Abstract
Several studies have shown that fibroblast growth factor-2 (FGF2) can directly affect axon regeneration after peripheral nerve damage. In this study, we performed sensory tests and histological analyses to study the effect of recombinant human FGF-2 (rhFGF2) treatment on damaged mental nerves. The mental nerves of 6-week-old male Sprague-Dawley rats were crush-injured for 1 minute and then treated with 10 or 50 μg/mL rhFGF2 or PBS in crush injury area with a mini Osmotic pump. Sensory test using von Frey filaments at 1 week revealed the presence of sensory degeneration based on decreased gap score and increased difference score. However, at 2 weeks, the gap score and difference score were significantly rebounded in the mental nerve crush group treated with 10 μg/mL rhFGF2. Interestingly, treatment with 10 μg/mL rhFGF had a more obviously positive effect on the gap score than treatment with 50 μg/mL rhFGF2. In addition, retrograde neuronal tracing with Dil revealed a significant increase in nerve regeneration in the trigeminal ganglion at 2 and 4 weeks in the rhFGF2 groups (10 μg/mL and 50 μg/mL) than in the PBS group. The 10 μg/mL rhFGF2 group also showed an obviously robust regeneration in axon density in the mental nerve at 4 weeks. Our results demonstrate that 10 μg/mL rhFGF induces mental nerve regeneration and sensory recovery after mental nerve crush injury.
Keywords: nerve regeneration, mental nerve, fibroblast growth factor, crush injury, sensory neuron, functional recovery, neural regeneration
Introduction
Damage to the inferior alveolar nerve often occurs during surgery or during other types of damage (Savignat et al., 2007; Sun et al., 2009; Kemp et al., 2011). For example, the inferior alveolar nerve (IVN) and mental nerve (MN) can be easily damaged during dental surgery, including dentoalveolar, tumor, and orthognathic surgeries (Savignat et al., 2008). Damage to the IVN can also occur during dental implantation (Alhassani and AlGhamdi, 2010). The MN originates from the trigeminal ganglion and innervates the lower lip (Imai et al., 2003). Several surgical procedures are used to treat peripheral nerve damage, including sutures, nerve grafting, electrical stimulation, and stem cell therapy; however, these treatments are insufficient in recovering nerve function (Sullivan et al., 2016).
For nerve regeneration, neurotrophic factors such as nerve growth factor (NGF), fibroblast growth factor (FGF), and brain-derived neurotrophic factor (BDNF) are effective treatments (Kerekes et al., 1997; Choi et al., 2008). These neurotrophic factors control the differentiation and proliferation of adult neuronal progenitor cells (Kuhn et al., 1997; Palmer et al., 1999; Binder and Scharfman, 2004; Werner et al., 2011; Li et al., 2012). FGF determines the size of the cerebral cortex during embryogenesis and reduces the glutamatergic pyramidal neurons in frontal and temporal cortex (Vaccarino et al., 1999; Shin et al., 2004; Rash et al., 2011). FGF also promotes the proliferation and differentiation of neurons, and regulates the repair of injured cervical root nerves (Li et al., 2012).
Basic fibroblast growth factor (bFGF) has been shown to promote regeneration of damaged or injured peripheral nerves (Fujimoto et al., 1997). Fujimoto et al. (1997) demonstrated that FGF directly affected axon regeneration after peripheral nerve damage. Grothe and Nikkhah (2001) demonstrated that exogenously applied bFGF mediates rescue effects on injured sensory neurons and supports neurite outgrowth of transectioned nerves.
The purpose of the present study was to identify regenerative pattern and sensory function changes in rats after a MN crush injury treated by recombinant human FGF-2 (rhFGF2). We also investigated the dose of rhFGF2 that is suitable for nerve regeneration and sensory functional recovery.
Materials and Methods
Animals
The study was approved by the Animal Care and Use Committee of Seoul National University, Korea (SNU-130201-2). Male Sprague-Dawley rats, aged 6 weeks, weighing 250–300 g, were purchased from Orient Bio (Gapyeong, Republic of Korea). The animals were housed at the Seoul National University School of Dentistry in a specific-pathogen-free (SPF) room of an experimental animal facility with the temperature maintained at 21 ± 1°C, humidity at 55%, and a 13.5: 10.5 light: dark cycle that began at 07:30. General feed (Purina Rodent Chow, Purina Co., Seoul, Republic of Korea) and filtered water were provided ad libitum. Prior to experimentation, the animals were quarantined in a cage for a week. Rats were randomly divided into sham-operated (sham), PBS (MN crush injury + PBS), 10 μg/mL rhFGF2 (MN crush injury + 10 μg/mL rhFGF2) and 50 μg/mL rhFGF2 (MN crush injury + 50 μg/mL rhFGF2) groups. A sensory test was performed 3 days before inducing the MN crush injury or sham injury.
