Abstract
Aging is a key risk factor for cognitive decline and age-related neurodegenerative disorders. Also, an age-related decrease in sex steroid hormones may have a negative impact on the formation of neurofibrillary tangles (NFTs); these hormones can regulate Tau phosphorylation and the principal kinase GSK3β involved in this process. Hormone replacement therapy decreases NFTs, but it increases the risk of some types of cancer. However, other synthetic hormones such as tibolone (TIB) have been used for hormone replacement therapy. The aim of this work was to evaluate the long-term effects of TIB (0.01 mg/kg and 1 mg/kg, intragastrically for 12 weeks) on the content of total and hyperphosphorylated Tau (PHF-1) proteins and the regulation of GSK3β/Akt/PI3K pathway and CDK5/p35/p25 complexes in the hippocampus of aged male mice. We observed that the content of PHF-1 decreased with TIB administration. In contrast, no changes were observed in the active form of GSK3β or PI3K. TIB decreased the expression of the total and phosphorylated form of Akt while increased that of p110 and p85. The content of CDK5 was differentially modified with TIB: it was increased at low doses and decreased at high doses. When we analyzed the content of CDK5 activators, an increase was found on p35; however, the content of p25 decreased with administration of low dose of TIB. Our results suggest a possible mechanism of action of TIB in the hippocampus of aged male mice. Through the regulation of Tau and GSK3β/Akt/PI3K pathway, and CDK5/p35/p25 complexes, TIB may modulate neuronal plasticity and regulate learning and memory processes.
Keywords: nerve regeneration, Tibolone, hippocampus, aged mice, sex steroids, Akt, GSK3β, PI3K, neural plasticity, Tau, neurofibrillary tangles, neural regeneration
Introduction
The association between low testosterone levels and increased risk for Alzheimer's disease (AD) in aged men may reflect the loss of beneficial androgen-mediated actions in the brain (Rosario et al., 2010). There are several androgen actions potentially relevant to AD, including promotion of neuron viability (Pike, 2001; Ramsden et al., 2003), synaptic plasticity (Leranth et al., 2003), selected aspects of cognition (Cherrier et al., 2005; Moffat, 2005; Janowsky, 2006), and reduction of Tau phosphorylation (Papasozomenos, 1997).
Neurofibrillary tangles (NFTs) are predominantly composed of post-translationally modified forms of the microtubule-associated protein Tau and can become widespread in hippocampal, neocortical, and limbic neurons in AD (Yankner et al., 2008).
Tau modulates the extent and rate of microtubule assembly and plays an essential role in morphogenetic processes, such as axonal growth (Drechsel et al., 1992). It also increases the rate of tubulin polymerization, decreases the rate of transit into the shrinking phase, and inhibits the rate of depolymerization (Drechsel et al., 1992).
In several diseases, hyperphosphorylation of Tau at multiple sites is related with its dissociation from microtubules and aggregation into oligomeric and fibrillar forms. This hyperphosphorylation may destabilize microtubules and impair anterograde axonal transport of essential macromolecules and organelles to synaptic endings (Skovronsky et al., 2006).
It has been shown that several kinases regulate Tau phosphorylation, but only two have been co-immunoprecipitated with microtubules, GSK3β and CDK5 (Ishiguro et al., 1992; Flaherty et al., 2000). CDK5 is a member of the cyclin-dependent protein kinase family. Whereas CDK5 does not have any role in the cell cycle, it participates in neuronal development and survival, phosphorylation of cytoskeletal proteins, and synaptic plasticity (Malumbres and Barbacid, 2005; Shukla et al., 2012).
Some evidence has suggested that GSK3β levels are increased in AD brains (Baum et al., 1996; Imahori and Uchida, 1997), and that the imbalance in GSK3β activation could compromise neurotrophin signaling. The abnormal Tau phosphorylation that results from increased activity of GSK3β could lead to sequestration of Tau, inhibition of microtubule formation, destabilization of the existing cytoskeleton, and promotion of assembly of large Tau-containing aggregates, and these would inhibit both anterograde and retrograde microtubule-based transport (Niewiadomska et al., 2006).
Some gonadal hormones such as estrogens, progestogens, and androgens can modulate the activation of Tau and GSK3β in female rats (Pinto-Almazán et al., 2012). In a mouse model of AD, dihydrotestosterone can prevent an increase of hyperphosphorylation of Tau as well (Rosario et al., 2010). In addition, different doses of estrogens have been shown to exert different effects (Espinosa-Raya et al., 2011).
Tibolone (TIB), a synthetic steroid, is widely prescribed to treat menopausal symptoms. TIB is metabolized into three biologically active metabolites: 3α-hydroxy and 3β-hydroxy (estrogenic), and Δ-4 keto isomer, which displays progestogenic and androgenic effects (Kloosterboer, 2001; Campisi and Marengo, 2007). TIB metabolites circulate as inactive forms, and its conversion to bioactive forms depends on tissue-specific desulfation (Verheuland Kloosterboer, 2006). TIB also exhibited neuroprotective effects when prevented oxidative stress neurodegeneration in male rats (Pinto-Almazán et al., 2014). TIB also exhibits different effects on memory and learning in a dose-dependent manner (Espinosa-Raya et al., 2012; Farfán-García et al., 2014). However, the effects of TIB on the activation of Tau protein and its signaling cascade in the brain of aged males are unknown. The aim of this study was to evaluate the long-term effects of two (low and high) doses of TIB on Tau hyperphosphorylation and PI3K/Akt/GSK3β signaling pathway, and CDK5/p35/p25 complexes.
