic K, Montecinos H, Caprile T. Subdivisions of chick diencephalic roof plate: implication in the formation of the posterior commissure. Dev Dyn. 2010;239:2584–2593. doi: 10.1002/dvdy.22387. [DOI] [PubMed] [Google Scholar]
- Stanic K, Saldivia N, Förstera B, Torrejón M, Montecinos H, Caprile T. Expression patterns of extracellular matrix proteins during posterior commissure development. Front Neuroanat. 2016;10:89. doi: 10.3389/fnana.2016.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera A, Stanic K, Montecinos H, Torrejón M, Marcellini S, Caprile T. SCO-spondin from embryonic cerebrospinal fluid is required for neurogenesis during early brain development. Front Cell Neurosci. 2013;7:80. doi: 10.3389/fncel.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]