Surgical procedures
Rats were anesthetized with an intraperitoneal injection of a 4:1 mixture of hetamine hydrochloride (100 mg/kg, Ketara®, Yuhan, Korea) and xylazine hydrochloride (25 mg/kg, Rumpun®, Bayer, Korea). A 4-mm crush injury was induced by exposing the left MN and tightening the area 5 mm away from the mental foramen with the second clip of HEMOSTATS forceps (Baby-mixter, No. 13013-14, Canada) for 1 minute. Tagging with the ETHILONS Suture (ETHICON Inc., Somerville, NJ, USA) on the distal of the injured area makes reference to the harvesting of the MN crush injury (Figure 1A–C). An osmotic pump (1004 ALZET® mini-Osmotic Pump, CA, USA) was inserted into the neck back. A Rat Jugular Catheter (ALZET®, CA, USA) was used to connect the osmotic pump and the MN (Figure 1D, E).
Figure 1.
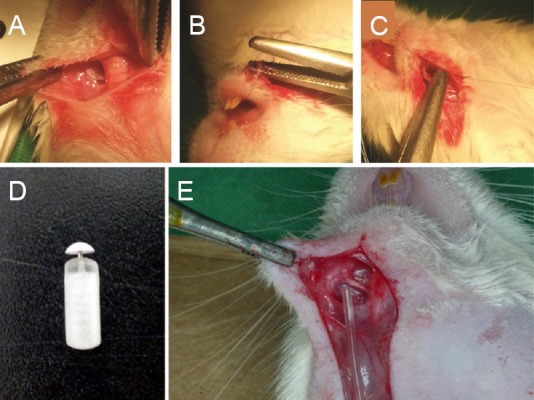
Animal surgery methods and materials.
(A) The mental nerve at the experimental site. (B) Mental nerve (MN) crush injury. (C) 9-0 suture tagged MN after crush injury. (D) Osmotic pump. (E) Connected MN and Rat Jugular Catheter. We did not systemically inject rhFGF2 in the osmotic pump but directly attached it to the mental nerve by connecting the Rat Jugular Catheter to the osmotic pump to affect only the mental nerve.
rhFGF-2 treatment and experimental design
rhFGF-2 (GENOSS Co., Ltd, Suwon, Republic of Korea) was injected at concentrations of 0.1 μg/mL (kg/d), 0.2 μg/mL (kg/d), and 0.4 μg/mL (kg/d) into the osmotic pumps for 1, 2, and 3 weeks in a preliminary experiment (n = 6 in each group at each time point, totally 54 rats). Afterwards, the inserted osmotic pumps in animals were used for administration with rhFGF-2 either at low dose (10 μg/mL (kg/d)) or at high dose (50 μg/mL (kg/d)) (n = 8 in each group at each time point, totally 64 rats). In the PBS and rhFGF groups, 84 μL of PBS or rhFGF was injected via the osmotic pump. In this manner, PBS or rhFGF was administered at 0.5 μL/h for 1, 2, 3, and 4 weeks (Lin et al., 2011). The osmotic pumps were refreshed once a week.
Sensory test
According to the method from Seinoa et al. (2009), a sensory test was performed to compare the touch sensitivity to mechanical stimulation using von Frey filaments (Semmes-Weinstein Monofilaments, North Coast Medical, Inc., Arcata, CA, USA). The rapid movement of the front legs of a rat towards its head or face due to the bending force of a von Frey filament (bending force of 0.008, 0.02, 0.04, 0.07, 0.016, 0.4, 0.6, 1.0, 1.4, 2.0, 4.0, and 6.0 g) indicated a positive response to stimulation (Figure 2A). The touch threshold to mechanical stimulation (in g) of the ipsilateral and contralateral sides was calculated by subtracting the value of the contralateral mental nerve area from the value of the ipsilateral mental nerve area. The gap score was defined as the difference between the medial and distal sensitivity of the MN to mechanical stimulation (Figure 2B), and the difference score was calculated by subtracting the value of the contralateral lip area from the value of the ipsilateral lip area (Figure 2B) (Seino et al., 2009). Decreased gap score and increased difference score mean the presence of sensory degeneration. The sensory test was performed before and at 1, 2, 3, and 4 weeks after surgery in each group.
Figure 2.
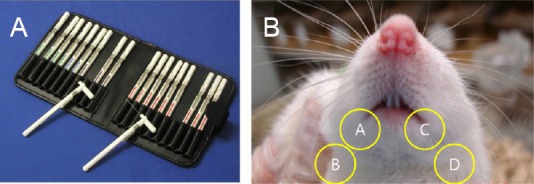
Calculation method of gap score and difference score using von Frey filaments.
(A) Von Frey filaments. (B) Screening site for measurement sensory test. Gap score: encircled A–B; difference score: encircled B–D.