Materials and Methods
Animals
Adult male aged (18 months) 129/C57BL/6 mice, weighing 25 ± 5 g, were housed five per cage, under a 12-hour light/dark cycle (lights on at 9:00 p.m.) and provided water and food ad libitum. All procedures were performed by the Mexican Guidelines for Animal Care and Handling (NOM-062-ZOO-1999). All efforts were made to minimize animal discomfort and reduce the number of animals used.
Treatments
Aged intact male mice were randomly divided into three groups (n = 6 per group). According to previous reports, mice in these groups were intregastrically administered vehicle (Veh), 0.01 mg/kg (low dose), or 1.0 mg/kg (high dose) of TIB (Sigma-Aldrich, San Luis, MO, USA), respectively once a day for 12 successive weeks (Espinosa-Raya et al., 2012; Farfán-García et al., 2014).
Western blot analysis
Twenty-four hours after the last TIB administration (at 10:00 a.m.), mice were decapitated and the brains were removed. The hippocampus was dissected according to the Atlas of Paxinos and Watson (1998) and then immediately homogenized in lysis buffer with protease inhibitors for protein extraction, as previously described (Espinosa-Raya et al., 2012). Protein samples were obtained by centrifugation and quantified using the Bradford method (Bio-Rad, Hercules, CA, USA). Proteins (50 μg) were separated by electrophoresis and transferred to Immobilon-P membranes (Millipore, Bedford, MA, USA). Membranes were blocked with Blocking Buffer (Bio-Rad) and incubated with primary antibodies (diluted 1:1,000). To determine the molecular weight of the proteins, broad range pertained protein markers were added.
The following antibodies were used: rabbit anti-tau (H150) polyclonal antibody, mouse anti-GSK3β monoclonal antibody, mouse anti-phosphorylated GSK3β (pSer9 GSK3β) monoclonal antibody, rabbit Akt polyclonal antibody, rabbit pAkt1/2/3 (Ser473) polyclonal antibody, rabbit PI3-kinase (p110) antibody, rabbit pPI3 kinase p110g (Tyr485) polyclonal antibody, mouse PI3-kinase (p85α) monoclonal antibody, goat pPI3 kinase p85a (Tyr508) antibody and rabbit CDK5 (J3) polyclonal antibody were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Rabbit p35/25 (C64B10) polyclonal antibody was purchased from Cell Signaling (Danvers, MA, USA). Mouse anti-glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) was purchased from Chemicon (Billerica, MA, USA). Rabbit anti-tau (PHF-1, phospho S396) monoclonal antibody was purchased from Abcam (Cambridge, UK).
The abovementioned membranes were incubated overnight at 4°C with the primary antibody, then washed and incubated with anti-mouse or anti-rabbit secondary antibody as appropriate for 2 hours at room temperature (Santa Cruz Biotechnology, diluted 1:15,000). Immunoreactive bands were detected using an enhanced chemiluminescence system (GE Healthcare, Piscataway, NJ, USA). Subsequently, the membranes were stripped with a commercial solution (Chemicon, Billerica, MA, USA) and reprobed with anti-GAPDH monoclonal antibody detected by the system mentioned above. The intensity of protein bands was quantified and analyzed by densitometry (KODAK 1D Image Analysis Software). The density of each band was normalized to its respective loading control (GAPDH). Samples from all animal groups were processed concurrently and under the same conditions in each experiment.
Statistical analysis
All data are presented as the mean ± standard error (SE) and analyzed by one-way analysis of variance to compare group means for each variable. Tukey's post-hoc test was used to determine differences between means. Prism 5.0 program (GraphPad, La Jolla, CA, USA) was used to calculate probability values. P values < 0.05 were considered statistically significant.
Results
Effect of TIB on the content of Tau and GSK3β
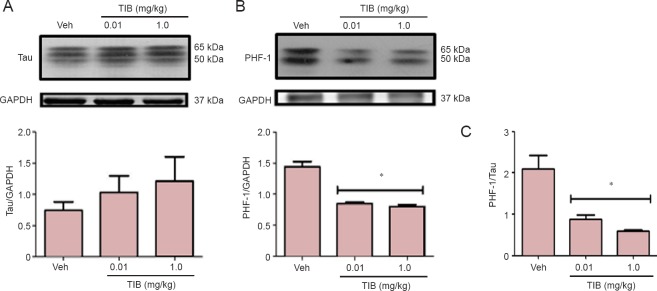
Western blot was used to analyze the effect of TIB on the content of PHF-1 and total Tau in hippocampal samples of aged mice. The expression of PHF-1 decreased with TIB treatment at both doses (Figure 1A); in contrast, the expression of total Tau was unaffected by TIB (Figure 1B). Furthermore, the ratio of hyperphosphorylated Tau vs. total Tau was significantly increased (P < 0.05) after TIB treatment at either dose (Figure 1C).
Figure 1.
Changes in the content of total Tau and PHF-1 in the hippocampus of aged mice treated with tibolone (TIB).
(A) Western blots for total Tau content in the hippocampus of aged mice. (B) PHF-1 levels in the hippocampus of aged mice treated with TIB. GAPDH was used to correct differences of total loaded protein. Proteins detected by western blot analysis from the hippocampus of mice treated with TIB were quantified by densitometric analysis and corrected using GAPDH protein content data. (C) The ratio of PHF-1 and Tau to their respective control (GAPDH) expression in the hippocampus of mice treated with low- and high-dose TIB. Results are expressed as the mean ± SE of six individual experiments. *P < 0.05, vs. vehicle (Veh).
