Abstract
Detailed mechanisms behind regeneration after nerve injury, in particular signal transduction and the fate of Schwann cells (SCs), are poorly understood. Here, we investigated axotomy-induced activation of extracellular-signal-regulated kinase-1/2 (ERK1/2; important for proliferation) and m-calpain in vitro, and the relation to Ca2+ deletion and Schwann cell proliferation and death after rat sciatic nerve axotomy. Nerve segments were cultured for up to 72 hours with and without ethylene glycol-bis(β-aminoethyl ether)-N, N, N’, N’-tetraacetic acid (EGTA). In some experiments, 5-bromo-2’-deoxyuridine (BrdU) was added during the last 24 hours to detect proliferating cells and propidium iodide (PI) was added at the last hour to detect dead and/or dying cells. Immunohistochemistry of sections of the cultured nerve segments was performed to label m-calpain and the phosphorylated and activated form of ERK1/2. The experiments revealed that immunoreactivity for p-ERK1/2 increased with time in organotypically cultured SCs. p-ERK1/2 and m-calpain were also observed in axons. A significant increase in the number of dead or dying SCs was observed in nerve segments cultured for 24 hours. When deprived of Ca2+, activation of axonal m-calpain was reduced, whereas p-ERK1/2 was increased in SCs. Ca2+ deprivation also significantly reduced the number of proliferating SCs, and instead increased the number of dead or dying SCs. Ca2+ seems to play an important role in activation of ERK1/2 in SCs and in SC survival and proliferation. In addition, extracellular Ca2+ levels are also required for m-calpain activation and up-regulation in axons. Thus, regulation of Ca2+ levels is likely to be a useful method to promote SC proliferation.
Keywords: nerve regeneration, p-ERK1/2, m-calpain, nerve injury, signal transduction, cell proliferation, cell death, activation, axotomy, sciatic nerve, neural regeneration
Introduction
When a peripheral nerve is injured, the supporting Schwann cells (SCs) near the site of injury are also damaged, and the SCs in the distal nerve segment become activated and start to proliferate in order to support the regeneration process in the specific microenvironment, i.e., tissue niche where cells can be modified by the milieu (Andersson-Sjoland et al., 2011). Even if many of the damaged SCs survive, some may, however, die as a result of the injury.
We, and others, have previously found that activation of the MAP-kinase ERK1/2 by phosphorylation may be part of the injury-induced response in SCs, ultimately leading to de-differentiation, survival, proliferation and regeneration of peripheral nerve (Martensson et al., 2007; Tsuda et al., 2011; Napoli et al., 2012). Furthermore, injury to the peripheral nerve induces a massive influx of Ca2+ into the damaged SCs and axons due to the difference between extracellular and intracellular Ca2+ levels (Finkbeiner and Greenberg, 1996; Agell et al., 2002; Cook and Lockyer, 2006; Soletti et al., 2010). The Ca2+ influx leads to an activation and up-regulation of calpains, a family of Ca2+-dependent proteases, and the subsequent degradation of neurofilaments distal to the injury (Cheng and Zochodne, 2002; Glass et al., 2002; Raff et al., 2002; Stoll et al., 2002). However, the result of the Ca2+ increase following cellular damage appears to differ between cell types and situations. Importantly, following a nerve injury, Ca2+ has been demonstrated to be involved in pathological events in neurons, such as apoptosis and autophagy (Gerschenson and Rotello, 1992; Cook and Lockyer, 2006; Knoferle et al., 2010), and in physiological regenerative processes, such as proliferation, and regulation of differentiation, regeneration and guidance (Shivakumar and Kumaran, 2001; Cook and Lockyer, 2006; Jacques-Fricke et al., 2006; Blackiston et al., 2009; Capiod, 2011). Less is, however, known about the outcome of injury-induced Ca2+ influx in SCs, although it has been demonstrated that ERK1/2 can become either activated or inhibited by the presence of Ca2+ in injured neurons and neuroblastoma cells (Finkbeiner and Greenberg, 1996; Agell et al., 2002; Schmitt et al., 2004; Cook and Lockyer, 2006; Soletti et al., 2010). Also, high levels of activated ERK1/2 leads to SC cell death instead of survival, which is an interesting contradictionary aspect of this kinase (Finkbeiner and Greenberg, 1996; Cook and Lockyer, 2006).