Retrograde nerve labeling
Retrograde labeling and counting of back-labeled sensory neurons were performed as described before (Li et al., 2012). The MN crush injury site was retrograde labeled with 1,1’-dioctadecyl-3,3’3’-tetramethylindocarbocyanine perchlorate (Dil, C59H97ClN2O4, Molecular Probes; Eugene, OR, USA) to measure nerve regeneration 2 and 4 weeks after the MN crush injury (n = 8 in each group at each time point). Five days after the Dil injection in mental nerve, the rats were rapidly anesthetized using an intraperitoneal (IP) injection of chloropent (3 mL/kg), and transcardially perfused using a 0.9% saline solution with 1% heparin (JW Pharmaceutical, Seoul, Korea) followed by 4% paraformaldehyde (Merck, Darmstadt, Germany) in 0.1 M sodium phosphate buffer (pH7.2). Immediately after fixation, the trigeminal ganglia were extracted and post-fixed with the same fixative at 4°C for 24 hours. To prevent the occurrence of freezing artifacts during the preparation of frozen tissue sections, the tissue was fixed in 30% sucrose (Sigma, St, Louis, MO, USA) at 4°C for 24 hours. The tissue sections were observed using a confocal laser-scanning microscope (CLSM, LSM700, Carl Zeiss, Oberkochen, Germany).
Histomorphometric evaluation
In the preliminiary experiment, animals were sacrificed at 1, 2 and 3 weeks (n = 6 in each group at each time point) and in the formal experiment, animals in each group were sacrificed at 2 and 4 weeks (n = 8 in each group at each time point). Once rats were euthanized, a 10-mm section of the MN including the MN crush injury site was incised and fixed in PBS (pH7.4) and 2.5% glutaraldehyde. Next, nerve sample was transversely cut at the center, and 2% osmium tetroxide solution was added. Subsequently, the nerve tissue sample was embedded with Epon 812 (Nisshin EM, Tokyo, Japan), cut into 1 μm-thick sections, and stained with 1% toluidine blue. Images were captured using a SPOT RT™-KE color mosaic, and digitized using SPOT software version 4.6 (Diagnostic Instruments, Inc., Sterling Heights, MI, USA). To simplify axon counting (total axon number), the total cross-section area of the nerve (total axon area) was measured at 40× magnification and 3 sampling fields were randomly selected at 200× magnification using a previously reported protocol (Li et al., 2012). The mean axon density was calculated by dividing the total number of nerve fibers within the sampling field by its area (N/mm2). The total axon number (N) was estimated by multiplying the mean fiber density by the entire cross-section area of the whole nerve, assuming a uniform distribution of nerve fibers across the entire section (Alrashdan et al., 2011).
To calculate myelin thickness, frozen sections were cut using the cryostat (Leica, Ultracut, UCT, Vienna, Austria) and double-stained with hematoxylin (Sigma, St. Louis, MO, USA) and eosin (Sigma). Then the sections were analyzed using OPTIMAS Ver. 6.5 (Media Cybernetics, Bethesda, MD, USA) to measure myelin thickness. G-ratio was defined as the ratio of axon diameter to the myelinated fiber diameter. The size of soma was measured as described before (Li et al., 2012). All measurements and histological observations were performed by individuals who were not involved in collecting samples for the experiment.
Statistical analysis
All variables were expressed as the mean ± SD. Data analysis was conducted using one-way analysis of variance with STATVIEW software (Abacus, Berkeley, CA, USA). The Mann-Whitney U test for nonparametric analysis was used. A level of P < 0.05 was considered statistically significant.
Results
Sensory function
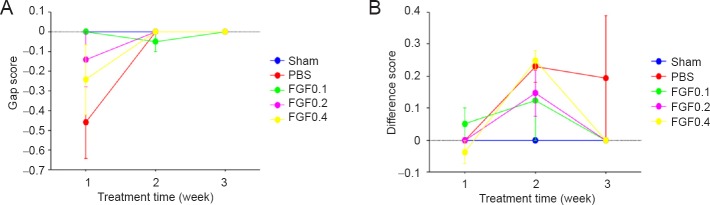
In a preliminary test, sensory nerve impairment and functional recovery were not observed in injured mental nerve after rhFGF2 treatment (Figure 3).
Figure 3.
Preliminary test of the sensory functional recovery of rats with mental nerve crush injury assessed by von Frey filaments test after recombinant human fibroblast growth factor-2 (rhFGF2) treatment.