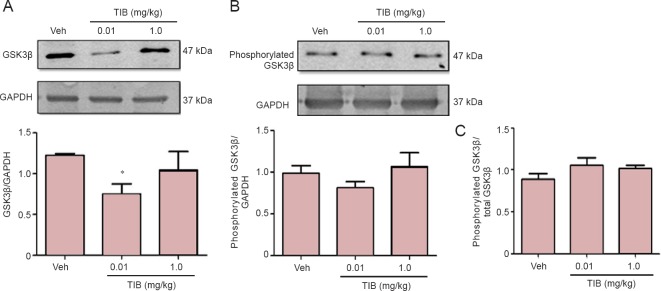
Tau phosphorylation is associated with the modification of GSK3β phosphorylation (Ser9) (Haque et al., 1999). Therefore, we analyzed the expression of total GSK3β, and the levels of phosphorylated GSK3β (Ser9) were assessed to determine if the expression of total GSK3β (Figure 2) was decreased in the hippocampus with the low-dose TIB treatment. However, TIB did not change the expression of phosphorylated GSK3β (Ser9). Therefore, there was no difference in the ratio of phosphorylated vs. total GSK3β.
Figure 2.
Changes in the content of total and phosphorylated GSK3β in the hippocampus of aged mice treated with tibolone (TIB).
Western blots for total (A) and phosphorylated (Ser9) (B) GSK3β content in the hippocampus of aged mice. GAPDH was used to correct differences of total loaded protein. Proteins detected by western blot analysis from the hippocampus of mice treated with TIB were quantified by densitometric analysis and corrected using GAPDH protein content data. (C) Ratio of phosphorylated (Ser9) GSK3β to GSK3β. Results are expressed as the mean ± SE of six individual experiments. *P < 0.05, vs. vehicle (Veh).
Effect of TIB on the content of PI3K/Akt pathway
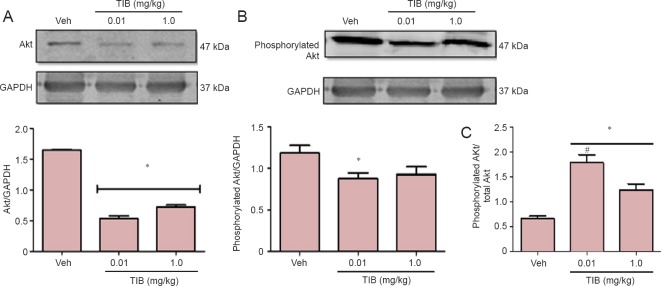
PI3K/Akt pathway is involved in the activation of GSK3β (Woodgett, 1990), so we investigated if there were any changes in Akt content. A decrease in the total content of Akt after treatment with TIB at both doses was observed (Figure 3A). Phosphorylated Akt content decreased only with the low-dose TIB treatment (Figure 3B). Figure 3C shows that the ratio of phosphorylated Akt and total Akt increased with the low-dose TIB while it decreased with treatment with high-dose TIB.
Figure 3.
Changes in the content of total and phosphorylated Akt in the hippocampus of aged mice treated with tibolone (TIB).
Western blots for total (A) and phosphorylated (B) Akt content in the hippocampus of aged mice. GAPDH was used to correct differences of total loaded protein. Proteins detected by western blot analysis from the hippocampus of mice treated with TIB were quantified by densitometric analysis and corrected using GAPDH protein content data. (C) Ratio of phosphorylated to total Akt. Results are expressed as the mean ± SE of six individual experiments. *P < 0.05 vs. vehicle (Veh); #P < 0.05, vs. 1.0 mg/kg TIB.
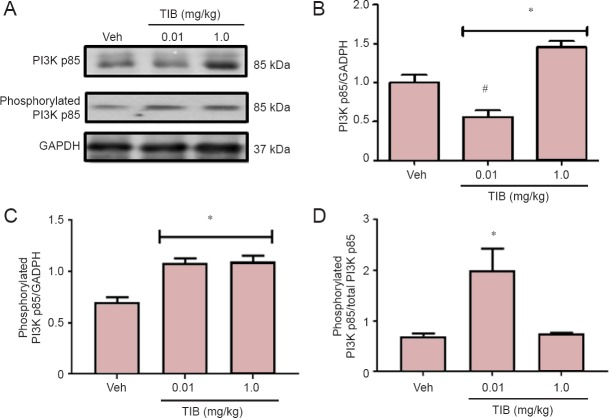
The content of the catalytic subunit of PI3 kinase, p110, and its phosphorylated form (pPI3 kinase), were also analyzed (Figure 4). No changes were observed for total PI3 kinase p110 (Figure 4B), whereas TIB increased the expression of pPI3 kinase p110 (Figure 4C). This effect was obvious when phosphorylated PI3K/PI3K ratio was analyzed (Figure 4D). The levels of the regulatory subunit of PI3 kinase p85 decreased with low-dose TIB treatment and increased with high-dose TIB treatment (Figure 5B). TIB at both doses increased the expression of pPI3 kinase p85 (Figure 5C), and the pPI3Kp85/PI3Kp85 ratio was the highest after treatment with TIB at 0.01 mg/kg (Figure 5D).
Figure 4.
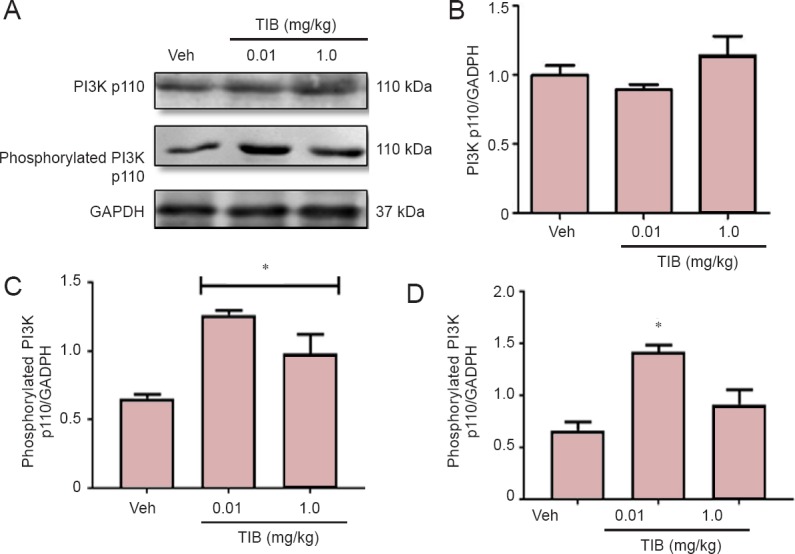
Changes in the content of the catalytic subunit PI3K p110 in the hippocampus of aged mice treated with tibolone (TIB).