In the present study, we investigated the activation of ERK1/2 and m-calpain (calpain II) in explanted rat sciatic nerve pieces and how such events were related to Ca2+ changes. To determine the effects of calcium on the organotypically cultured SCs, we chelated extracellular Ca2+ by adding ethylene glycol tetra-acetic acid (EGTA) to the culture medium.
Materials and Methods
Animals
Adult female Sprague-Dawley rats (Møllegaard Breeding Center, Copenhagen, Denmark), weighing 200 g, were used in all experiments. The experimental procedures were approved by the ethical committee on animal welfare in Lund, Sweden (approval No. M131-14). The animals were kept on a 12-hour light/dark cycle with water and food ad libitum. Totally nine rats were used.
Organotypic culture of sciatic nerve segments
All animals were sacrificed by an intraperitoneal overdose of sodium pentobarbital (60 mg/mL; Apoteksbolaget, Sweden) followed by heart puncture. The sciatic nerves on both sides were exposed, dissected and then cut into 4 mm-long segments. The pieces of sciatic nerve were incubated free-floating in serum-free RPMI-1640 (Roswell Park Memorial Institute 1640) medium supplemented with penicillin/streptomycin (Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C for 24–72 hours (Martensson et al., 2007; Blom et al., 2014; Park et al., 2015). In our experimental setup, some nerve segments were cultured for 2, 24 or 48 hours in medium supplemented with ethylene glycol bis(beta-aminoethyl ether)-N,N,N’,N’-tetraacetic acid (EGTA; Sigma-Aldrich, St. Louis, MO, USA) at a final concentration of 2 mM, in order to inhibit the effect of Ca2+. For the proliferation studies, 5-bromo-2’-deoxyuridine (BrdU) (Molecular Probes, Thermo-Fisher, Waltham, MA, USA) was added to the medium for the final 24 hour culture at a final concentration of 55 μM. In some cases, propidium iodide (PI; Sigma-Aldrich) was added to the culture medium during the last hour of tissue culture at a final concentration of 10 μg/mL to assess cell viability.
Immunohistochemistry
For immunohistochemical detection, the frozen sciatic nerve segments were cut into longitudinal 10 μm-thick sections on a cryostat. The sections were immediately mounted on objective slides and left to dry. Sections were then washed with phosphate buffered saline (PBS) and incubated overnight at 4°C with primary antibodies (Table 1) diluted in PBS with 0.25% bovine serum albumin (BSA) and 0.25% Triton X-100. The sections were then washed again with PBS, followed by incubation with a secondary antibody for 1 hour at room temperature (Table 1) diluted as described for primary antibodies. Finally, the sections were washed and the nuclei were counterstained with bisbenzimide (1:10,000 in PBS). The sections were washed again and coverslipped with PBS/glycerol (1:1, v:v).
Table 1.
Primary and secondary antibodies
Photography and image analysis
Images were captured using an Olympus AX70 fluorescence microscope (Olympus, Japan) equipped with a Nikon DS-Ri1 camera and the NIS-Elements BR3.0 image acquisition program (Nikon, Japan). The images were converted to 8-bit greyscale TIFF using Adobe Photoshop 9.0.2 (Adobe Systems Incorporated, San Jose, CA, USA) and imported into ImageJ 1.40g (a public domain image analysis program developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image). In ImageJ, the tool threshold was used to determine the immunostained area of the nerve. The image analyses were performed on images captured at 10× objective magnification.