(A, B) A comparison of the gap score (A) and difference score (B) shows little sensory functional recovery in rats with mental nerve crush injury after administration of rhFGF2 at low doses (0.1, 0.2 and 0.4 μg/mL). Decreased gap score and increased difference score mean the presence of sensory degeneration. Sham: Sham-operated (normal control); PBS: PBS treatment after mental nerve crush injury; rhFGF0.1, rhFGF0.2 and rhFGF0.4: rhFGF2 treatment (0.1, 0.2, 0.4 μg/mL, respectively) after mental nerve crush injury. There were six rats per group.
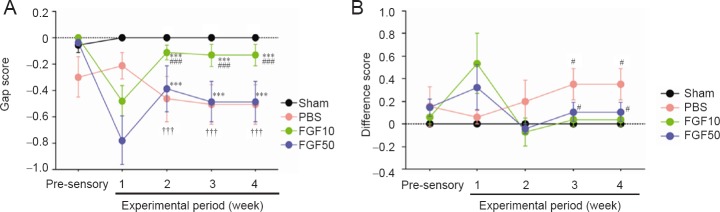
A decline in sensory function was indicated by the gap score in the rhFGF groups at 1 week after the crush injury. However, a sensory functional recovery was observed at 2–4 weeks (Figure 4A; P < 0.05). From the viewpoint of difference score, sensory function was improved at 1 week but it maintained a stable level from week 2 from which the nerve injury began to recover as indicated by the gap score. However, sensory function was significantly different at 3 and 4 weeks between 10 μg/mL rhFGF2, 50 μg/mL rhFGF2 and PBS groups (Figure 4B; P < 0.05).
Figure 4.
Effect of recombinant human fibroblast growth factor-2 (rhFGF2) treatment on touch sensory recovery of rats with mental nerve crush injury.
(A) Gap score. At 1 week, sensory function was impaired by mental nerve crush injury. However, the sensory test showed that at 2 weeks, sensory function in the rhFGF2-treated groups recovered (FGF10 and FGF50 groups vs. sham group ***P < 0.001; FGF10 group vs. PBS group ###P < 0.001; FGF10 group vs. FGF50 group †††P < 0.001). (B) Difference score (FGF10 and FGF50 groups vs. PBS group #P < 0.05). Data in (A) and (B) are expressed as the mean ± SEM. Decreased gap score and increased different score mean the presence of sensory degeneration. Sham: Sham-operated (normal control); PBS: PBS treatment after mental nerve crush injury; FGF10 and FGF50: rhFGF2 treatment (10 and 50 μg/mL, respectively) after mental nerve crush injury. There were eight rats in each group at each time point.
Nerve regeneration in the trigeminal ganglia of rats treated with rhFGF2 after mental nerve crush injury
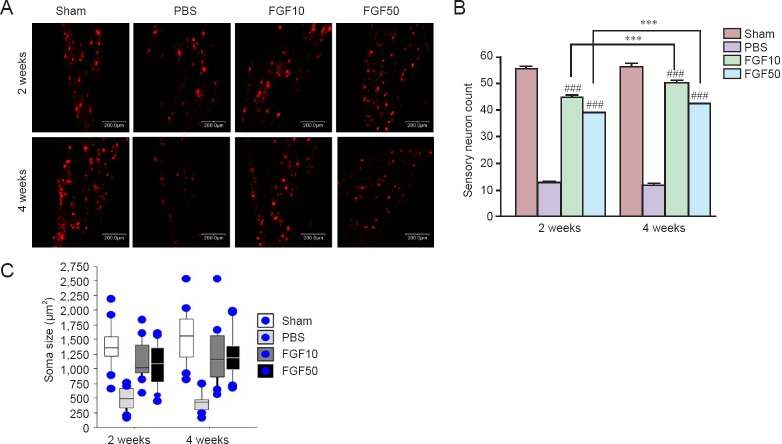
After rhFGF2 treatment in rats with mental nerve crush injury, Dil staining was performed to investigate retrogradely labeled neurons in the trigeminal ganglion (Figure 5A). At 2 and 4 weeks after treatment, the number of sensory neurons in the rhFGF2 groups was significantly greater than in the PBS group (Figure 5B; P < 0.001). There was no significant difference in the size of soma between rhFGF2 groups and sham group, but the size of soma was significantly larger in the rhFGF2 groups than in the PBS group (Figure 5C; P < 0.001).
Figure 5.
Retrograde tracing of the trigeminal ganglion with Dil.
(A) Sensory neurons (red) determined by DiI staining in the trigeminal ganglion. (B) Quantification and statistical significance of the cell count (###P < 0.001, vs. PBS group; ***P < 0.001). (C) Soma sizes (μm2) in each group. The retrogradely labeled neurons had larger soma in the rhFGF2-treated groups than in the PBS group (P < 0.001, FGF10 and FGF50 groups vs. PBS group at 2 and 4 weeks). Scale bars: 200 μm. Data in (B) and (C) are expressed as the mean ± SEM. Sham: Sham-operated (normal control); PBS: PBS treatment after mental nerve crush injury; FGF10 and FGF50: rhFGF2 treatment (10 and 50 μg/mL, respectively) after mental nerve crush injury.