Representative western blots for PI3K p110 and phosphorylated p110 content in the hippocampus of aged mice. GAPDH was used to correct differences of total loaded protein. Proteins detected by western blot analysis from the hippocampus of mice treated with TIB were quantified by densitometric analysis and corrected using GAPDH protein content data. Densitometric analysis of total (B) and phosphorylated (C) catalytic subunit PI3K p110. (D) Ratio of phosphorylated versus total PI3K p100. Results are expressed as the mean ± SE of six individual experiments. *P < 0.05, vs. vehicle (Veh).
Figure 5.
Changes in the content of regulatory subunit PI3K p85 in the hippocampus of aged mice treated with tibolone (TIB).
Representative western blots for total PI3K p85 and phosphorylated p85 content in the hippocampus of aged mice. GAPDH was used to correct differences of total loaded protein. Proteins detected by western blot analysis from the hippocampus of mice treated with TIB were quantified by densitometric analysis and corrected using GAPDH protein content data. Densitometric analysis of total (B) and (C) phosphorylated catalytic subunit PI3K p85. (D) Ratio of phosphorylated versus total PI3K p85. Results are expressed as the mean ± SE of six individual experiments.*P < 0.05, vs. vehicle (Veh); #P < 0.05, vs. 1.0 mg/kg TIB.
Effect of TIB on the content of CDK5/p35/p25 complexes
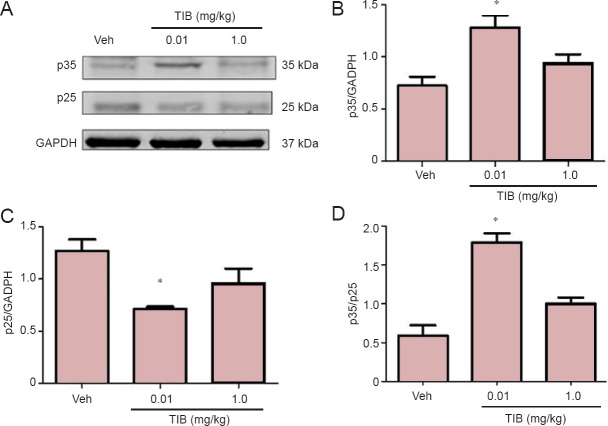
Another kinase involved in the regulation of Tau hyperpho- sphorylation is CDK5. We analyzed the expression of CDK5 and its activators p35 and p25. The content of CDK5 was differentially modified with TIB treatment: it increased with treatment with low dose TIB, and with treatment with high dose TIB, decreased CDK5 content was observed (Figure 6). When we analyzed the content of the activators, an increase was observed in the content of p35. In contrast, the content of p25 decreased with treatment with low dose TIB (Figure 7).
Figure 6.
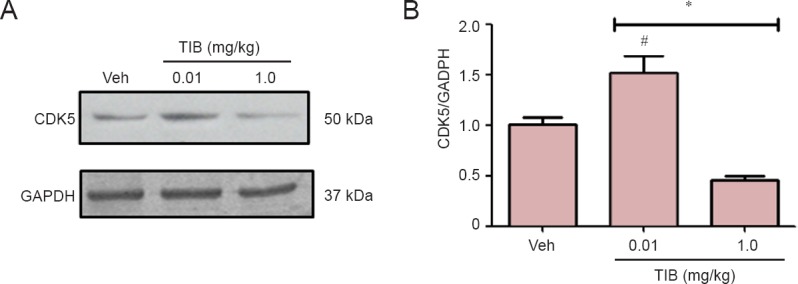
Changes in the content of CDK5 in the hippocampus of aged mice treated with tibolone (TIB).
Western blots for CDK5 content in the hippocampus of aged mice. GAPDH was used to correct differences of total loaded protein. Proteins detected by western blot analysis from the hippocampus of mice treated with TIB were quantified by densitometric analysis and corrected using GAPDH protein content data. Results are expressed as the mean ± SE of six individual experiments. *P < 0.05, vs. vehicle (Veh); #P < 0.05, vs. 1.0 mg/kg TIB.
Figure 7.
Changes in the content of p35 and p25 in the hippocampus of aged mice treated with tibolone (TIB).
(A) Representative western blots for p35 and p25 content in the hippocampus of aged mice. GAPDH was used to correct differences of total loaded protein. Proteins detected by western blot analysis from the hippocampus of mice treated with TIB were quantified by densitometric analysis and corrected using GAPDH protein content data. (B–D) The densitometric analysis of p35 (B) and p25 (C) and the ratio of p35 versus p25 (D). Results are expressed as the mean ± SE of six individual experiments. *P < 0.05, vs. vehicle (Veh).
Discussion
Our results suggest that TIB can modulate the content of total and phosphorylated microtubule-associated protein Tau through the PI3K/Akt/GSK3β pathway and CDK5/p35/p25 complexes in the hippocampus of aged male mice.