A region of interest (ROI) (100 × 100 pixels) was selected in the endoneurial area furthest away from the transection site in order to estimate the immunoflourescent intensity of the background. To determine the level of immunolabeling, the threshold was set to ±3 standard deviations of the background. The immunostained area was then measured on the entire 10 μm sections and expressed in percentage of the total area of the nerve section (a method previously described (Martensson et al., 2007)). The number of immunostained and bizbenzimide stained nuclei was counted using the ImageJ tool “particle analysis”, where the minimum and maximum particle sizes were set to 20 and 200 pixels, respectively, using the10× objective. The number of immunostained nuclei was expressed as a percentage of the total number of nuclei.
Statistical analysis
Data were expressed as the mean ± SEM. One way analysis of variance (ANOVA) was used to determine the significance of the fluctuations in PI and m-calpain immunoreactivity. The two-tailed t-test was used to evaluate if there were any significant changes in immunoreactivity. A P-value of < 0.05 was considered statistically significant. The software used for the statistical analyses was StatView 5.0.1 (SAS Institute Inc., Cary, NC, USA) and Microsoft Excel 2010 (Microsoft Cooperation, Redmond, WA, USA).
Results
p-ERK1/2
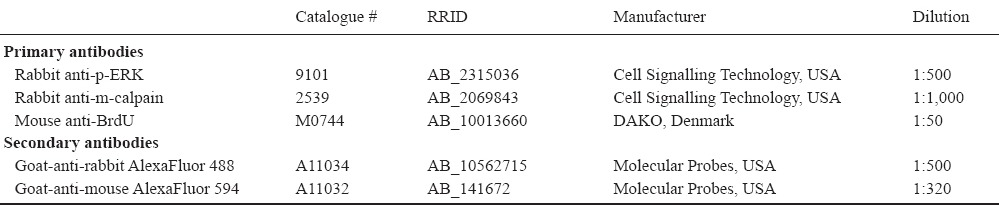
The activation of ERK1/2 along the length of the cultured nerve segments was investigated. As expected from our previous work (Martensson et al., 2007), p-ERK1/2 labeling was increased at the end of the nerve segments already at 2 hours (6.0%; P = 0.0144) and still at 24 hours (10.7%; P < 0.0001) as compared to the 0 hour control (2.8%) (Figure 1A).
Figure 1.
Amount of p-ERK1/2 and BrdU labeling over time in cultured sciatic nerve segments.
(A, B) The amount of p-ERK1/2 immunopositive area (A) expressed as %, and the amount of BrdU positive nuclei (B) in the sciatic nerve segments over time in vitro. Values are expressed as the mean ± SEM (two-tailed t-test). *P < 0.05, **P < 0.01, ***P < 0.001; n = 5. BrdU: 5-Bromo-2’-deoxyuridine; h: hour(s).
BrdU
The proliferation of SCs was quantified by analyzing the BrdU incorporation. After 24 hour culture, the amount of BrdU positive nuclei was 0.9% and after 48 hours this amount had increased to 10.5% (P = 0.0041) of the total number of nuclei in the nerve section (Figure 1B).
PI
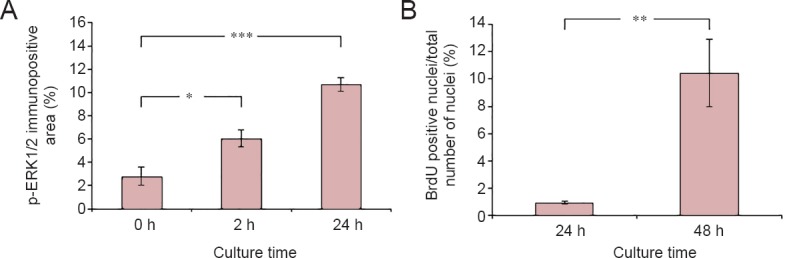
When PI was added to the culture medium, the number of labeled SCs significantly increased from 0.7% in control sections to 9.2% (P = 0.0132) at the edge of the cultured sciatic nerve segments (i.e., the “site of injury”) after 24 hour culture. A further increase to 15.5% (P = 0.0002) was found after 48 hours, and at this time point PI stained cells were also found further in the nerve segment. At 72 hours, there was still a significantly higher number of PI stained SCs in the damaged nerve segment as compared to the numbers in control (i.e., freshly dissected; 0 hour) segments (11.5%; P = 0.0027), although slightly less than at 48 hours (Figure 2A).