Histomorphometric evaluation results
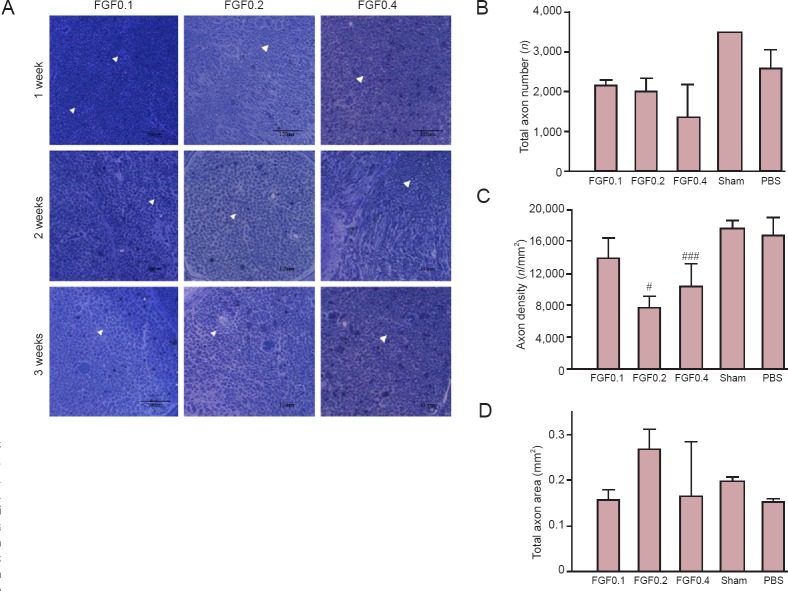
Although axonal changes were difficult to be observed (Figure 6A), rhFGF2 treatments did not induce rebounding of total axonal number (Figure 6B). Comparisons of axon density and total area between groups showed that rhFGF2 treatment did not lead to recovery from nerve injury (Figure 6C, D).
Figure 6.
Preliminary test of axonal changes in injured mental nerve after recombinant human fibroblast growth factor-2 (rhFGF2) administration.
(A) Light microscopy images of the axons in the crush injury area. Scale bars: 100 μm. A simple example of axon was shown by an arrowhead, which shows the quantity, density, and statistical total axon area. (B–D) The quantitative results were obtained at 4 weeks. (B) Total axon number. (C) Axon density based on the total axon number and total area (#P < 0.05, ###P < 0.001, vs. PBS group). (D) Area statistics for the axons in the crush injury area. Data in (B), (C) and (D) are expressed as the mean ± SEM. Sham: Sham-operated (normal control); PBS: PBS treatment after mental nerve crush injury; FGF0.1, FGF0.2 and FGF0.4: rhFGF2 treatment (0.1, 0.2 and 0.4 μg/mL, respectively) after mental nerve crush injury.
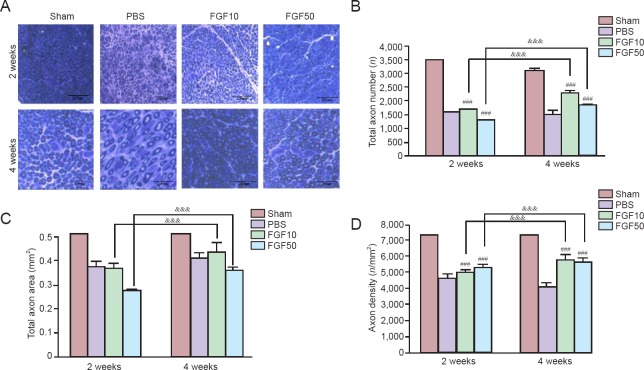
No obvious axonal changes were observed in the PBS group, but total axon numbers increased in the FGF10 and FGF50 groups (Figure 7A). The total axon numbers were significantly different between the PBS, FGF10, and FGF50 groups at 2 and 4 weeks (Figure 7B; P < 0.001). There was statistical significance in total axon number between FGF10 and FGF50 groups and PBS group at 2 and 4 weeks (Figure 7B; P < 0.001). Total axon area increased with rhFGF2 treatment and there was significant difference between FGF10 and FGF50 groups at 2 and 4 weeks (Figure 7C; P < 0.001). Axon density (total axon numbers/total axon area) was calculated to verify the increase in axons per unit area. Axon density was significantly different in both the FGF10 and FGF50 groups compared to the PBS group at 2 and 4 weeks (Figure 7D; P < 0.001), and axon density increased in the FGF10 and FGF50 groups than in the PBS group at 2 and 4 weeks (Figure 7D; P < 0.001).
Figure 7.