Our results showed that aging increased the phosphorylation of Tau and TIB administration decreased the phosphorylation of Tau in the hippocampus of the aged mice. These results were in accordance with previous studies showing that aging increased the phosphorylation of Tau in the brain tissue of monkeys and rats (Niewiadomska et al., 2006; Carlyle et al., 2014) and that TIB decreased the phosphorylation of Tau in the hippocampus of ovariectomized rats (Pinto-Almazán et al., 2012)
Tau phosphorylation reduces the affinity for microtubules and its ability to promote microtubule assembly in vitro (Lindwall and Cole, 1984; Biernat et al., 1993; Lu and Wood, 1993). Many of the phosphorylated residues in Tau during the development of paired helical filaments in Alzheimer's disease are serines and threonines preceding a proline (Mietelska-Porowska et al., 2014). GSK3β, a proline-directed kinase, induces phosphorylation of Tau at least on some of these residues. Co-transfection of Tau with GSK3β elevates GSK3β activity in fibroblast cells, so as to phosphorylate Tau (Lovestone et al., 1994; Sperber et al., 1995). Androgen depletion has been shown to significantly accelerate the development of Alzheimer's disease-like neuropathology in the triple-transgenic mouse model of AD (3xTg-AD). This effect is prevented by androgen treatment. It was observed that the levels of Tau hyperphosphorylation in sham gonadectomy (GDX) 3xTg-AD male mice were modest and only increased slightly by GDX. In GDX male mice, treatment with testosterone (T) or 17β-estradiol (E2) but not with dihydrotestosterone (DHT) reduced Tau hyperphosphorylation to levels lower than observed in sham animals. Because T is metabolized into both the androgen DHT and the estrogen E2 in the brain, T has been shown to mediate its effects through androgen or estrogen metabolic pathways (Rosario et al., 2010).
The most abundant free TIB metabolite in the hippocampus and cerebellum of cynomolgus monkeys is the 3-hydroxy tibolone, which has estrogenic activity (Verheul and Kloosterboer, 2006). These data suggest that TIB modulates Tau phosphorylation through this metabolite in both the hippocampus and cerebellum. Our results suggest that the decrease in the phosphorylation of Tau in the hippocampus of aged male mice may involve estrogen-regulated pathways. Nevertheless, further research needs to be undertaken in this area.
Considering that GSK3β plays a significant role in the regulation of Tau phosphorylation in vivo, the understanding of signaling pathways that modulate the activity of GSK3β is of critical importance. It is well known that phosphorylation on the Ser9 residue of GSK3β inhibits its activity, while phosphorylation on Tyr216 increases it (Jope and Johnson, 2004). GSK3β regulates Tau hyperphosphorylation at Ser198/Ser199/Ser202 sites and Ser396/Ser404 sites (Haque et al., 1999). Furthermore, as a down-regulator of insulin signaling, GSK3β is regulated by Akt (Beurel et al., 2015). In addition, long-term treatment with insulin or IGF-1 results in Akt activation, which in turn phosphorylates GSK3β on Ser9 (Hong and Lee, 1997; Lesort and Johnson, 2000).
Previous results have shown that TIB treatment increases only the phosphorylation of GSK3β, the inactive form of this protein, in the hippocampus. These results correlate with the decrease of Tau hyperphosphorylation in female rats (Pinto-Almazán et al., 2012), and suggest that TIB may decrease Tau phosphorylation through the inactivation of GSK3β in females. In contrast, our results indicate that, despite a decrease in the total GSK3β content with the low dose of TIB, this hormone does not modify the content of the active form of GSK3β in the hippocampus of aged male mice. Unlike the previous investigation, where a model with young, ovariectomized female rats was used (Pinto-Almazán et al., 2012), we used aged male mice as a model in the present investigation. The differences in the observed results may be because ovariectomy in female rats modifies the hypothalamic-pituitary-gonadal axis.
Furthermore, we investigated Akt activation, which can be achieved by the binding of neurotransmitters or growth factors on many specific cell-surface receptors, which in turn initiate a cascade of second messengers related to the PI3K pathway (Cardona-Gomez et al., 2001). Moreover, these two signaling pathways converge downstream to GSK3β, which is inhibited by Akt (Woodgett, 1990). PI3K signaling results in the activation of Akt following its phosphorylation on the Thr308 and Ser473 residues (Beaulieu, 2010). A previous report showed that in the rat cerebellum, E2 increased the phosphorylation of Akt at 6 and 12 hours after its administration (Morissette et al., 2008). However, in this study, we observed that TIB decreased the content and phosphorylation of Akt in the hippocampus of aged males. These results, together with those observed for GSK3β, indicate that the GSK3β/Akt signaling pathway is likely not involved in the regulation of TIB-mediated Tau phosphorylation in the hippocampus of the old mice. The negative regulation of TIB on Akt may have an effect on the decrease in Tau phosphorylation through inactivating another kinase involved in this process such as CDK5. Overall, these results indicate the need for the analysis of the effect of TIB on the CDK5 signaling pathway in the old mouse hippocampus.
It has been observed that the activation of PI3K by the phosphorylation of tyrosine residues of its regulatory subunit p85 leads to the activation of the downstream kinase 3-phosphoinositide-dependent protein kinase-1 (PDK1) by phosphorylating PDK1 at Ser241 (Fyffe and Falasca, 2013). Activated PDK1 then activates Akt by its phosphorylation at Thr308. Full activation of Akt also requires its phosphorylation at Ser473. A major target of Akt is GSK3. The activity of GSK3 is inhibited when it is phosphorylated at Ser21 in GSK3α or at Ser9 in GSK3β by Akt, which results in glycogen synthesis. GSK3β is also a major Tau kinase (Takashima, 2006; Avila and Hernández, 2007). We observed that TIB increased the expression of the catalytic p110 and regulatory p85 subunits. Therefore, these modifications may lead to a down-regulation of the Tau phosphorylation pathway.