Figure 2.
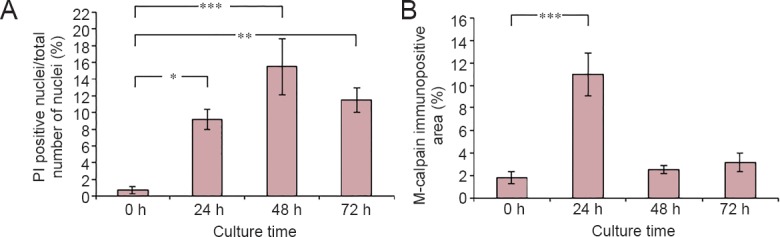
Amount of propidium iodide (PI) and m-calpain labeling over time in cultured sciatic nerve segments.
(A, B) The amount of PI (A) positive nuclei, expressed as %, and the relative m-calpain immunopositive area (B) in the sciatic nerve segments over time in vitro. Values are expressed as the mean ± SEM (two-tailed t-test). *P < 0.05, **P < 0.01, ***P < 0.001; n = 5. h: Hour(s).
M-calpain
The m-calpain immunostained area significantly increased from 1.9% in the 0 hour control to 10.6% (P < 0.0001) after 24 hour culture, but at 48 and 72 hours, the m-calpain immunoreactivity was again reduced to the levels in control nerves (Figure 2B). The m-calpain immunoreactivity was localized to axons at the site of transection.
Effects of Ca2+ deprivation
p-ERK1/2
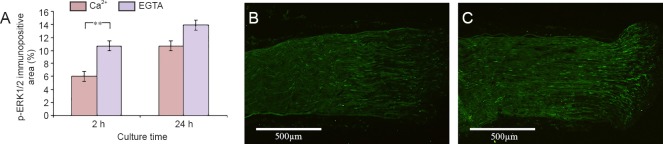
When the nerve segments were treated with EGTA, the number of p-ERK1/2 labeled cells had increased, as compared to the segments cultured in regular medium, with a statistically significant increase at 2 hours (10.7%; P = 0.009) as compared to 6.0%, but not later, indicating that this effect was immediate (Figure 3).
Figure 3.
Effect of Ca2+ deprivation on the activation of ERK1/2 in cultured sciatic nerve segments.
(A) Effect of EGTA treatment (to achieve Ca2+ deprivation) on p-ERK1/2 immunopositive area in the cultured sciatic nerve segments. (B, C) p-ERK1/2 immunolabeling (green) in the rat sciatic nerve segments cultured for 2 hours in the medium without (B) and with EGTA (C). Values are expressed as the mean ± SEM (two-tailed t-test) **P < 0.01. n = 5. EGTA: Ethylene glycol tetra-acetic acid; h: hours.
BrdU
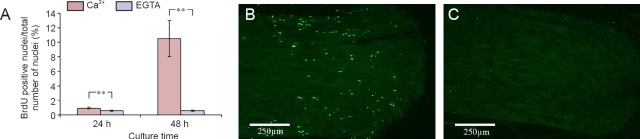
When the nerve segments were cultured in the presence of EGTA, the number of BrdU immunostained cells was significantly reduced both at 24 hours (0.6%; P = 0.009) and at 48 hours (0.6%; P = 0.003) as compared to nerve segments cultured in Ca2+ containing medium (24 hours: 0.9% and 48 hours: 10.5%) (Figure 4).
Figure 4.