Axon observation in injured mental nerve after recombinant human fibroblast growth factor-2 (rhFGF2) treatment.
The mental nerve was collected at 2 and 4 weeks after injury. Scale bars: 200 μm. (A) Light microscopy images of a harvested mental nerve. (B) Total axon number (&&&P < 0.001; ###P < 0.001, vs. PBS group). (C) Total axon area (&&&P < 0.001). (D) Axon density (&&&P < 0.001; ###P < 0.001, vs. PBS group). Data in (B), (C) and (D) are expressed as the mean ± SEM. Sham: Sham-operated (normal control); PBS: PBS treatment after mental nerve crush injury; FGF10 and FGF50: rhFGF2 treatment (10 and 50 μg/mL, respectively) after mental nerve crush injury.
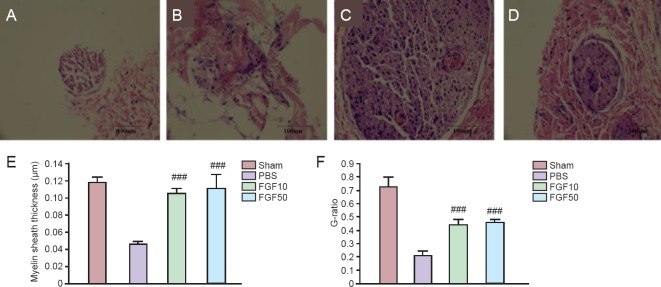
According to a previous report by our laboratory (Li et al., 2012), the thickness of myelin sheath was measured in each group at 4 weeks (Figure 8A–D). For measurement of myelin sheath thickness, axon surrounded by uniform-thickness myelin layer was chosen. Mean myelin thickness and G-ratio in the FGF10 and FGF50 groups were significantly increased than in the PBS group (Figure 8E and F, P < 0.001).
Figure 8.
Changes in myelin sheath thickness in injured mental nerve.
(A–D) Hematoxylin-eosin staining in the sham (A), PBS (B), FGF10 (C) and FGF50 groups (D). (E) Myelin sheath thickness (###P < 0.001, vs. PBS group). (F) G-ratio (###P < 0.001, vs. PBS group). Data in (E) and (F) are expressed as the mean ± SEM. Sham: Sham-operated (normal control); PBS: PBS treatment after mental nerve crush injury; FGF10 and FGF50: rhFGF2 treatment (10 and 50 μg/mL, respectively) after mental nerve crush injury.
Discussion
FGF2 triggers mitogenesis and the proliferation of neural stem cells in vitro (Vescovi et al., 1993; Palmer et al., 1999). FGF2 also plays important physiological roles in vivo, including neurogenesis that occurs in the subventricular zone and dentate gyrus of the hippocampus (Vescovi et al., 1993; Craig et al., 1996; Kuhn et al., 1997; Nakagami et al., 1997; Palmer et al., 1999; Yoshimura et al., 2001). Lin et al. (2011) found that FGF2 administration reduced allodynia, and Collombet et al. (2011) reported that cytokine treatment using FGF2 or epidermal growth factor is effective for nerve regeneration and cognitive behavior recovery. To induce long distance regeneration, leupeptin promotes FGF2-induced axonal growth (Hausott et al., 2008), and inhibition of FGFR1 endocytosis stimulates the axonal branching of sensory neurons (Hausott et al., 2011).
Here, we provide evidence that administration of rhFGF2 can be used to treat MN crush injury in rats. Typically, for nerve injuries, neurotrophic factors are primarily administered subcutaneously, intramuscularly or intravenously via an osmotic pump or a Hamilton syringe. In the current study, we directly and constitutively administered rhFGF2 to the crush injury area using an osmotic pump.
The purpose of our study was to investigate the possible recovery of sensory neurons by administering rhFGF2 to the nerve injury area. Because the rhFGF2 that we used had not been used previously in clinical or pre-clinical tests, a preliminary experiment was performed using low doses of rhFGF2 to determine a suitable dose for sensory recovery. However, these low doses were not associated with functional recovery in the sensory neurons.
Based on previous studies (Hausott et al., 2008; Collombet et al., 2011; Lin et al., 2011; Li et al., 2012), we performed rhFGF2 treatments using 2 dosage groups: a low dose group that was treated with 10 μg/mL rhFGF2 (FGF10) and a high dose group that was treated with 50 μg/mL rhFGF2 (FGF50). We investigated the functional recovery of sensory neurons after rhFGF2 treatments in rats with a MN crush injury.