These differences may be because the regulation of Tau phosphorylation is subject to a balance between the action of kinases and phosphatases. Mitogen-activated protein kinases (MAPKs) and CDK5 are involved in the regulation of Tau phosphorylation in the hippocampus (Hyman et al., 1994; Crespo-Biel et al., 2007), and steroid hormones can modulate its activation (Guerra-Araiza et al., 2009; Harburger et al., 2009). In 2010, Amorim et al. reported that the phosphorylation of protein phosphatase 2A (PP2A) was regulated by hormones. PP2A is also capable of dephosphorylating Akt at Thr308 (Millward et al., 1999), and it regulates Tau phosphorylation directly or via GSK3β (Qian et al., 2010).
Another kinase involved in the hyperphosphorylation of Tau is CDK5. This kinase works with its co-activators, named p39/p35 and p29/p25. Without these activators, the monomeric form of CDK5 is enzymatically inactive (Gong and Iqbal, 2008).
Under pathological conditions, there is a cleavage of the activator p35 that leads to p25. The complex CDK5/p25 has been shown to induce the hyperphosphorylation of Tau, and also causes neurodegeneration. This complex was found in patients with Alzheimer's disease and co-localized with Tau aggregates (Imahori and Uchida, 1997). Moreover, the accumulation of this complex is located in several areas, including the frontal cortex, inferior parietal cortex, and hippocampus. Hyperphosphorylation of Tau and neurofilaments have been observed in mice overexpressing human p25, an activator of CDK5 (Tseng et al., 2002; Shelton and Johnson, 2004; Martina et al., 2013).
Our results showed that at a low dose, TIB increased the content of CDK5. On the contrary, at a high dose, the content of CDK5 decreased. When we analyzed the content of the p35 and p25 activators, it was observed that low doses of TIB increased the content of p35 and decreased the content of p25. As mentioned above, in mice overexpressing human p25, the hyperphosphorylation of Tau and neurofilaments have been observed in some areas, such as frontal cortex, cerebellum, and amygdala (Ahlijanian et al., 2000).
The effects of E2 on the regulation of CDK5 were demonstrated in SH-SY5Y cells using okadaic acid to induce Tau phosphorylation. Zhang and Simpkins (2010) reported that the hyperphosphorylation of Tau and a decrease in the upregulation of CDK5 were prevented by E2 in a dose-dependent manner, and this effect was blocked when the antagonist of estrogen receptor, ICI 182 780, was added.
Our results provide a possible mechanism of action of TIB in the aged hippocampus. Through the regulation of Tau, GSK3β/Akt/PI3K pathway and CDK5 p35/p25 complexes, TIB can modulate neuronal plasticity and participate in the regulation of learning and memory processes. Further studies are needed to determine whether TIB could be used as a neuroprotective agent for the prevention of tauopathies or other neurodegenerative diseases in aged males.
Acknowledgments
This work was submitted in partial fulfillment of the requirements for the Ph.D. degree of Teresa Neri-Gómez at Doctorado en Investigación en Medicina (ESM/IPN). Christian Guerra-Araiza received Beca de Excelencia en Investigación by Fundación IMSS, A. C.
Footnotes
Funding: This study was supported by FIS/IMSS project No. FIS/IMSS/PROT/G13/1216, COFAA, SIP-IPN and by DGAPA-UNAM IN203616.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Li CH, Song LP, Zhao M
References
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowsk A, Kowsz K, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, John D, McNeish JD. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci U S A. 2000;97:2910–2915. doi: 10.1073/pnas.040577797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim MA, Guerra-Araiza C, Pernía O, da Cruz e Silva EF, Garcia-Segura LM. Progesterone regulates the phosphorylation of protein phosphatases in the brain. J Neurosci Res. 2010;88:2826–2832. doi: 10.1002/jnr.22442. [DOI] [PubMed] [Google Scholar]
- Avila J, Hernández F. GSK-3 inhibitors for Alzheimer's disease. Expert Rev Neurother. 2007;7:1527–1533. doi: 10.1586/14737175.7.11.1527. [DOI] [PubMed] [Google Scholar]
- Baum L, Hansen L, Masliah E, Saitoh T. Glycogen synthase kinase 3 alteration in Alzheimer disease is related to neurofibrillary tangle formation. Mol Chem Neuropathol. 1996;29:253–261. doi: 10.1007/BF02815006. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J Psychiatry Neurosci. 2010;37:7–16. doi: 10.1503/jpn.110011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernat J, Gustke N, Drewes G, Mandelkow EM, Mandelkow E. Phosphorylation of Ser262 strongly reduces binding of tau to microtubules: distinction between PHF-like immunoreactivity and microtubule binding. Neuron. 1993;11:153–163. doi: 10.1016/0896-6273(93)90279-z. [DOI] [PubMed] [Google Scholar]
- Campisi R, Marengo FD. Cardiovascular effects of tibolone: a selective tissue estrogenic activity regulator. Cardiovasc Drug Rev. 2007;25:132–145. doi: 10.1111/j.1527-3466.2007.00007.x. [DOI] [PubMed] [Google Scholar]
- Cardona-Gómez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogens and insulin-like growth factor-I in the brain: implications for neuroprotection. Brain Res Brain Res Rev. 2001;37:320–334. doi: 10.1016/s0165-0173(01)00137-0. [DOI] [PubMed] [Google Scholar]