Effect of Ca2+ deprivation on SC proliferation in cultured sciatic nerve segments.
(A) Effect of EGTA treatment on the number of BrdU stained nuclei in the cultured sciatic nerve segment. (B, C) BrdU labeled nuclei (green) in the rat sciatic nerve segments cultured for 48 hours in medium without (B) and with EGTA (C). Values are expressed as the mean ± SEM (two-tailed t-test). **P < 0.01. n = 4. EGTA: Ethylene glycol tetra-acetic acid; BrdU: 5-bromo-2’-deoxyuridine; h: hours.
PI
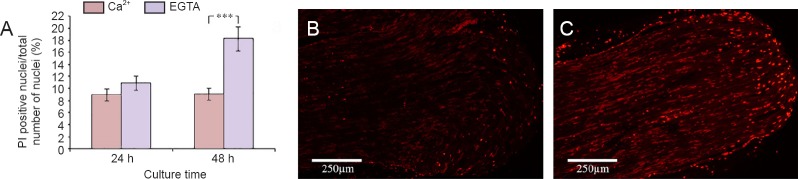
When deprived of extracellular Ca2+, the SCs showed no significant increase in PI incorporation after 24 hours, but the effect of EGTA caused a significant increase in dead or dying SCs after 48 hours of culture (18.3%; P < 0.001) as compared to those cultured with Ca2+ (9.1%) (Figure 5).
Figure 5.
Effect of Ca2+ deprivation on Schwann cell death in cultured sciatic nerve segments.
(A) Effect of EGTA treatment on the number of PI positive nuclei in the cultured sciatic nerve segment. (B, C) The images illustrate PI labeled nuclei (red) in the rat sciatic nerve segments cultured for 48 hours in medium without (B) and with EGTA (C). Values are expressed as the mean ± SEM (two-tailed t-test). ***P < 0.001. n = 4. EGTA: Ethylene glycol tetra-acetic acid; PI: propidium iodid; h: hours.
M-calpain
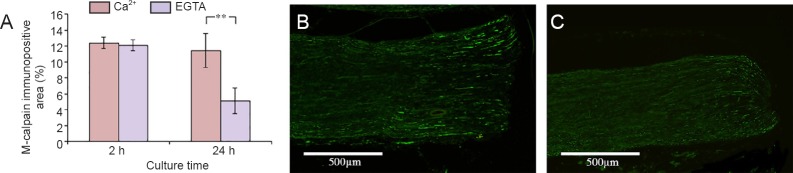
There was no difference in m-calpain level in the nerve segments cultured for 2 hours in the presence and absence of EGTA. At 24 hours, however, the m-calpain immunoreactivity was significantly reduced in the nerve segments deprived of extracellular Ca2+ (5.1%; P = 0.009) compared to those maintained in the control medium (11.4%) (Figure 6).
Figure 6.
Effect of Ca2+ deprivation on the activation of m-calpain in cultured sciatic nerve segments.
(A) Effect of EGTA treatment on the m-calpain positive area in the cultured sciatic nerve segments. (B, C) M-calpain positive axons (green) in the rat sciatic nerve cultured for 24 hours in medium without (B) and with EGTA (C). Values are expressed as the mean ± SEM (two-tailed t-test). **P < 0.01. n = 5.