In the sensory test that we used based on the method from Seino et al. (2009), the subjective will of an examiner can affect the experimental results. To avoid this issue, the examiner was blinded to the experimental groups. We recorded the absolute values of the results from the sensory test for each rat to reveal the effects of the experimental conditions (Chaplan et al., 1994). However, the influence of the experimental conditions of the rats was minimized by measuring each score not as an absolute value but as the mean value for each group. Raising a front leg to touch the filament indicated a ‘positive’ touch response because the lower lip of the rat was pierced so that the animal could not turn its head and bite the filament with its teeth. The sensory test was performed once 3 days before the surgery and once a week from weeks 1 to 4 after the surgery. The sensory test score was expressed as a gap score and a difference score. Although sensory loss based on a comparison of the pre- and post-surgery gap score at week 1 occurred in all groups except the sham group, sensory recovery indicated by a gap score closer to 0 was observed in the rhFGF2 group at 2 weeks. In contrast, the difference score increased in the rhFGF2 group at 1 week and began to decrease at 2 weeks. The symmetric graphs of the difference score and gap score show that the extent of the recovery in sensory function progressed over time in the rhFGF2-treated groups.
Nerve regeneration of the trigeminal ganglia decreased over time in the PBS group. Compared to the PBS group, the rhFGF2 group had significantly more nerve regeneration over time. Axonal density and total axon number were significantly greater in the rhFGF2 groups than in the PBS group. Moreover, over time, the total axon number, total axon area, and axon density increased in the rhFGF2 groups.
There are many studies reporting that stem cells can be used to repair peripheral nerve injury. But there are few clinical studies about it. There is evidence that at 8 weeks after transplantation of human hair follicle stem cells for repair of sciatic nerve injury, the growth of spindle cells was observed (Amoh et al., 2005, 2008, 2009, 2012; Mii et al., 2013; Hoffman, 2015). Li et al. (2012) recombinant human nerve growth factor-β gene transfer promotes regeneration of crush-injured mental nerve in rats. As in the abovementioned experiments, stem cells or stem cell growth factors have been reported to repair peripheral nerve damage. However, we evaluated neurological recovery after intravenous injection of growth factor for mental nerve crush injury.
Our results suggest that rhFGF2 treatment affects the recovery of sensory function from a MN crush injury. However, the rhFGF2 used in our study had not been tested clinically; therefore, a more accurate dose calculation is required for the clinical application of rhFGF2. In addition, future studies should investigate the mechanism underlying down-regulation of FGF activity and the mechanism by which rhFGF2 directly affects nerve regeneration and the functional recovery of sensory neurons.
Footnotes
Funding: This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea, No. A101578.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using CrossCheck to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Copyedited by Li CH, Song LP, Zhao M
References
- Alhassani AA, AlGhamdi AST. Inferior alveolar nerve injury in implant dentistry: diagnosis, causes, prevention, and management. J Oral Implantol. 2010;36:401–407. doi: 10.1563/AAID-JOI-D-09-00059. [DOI] [PubMed] [Google Scholar]
- Alrashdan MS, Sung MA, Kwon YK, Chung HJ, Kim SJ, Lee JH. Effects of combining electrical stimulation with BDNF gene transfer on the regeneration of crushed rat sciatic nerve. Acta Neurochir (Wien) 2011;153:2021–2029. doi: 10.1007/s00701-011-1054-x. [DOI] [PubMed] [Google Scholar]
- Amoh Y, Li L, Katsuoka K, Hoffman RM. Multipotent hair follicle stem cells promote repair of spinal cord injury and recovery of walking function. Cell Cycle. 2008;7:1865–1869. doi: 10.4161/cc.7.12.6056. [DOI] [PubMed] [Google Scholar]
- Amoh Y, Li L, Campillo R, Kawahara K, Katsuoka K, Penman S, Hoffman RM. Implanted hair follicle stem cells form Schwann cells that support repair of severed peripheral nerves. Proc Natl Acad Sci U S A. 2005;102:17734–17738. doi: 10.1073/pnas.0508440102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoh Y, Kanoh M, Niiyama S, Hamada Y, Kawahara K, Sato Y, Hoffman RM, Katsuoka K. Human hair follicle pluripotent stem (hfPS) cells promote regeneration of peripheral-nerve injury: An advantageous alternative to ES and iPS cells. J Cell Biochem. 2009;107:1016–1020. doi: 10.1002/jcb.22204. [DOI] [PubMed] [Google Scholar]