- Carlyle BC, Nairn AC, Wang M, Yang Y, Jin LE, Simen AA, Ramos BP, Bordner KA, Craft GE, Davies P, Pletikos M, Sestan N, Arnsten AFT, Paspalas CD. cAMP-PKA phosphorylation of tau confers risk for degeneration in aging association cortex. Proc Natl Acad Sci U S A. 2014;111:5036–5041. doi: 10.1073/pnas.1322360111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- Crespo-Biel N, Canudas AM, Camins A, Palla's M. Kainate induces AKT, ERK and cdk5/GSK3beta pathway deregulation, phosphorylates tau protein in mouse hippocampus. Neurochem Int. 2007;50:435–442. doi: 10.1016/j.neuint.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Drechsel DN, Hyman AA, Cobb MH, Kirschner MW. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992;3:1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa-Raya J, Plata-Cruz N, Neri-Gómez T, Camacho-Arroyo I, Picazo O. Effects of short-term hormonal replacement on learning and on basal forebrain ChAT and TrkA content in ovariectomized rats. Brain Res. 2011;1375:77–84. doi: 10.1016/j.brainres.2010.12.044. [DOI] [PubMed] [Google Scholar]
- Espinosa-Raya J, Neri-Gómez T, Orozco-Suárez S, Campos MG, Guerra-Araiza C. Chronic administration of tibolone modulates anxiety-like behavior and enhances cognitive performance in ovariectomized rats. Horm Behav. 2012;61:76–83. doi: 10.1016/j.yhbeh.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Farfán-García ED, Castillo-Hernández MC, Pinto-Almazán R, Rivas-Arancibia S, Gallardo JM, Guerra-Araiza C. Tibolone prevents oxidation and ameliorates cholinergic deficit induced by ozone exposure in the male rat hippocampus. Neurochem Res. 2014;39:1776–1786. doi: 10.1007/s11064-014-1385-0. [DOI] [PubMed] [Google Scholar]
- Flaherty DB1, Soria JP, Tomasiewicz HG, Wood JG. Phosphorylation of human tau protein by microtubule-associated kinases: GSK3beta and cdk5 are key participants. J Neurosci Res. 2000;62:463–472. doi: 10.1002/1097-4547(20001101)62:3<463::AID-JNR16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Fyffe C, Falasca M. 3-Phosphoinositide-dependent protein kinase-1 as an emerging target in the management of breast cancer. Cancer Manag Res. 2013;5:271–280. doi: 10.2147/CMAR.S35026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong CX, Iqbal K. Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr Med Chem. 2008;15:2321–2328. doi: 10.2174/092986708785909111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra-Araiza C, Amorim MA, Pinto-Almazán R, González-Arenas A, Campos M, Garcia-Segura LM. Regulation of the phosphoinositide-3 kinase and mitogen-activated protein kinase signaling pathways by progesterone and its reduced metabolites in the rat brain. J Neurosci Res. 2009;87:470–481. doi: 10.1002/jnr.21848. [DOI] [PubMed] [Google Scholar]
- Haque N, Tanaka T, Iqbal K, Grundke-Iqbal I. Regulation of expression, phosphorylation and biological activity of tau during differentiation in SY5Y cells. Brain Res. 1999;838:69–77. doi: 10.1016/s0006-8993(99)01622-4. [DOI] [PubMed] [Google Scholar]
- Harburger LL, Saadi A, Frick KM. Dose-dependent effects of post training estradiol plus progesterone treatment on object memory consolidation and hippocampal extracellular signal-regulated kinase activation in young ovariectomized mice. Neuroscience. 2009;160:6–12. doi: 10.1016/j.neuroscience.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Elvhage TE, Reiter J. Extracellular signal regulated kinases. Localization of protein and mRNA in the human hippocampal formation in Alzheimer's disease. Am J Pathol. 1994;144:565–572. [PMC free article] [PubMed] [Google Scholar]
- Imahori K, Uchida T. Physiology and pathology of tau protein kinases in relation to Alzheimer's disease. J Biochem (Tokyo) 1997;121:179–188. [PubMed] [Google Scholar]
- Ishiguro K1, Omori A, Takamatsu M, Sato K, Arioka M, Uchida T, Imahori K. Phosphorylation sites on tau by tau protein kinase I, a bovine derived kinase generating an epitope of paired helical filaments. Neurosci Lett. 1992;148:202–206. doi: 10.1016/0304-3940(92)90839-y. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. Thinking with your gonads: testosterone and cognition. Trends Cogn Sci. 2006;10:77–82. doi: 10.1016/j.tics.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Jope RS, Johnson GV. The glamour and gloom of glycogen synthase kinase 3 (GSK3) Trends Biol Sci. 2004;29:95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kloosterboer HJ. Tibolone: a steroid with a tissue-specific mode of action. J Steroid Biochem Mol Biol. 2001;76:231–238. doi: 10.1016/s0960-0760(01)00044-9. [DOI] [PubMed] [Google Scholar]
- Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23:1588–1592. doi: 10.1523/JNEUROSCI.23-05-01588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesort M, Johnson GV. Insulin-like growth factor-1 and insulin mediate transient site-selective increases in tau phosphorylation in primary cortical neurons. Neuroscience. 2000;99:305–316. doi: 10.1016/s0306-4522(00)00200-1. [DOI] [PubMed] [Google Scholar]
- Lindwall G, Cole RD. Phosphorylation affects the ability of Tau protein to promote microtubule assembly. J Biol Chem. 1984;259:5301–5305. [PubMed] [Google Scholar]
- Lovestone S, Reynolds CH, Latimer D, Davis DR, Anderton BH, Gallo JM, Hanger D, Mulot S, Marquardt B, Stabel S. Alzheimer's disease-like phosphorylation of the microtubule-associated protein tau by glycogen synthase kinase-3 in transfected mammalian cells. Curr Biol. 1994;4:1077–1086. doi: 10.1016/s0960-9822(00)00246-3. [DOI] [PubMed] [Google Scholar]