Discussion
The present study corroborates our earlier findings that p-ERK1/2, as previously reported, increases in SCs and in axons as a response to nerve injury (Martensson et al., 2007). The levels of m-calpain, which were also observed in axons, were reduced, whereas p-ERK1/2 was increased when the SCs were deprived of extracellular Ca2+. In addition, the Ca2+ deprivation reduced the number of proliferating SCs and it also increased the number of dead or dying SCs. These are observations that may be relevant in the regeneration process after nerve injury due to the importance of SCs in this process. A number of opposing findings on the effect of Ca2+-dependent activation of the mitogen-activated protein kinase (MAPK) pathways in somatic cells have been published, indicating at least four different Ca2+-dependent pathways leading to MAPK-pathway activation. There are also different responses to activation of the MAPK pathway depending on cell type, localization, level and duration of the activation. All these options lead to different responses, such as survival, differentiation, proliferation, cell death or growth cone formation (Finkbeiner and Greenberg, 1996; Agell et al., 2002; Cook and Lockyer, 2006; Blackiston et al., 2009; Napoli et al., 2012). The suggested involvements of Ca2+ in both cell death and survival following nerve injury prompted us to study ERK1/2 activation in relation to levels of Ca2+ in SCs. To this end, we used organotypic sciatic nerve segments that were cultured in vitro in the presence or absence of Ca2+. By immunocytochemistry, it was possible to identify the cells in their environment in the nerve segments. The relationship between Ca2+ and levels of p-ERK1/2 or m-calpain can also be gained by performing a western blot analysis, but it would not be able to distinguish between expression in specific types of cells or structures (i.e., Schwann cells and axonal expression or even mast cells, fibroblasts and endothelial cells, etc.), particularly in the Schwann cells that are crucial for the regeneration process (Saito and Dahlin, 2008; Saito et al., 2009). This selective expression pattern in, and function of, the various cells in their local environment - the tissue niche - is crucial for different regeneration processes (Andersson-Sjoland et al., 2011). Furthermore, we examined the processes by depleting the Ca2+ level, but not focusing on different Ca2+- levels in accordance with previous studies (Finkbeiner and Greenberg, 1996; Agell et al., 2002; Schmitt et al., 2004; Cook and Lockyer, 2006; Soletti et al., 2010).
The levels of Ca2+-related enzyme m-calpain increased significantly after axotomy, but only in axons and not in SCs, which is in accordance with the findings of Glass et al. (2002). In this study, m-calpain increased dramatically in neurites at 24 hours after injury, and then the levels decrease again, before the onset of axonal degeneration. M-calpain is activated and upregulated by high concentrations of Ca2+, and can only be attained intracellularly if the cell membrane is disrupted. The axons in the cultured nerve pieces are destined to degenerate since they have lost contact with their cell bodies; thus, similar to the events in the distal nerve segment after a nerve injury. Therefore, if such an increase in m-calpainis related to SC death, it would be via the neuron-SC communication. However, this issue needs to be investigated more thoroughly in order to establish a connection between injury-induced neurite Ca2+ fluctuations to SC death, although Ca2+ regulated Schwann cell death may very well be an event separated from the Ca2+ dependent cytoskeletal reconstruction in regenerating neurons.
A main finding in the present study was that the levels of activated ERK1/2 increased in the nerve pieces cultured with reduced levels of calcium as compared to the pieces cultured in normal medium, which is in accordance with previous studies (Finkbeiner and Greenberg, 1996; Agell et al., 2002; Schmitt et al., 2004; Cook and Lockyer, 2006; Soletti et al., 2010). Although we did not measure intracellular Ca2+, it is reasonable to assume that a decrease in intracellular Ca2+ is responsible for the present effect. The results are in accordance with studies in fibroblasts, where Ca2+ has been shown to have an inhibitory effect on ERK1/2; thus, keeping the ERK1/2 at levels appropriate for cell survival and proliferation (Ji and Carpenter, 2000; Agell et al., 2002). When Ca2+ levels are lowered, the ERK1/2 pathway is on the other hand over-stimulated leading to cell death instead of proliferation and survival. This effect has also been demonstrated in experiments by Widerberg et al. (1997), where a slight decrease in systemic Ca2+ levels increased nerve regeneration, while inhibition of Ca2+ uptake significantly reduced nerve regeneration. This finding of a detrimental increase in p-ERK1/2 is supported by the decrease of proliferating SCs and increase of SC death that we observed when nerve segments were cultured in Ca2+ free medium.