- Lu Q, Wood JG. Functional studies of Alzheimer's disease tau protein. J Neurosci. 1993;13:508–515. doi: 10.1523/JNEUROSCI.13-02-00508.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Mammalian cyclin-dependent kinases. Trends Biochem Sci. 2005;30:630–641. doi: 10.1016/j.tibs.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Martina L, Latypovac X, Wilsona CM, Magnaudeixa A, Perrina ML, Yardina C, Terroa F. Tau protein kinases: Involvement in Alzheimer's disease. Ageing Res Rev. 2013;12:289–309. doi: 10.1016/j.arr.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Mietelska-Porowska A, Wasik U, Goras M, Filipek A, Niewiadomska G. Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci. 2014;15:4671–4713. doi: 10.3390/ijms15034671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24:186–191. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- Moffat SD. Effects of testosterone on cognitive and brain aging in elderly men. Ann N Y Acad Sci. 2005;1055:80–92. doi: 10.1196/annals.1323.014. [DOI] [PubMed] [Google Scholar]
- Morissette M, Le Saux M, D’Astous M, Jourdain S, Al Sweidi S, Morin N, Estrada-Camarena E, Mendez P, Garcia-Segura LM, Di Paolo T. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Steroid Biochem Mol Biol. 2008;108:327–338. doi: 10.1016/j.jsbmb.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Niewiadomska G, Baksalerska-Pazera M, Lenarcik I, Riede G. Compartmental protein expression of Tau, GSK-3b and TrkA in cholinergic neurons of aged rats. J Neural Transm. 2006;113:1733–1746. doi: 10.1007/s00702-006-0488-4. [DOI] [PubMed] [Google Scholar]
- Papasozomenos SC. The heat shock-induced hyperphosphorylation of tau is estrogen-independent and prevented by androgens: implications for Alzheimer disease. Proc Natl Acad Sci U S A. 1997;94:6612–6617. doi: 10.1073/pnas.94.13.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4th ed. San Diego: Academic Press; 1998. [Google Scholar]
- Pike CJ. Testosterone attenuates beta-amyloid toxicity in cultured hippocampal neurons. Brain Res. 2001;919:160–165. doi: 10.1016/s0006-8993(01)03024-4. [DOI] [PubMed] [Google Scholar]
- Pinto-Almazán R, Calzada-Mendoza CC, Campos-Lara MG, Guerra-Araiza C. Effect of chronic administration of estradiol, progesterone, and tibolone on the expression and phosphorylation of glycogen synthase kinase-3β and the microtubule-associated protein Tau in the hippocampus and cerebellum of female rat. J Neurosci Res. 2012;90:878–886. doi: 10.1002/jnr.22808. [DOI] [PubMed] [Google Scholar]
- Pinto-Almazán R, Rivas-Arancibia S, Farfán-García ED, Rodríguez-Martínez E, Guerra-Araiza C. Neuroprotective effects of tibolone against oxidative stress induced by ozone exposure. Rev Neurol. 2014;58:441–448. [PubMed] [Google Scholar]
- Qian W, Shi J, Yin X, Iqbal K, Grundke-Iqbal I, Gong CX, Liu F. PP2A regulates tau phosphorylation directly and also indirectly via activating GSK-3beta. J Alzheimers Dis. 2010;9:1221–1229. doi: 10.3233/JAD-2010-1317. [DOI] [PubMed] [Google Scholar]
- Ramsden M, Shin TM, Pike CJ. Androgens modulate neuronal vulnerability to kainate lesion. Neuroscience. 2003;122:573–578. doi: 10.1016/j.neuroscience.2003.08.048. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Carroll J, Pike CJ. Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain Res. 2010;1359:281–290. doi: 10.1016/j.brainres.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton SB, Johnson GV. Cyclin-dependent kinase-5 in neurodegeneration. J Neurochem. 2004;88:1313–1326. doi: 10.1111/j.1471-4159.2003.02328.x. [DOI] [PubMed] [Google Scholar]
- Shukla V, Skuntz S, Pant HC. Deregulated cdk5 activity is involved in inducing Alzheimer's disease. Arch Med Res. 2012;43:655–662. doi: 10.1016/j.arcmed.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovronsky DM, Lee VM, Trojanowski JQ. Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu Rev Pathol. 2006;1:151–170. doi: 10.1146/annurev.pathol.1.110304.100113. [DOI] [PubMed] [Google Scholar]
- Sperber BR, Leight S, Goedert M, Lee VM. Glycogen synthase kinase-3 beta phosphorylates tau protein at multiple sites in intact cells. Neurosci Lett. 1995;197:149–153. doi: 10.1016/0304-3940(95)11902-9. [DOI] [PubMed] [Google Scholar]
- Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer's disease. J Alzheimers Dis. 2006;9(Suppl):309–317. doi: 10.3233/jad-2006-9s335. [DOI] [PubMed] [Google Scholar]
- Tseng HC, Zhou Y, Shen Y, Tsai LH. A survey of Cdk5 activator p35 and p25 levels in Alzheimer's disease brains. FEBS Lett. 2002;523:58–62. doi: 10.1016/s0014-5793(02)02934-4. [DOI] [PubMed] [Google Scholar]
- Verheul HA, Kloosterboer HJ. Metabolism of exogenous sex steroids and effect on brain functions with a focus on tibolone. J Steroid Biochem Mol Biol. 2006;102:195–204. doi: 10.1016/j.jsbmb.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev PatholMech. 2008;3:41–66. doi: 10.1146/annurev.pathmechdis.2.010506.092044. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Simpkins JW. Okadaic acid induces tau phosphorylation in SH-SY5Y cells in an estrogen-preventable manner. Brain Res. 2010;1345:176–181. doi: 10.1016/j.brainres.2010.04.074. [DOI] [PMC free article] [PubMed] [Google Scholar]