EGTA treatment of cultured nerve segments also attenuated the levels of axonal m-calpain after culture for 24 hours. Again, this illustrates a possible link between Ca2+ and the calpains. However, since the increase of m-calpain, as a response to a nerve injury, only occurred in axons that were degenerating and not in SCs, we cannot draw any conclusions on what impact this has on the SCs.
As a response to cellular damage, there is an activation of both ERK1/2 and m-calpain in cultured nerve pieces. Here, we show that Ca2+ regulates the activation of ERK1/2 in SCs and the activation and upregulation of m-calpain in degenerating axons. Depletion of Ca2+ increases ERK1/2 activation in SCs to a point where it increases the number of dead or dying SCs and reduces SC proliferation. Removal of extracellular Ca2+ decreases the activation of m-calpain in degenerating axons. We conclude that the Ca2+ flux into the damaged SCs is important for survival and proliferation and that gaining control over this flux can be vital for successful nerve regeneration.
Footnotes
Funding: This study was supported by the Research School in Pharmaceutical Science in Lund, The Royal Physiographic Society in Lund, The Swedish Research Council (Medicine), the Craaford's and Thure Nilsson's Funds for Medical Research, Funds for diabetic research, Lund University and Region Skåne.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Li CH, Song LP, Zhao M
References
- Agell N, Bachs O, Rocamora N, Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca(2+), and calmodulin. Cell Signal. 2002;14:649–654. doi: 10.1016/s0898-6568(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Andersson-Sjoland A, Nihlberg K, Eriksson L, Bjermer L, Westergren-Thorsson G. Fibrocytes and the tissue niche in lung repair. Respir Res. 2011;12:76. doi: 10.1186/1465-9921-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston DJ, McLaughlin KA, Levin M. Bioelectric controls of cell proliferation: ion channels, membrane voltage and the cell cycle. Cell Cycle. 2009;8:3527–3536. doi: 10.4161/cc.8.21.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blom CL, Martensson LB, Dahlin LB. Nerve injury-induced c-Jun activation in Schwann cells is JNK independent. Biomed Res Int 2014. 2014 doi: 10.1155/2014/392971. 392971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capiod T. Cell proliferation, calcium influx and calcium channels. Biochimie. 2011;93:2075–2079. doi: 10.1016/j.biochi.2011.07.015. [DOI] [PubMed] [Google Scholar]
- Cheng C, Zochodne DW. In vivo proliferation, migration and phenotypic changes of Schwann cells in the presence of myelinated fibers. Neuroscience. 2002;115:321–329. doi: 10.1016/s0306-4522(02)00291-9. [DOI] [PubMed] [Google Scholar]
- Cook SJ, Lockyer PJ. Recent advances in Ca(2+)-dependent Ras regulation and cell proliferation. Cell Calcium. 2006;39:101–112. doi: 10.1016/j.ceca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Finkbeiner S, Greenberg ME. Ca(2+)-dependent routes to Ras: mechanisms for neuronal survival, differentiation, and plasticity? Neuron. 1996;16:233–236. doi: 10.1016/s0896-6273(00)80040-9. [DOI] [PubMed] [Google Scholar]
- Gerschenson LE, Rotello RJ. Apoptosis: a different type of cell death. FASEB J. 1992;6:2450–2455. doi: 10.1096/fasebj.6.7.1563596. [DOI] [PubMed] [Google Scholar]
- Glass JD, Culver DG, Levey AI, Nash NR. Very early activation of m-calpain in peripheral nerve during Wallerian degeneration. J Neurol Sci. 2002;196:9–20. doi: 10.1016/s0022-510x(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Jacques-Fricke BT, Seow Y, Gottlieb PA, Sachs F, Gomez TM. Ca2+ influx through mechanosensitive channels inhibits neurite outgrowth in opposition to other influx pathways and release from intracellular stores. J Neurosci. 2006;26:5656–5664. doi: 10.1523/JNEUROSCI.0675-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji QS, Carpenter G. Role of basal calcium in the EGF activation of MAP kinases. Oncogene. 2000;19:1853–1856. doi: 10.1038/sj.onc.1203517. [DOI] [PubMed] [Google Scholar]
- Knoferle J, Koch JC, Ostendorf T, Michel U, Planchamp V, Vutova P, Tonges L, Stadelmann C, Bruck W, Bahr M, Lingor P. Mechanisms of acute axonal degeneration in the optic nerve in vivo. Proc Natl Acad Sci U S A. 2010;107:6064–6069. doi: 10.1073/pnas.0909794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensson L, Gustavsson P, Dahlin LB, Kanje M. Activation of extracellular-signal-regulated kinase-1/2 precedes and is required for injury-induced Schwann cell proliferation. Neuroreport. 2007;18:957–961. doi: 10.1097/WNR.0b013e32819f8f27. [DOI] [PubMed] [Google Scholar]
- Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, Lloyd AC. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Park BS, Kim HW, Rhyu IJ, Park C, Yeo SG, Huh Y, Jeong NY, Jung J. Hydrogen sulfide is essential for Schwann cell responses to peripheral nerve injury. J Neurochem. 2015;132:230–242. doi: 10.1111/jnc.12932. [DOI] [PubMed] [Google Scholar]
- Raff MC, Whitmore AV, Finn JT. Axonal self-destruction and neurodegeneration. Science. 2002;296:868–871. doi: 10.1126/science.1068613. [DOI] [PubMed] [Google Scholar]
- Saito H, Dahlin LB. Expression of ATF3 and axonal outgrowth are impaired after delayed nerve repair. BMC Neurosci. 2008;9:88. doi: 10.1186/1471-2202-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Kanje M, Dahlin LB. Delayed nerve repair increases number of caspase 3 stained Schwann cells. Neurosci Lett. 2009;456:30–33. doi: 10.1016/j.neulet.2009.03.075. [DOI] [PubMed] [Google Scholar]
- Schmitt JM, Wayman GA, Nozaki N, Soderling TR. Calcium activation of ERK mediated by calmodulin kinase I. J Biol Chem. 2004;279:24064–24072. doi: 10.1074/jbc.M401501200. [DOI] [PubMed] [Google Scholar]
- Shivakumar K, Kumaran C. L-type calcium channel blockers and EGTA enhance superoxide production in cardiac fibroblasts. J Mol Cell Cardiol. 2001;33:373–377. doi: 10.1006/jmcc.2000.1309. [DOI] [PubMed] [Google Scholar]
- Soletti RC, del Barrio L, Daffre S, Miranda A, Borges HL, Moura-Neto V, Lopez MG, Gabilan NH. Peptide gomesin triggers cell death through L-type channel calcium influx, MAPK/ERK, PKC and PI3K signaling and generation of reactive oxygen species. Chem Biol Interact. 2010;186:135–143. doi: 10.1016/j.cbi.2010.04.012. [DOI] [PubMed] [Google Scholar]
- Stoll G, Jander S, Myers RR. Degeneration and regeneration of the peripheral nervous system: from Augustus Waller's observations to neuroinflammation. J Peripher Nerv Syst. 2002;7:13–27. doi: 10.1046/j.1529-8027.2002.02002.x. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Kanje M, Dahlin LB. Axonal outgrowth is associated with increased ERK 1/2 activation but decreased caspase 3 linked cell death in Schwann cells after immediate nerve repair in rats. BMC Neurosci. 2011;12:12. doi: 10.1186/1471-2202-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widerberg A, Bergman S, Danielsen N, Lundborg G, Dahlin LB. Nerve injury induced by vibration: prevention of the effect of a conditioning lesion by D600, a Ca2+ channel blocker. Occup Environ Med. 1997;54:312–315. doi: 10.1136/oem.54.5.312. [DOI] [PMC free article] [PubMed] [Google Scholar]