- Amoh Y, Aki R, Hamada Y, Niiyama S, Eshima K, Kawahara K, Sato Y, Tani Y, Hoffman RM, Katsuoka K. Nestin-positive hair follicle pluripotent stem cells can promote regeneration of impinged peripheral nerve injury. J Dermatol. 2012;39:33–38. doi: 10.1111/j.1346-8138.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE. Mini review. Growth factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach F, Pogrel J, Chung J, Yaksh T. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Choi KC, Yoo DS, Cho KS, Huh PW, Kim DS, Park CK. Effect of single growth factor and growth factor combinations on differentiation of neural stem cells. J Korean Neurosurg Soc. 2008;44:375–381. doi: 10.3340/jkns.2008.44.6.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombet JM, Béracochéa D, Liscia P, Piérard C, Lallement G, Filliat P. Long-term effects of cytokine treatment on cognitive behavioral recovery and neuronal regeneration in soman-poisoned mice. Behav Brain Res. 2011;221:261–270. doi: 10.1016/j.bbr.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Craig CG, Tropepe V, Morshead CM, Reynolds BA, Weiss S, Van der Kooy D. In vivo growth factor expansion of endogenous subependymal neural precursor cell populations in the adult mouse brain. J Neurosci. 1996;16:2649–2658. doi: 10.1523/JNEUROSCI.16-08-02649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto E, Mizoguchi A, Hanada K, Yahima M, Ide C. Basic fibroblast growth factor promotes extension of regenerating axons of peripheral nerve. In vivo experiments using a Schwann cell basal lamina tube model. J Neurocytol. 1997;26:511–528. doi: 10.1023/a:1015410023132. [DOI] [PubMed] [Google Scholar]
- Grothe C, Nikkhah G. The role of basic fibroblast growth factor in peripheral nerve regeneration. Anat Embryol (Berl) 2001;204:171–177. doi: 10.1007/s004290100205. [DOI] [PubMed] [Google Scholar]
- Hausott B, Schlick B, Vallant N, Dorn R, Klimaschewski L. Promotion of neurite outgrowth by fibroblast growth factor receptor 1 overexpression and lysosomal inhibition of receptor degradation in pheochromocytoma cells and adult sensory neurons. Neuroscience. 2008;153:461–473. doi: 10.1016/j.neuroscience.2008.01.083. [DOI] [PubMed] [Google Scholar]
- Hausott B, Rietzler A, Vallant N, Auer M, Haller I, Perkhofer S, Klimaschewski L. Inhibition of fibroblast growth factor receptor 1 endocytosis promotes axonal branching of adult sensory neurons. Neuroscience. 2011;188:13–22. doi: 10.1016/j.neuroscience.2011.04.064. [DOI] [PubMed] [Google Scholar]
- Hoffman RM. Nestin-expressing hair follicle-accessible pluripotent stem cells for nerve and spinal cord repair. Cells Tissues Organs. 2015;200:42–47. doi: 10.1159/000366098. [DOI] [PubMed] [Google Scholar]
- Imai T, Atsumi Y, Matsumoto K, Yura Y, Wakisaka S. Regeneration of periodontal Ruffini endings of rat lower incisors following nerve cross-anastomosis with mental nerve. Brain Res. 2003;992:20–29. doi: 10.1016/j.brainres.2003.08.023. [DOI] [PubMed] [Google Scholar]
- Kemp SW, Webb AA, Dhaliwal S, Syed S, Walsh SK, Midha R. Dose and duration of nerve growth factor (NGF) administration determine the extent of behavioral recovery following peripheral nerve injury in the rat. Exp Neurol. 2011;229:460–470. doi: 10.1016/j.expneurol.2011.03.017. [DOI] [PubMed] [Google Scholar]
- Kerekes N, Landry M, Rydh-Rinder M, Hkfelt T. The effect of NGF, BDNF and bFGF on expression of galanin in cultured rat dorsal root ganglia. Brain Res. 1997;754:131–141. doi: 10.1016/s0006-8993(97)00056-5. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BH, Kim SM, Yoo SB, Kim MJ, Jahng JW, Lee JH. Recombinant human nerve growth factor (rhNGF-β) gene transfer promotes regeneration of crush-injured mental nerve in rats. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:e26–34. doi: 10.1016/j.tripleo.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Lin YL, Kuo HS, Lo MJ, Tsai MJ, Lee MJ, Huang WC, Kuo WC, Shih YH, Cheng H, Huang MC. Treatment with nerve grafts and aFGF attenuates allodynia caused by cervical root transection injuries. Restor Neurol Neurosci. 2011;29:265–274. doi: 10.3233/RNN-2011-0597. [DOI] [PubMed] [Google Scholar]
- Mii S, Duong J, Tome Y, Uchugonova A, Liu F, Amoh Y, Saito N, Katsuoka K, Hoffman RM. The role of hair follicle nestin-expressing stem cells during whisker sensory-nerve growth in long-term 3D culture. J Cell Biochem. 2013;114:1674–1684. doi: 10.1002/jcb.24509. [DOI] [PubMed] [Google Scholar]
- Nakagami Y, Saito H, Matsuki N. Basic fibroblast growth factor and brain-derived neurotrophic factor promote survival and neuronal circuit formation in organotypic hippocampal culture. Jpn J Pharmacol. 1997;75:319–326. doi: 10.1254/jjp.75.319. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rash BG, Lim HD, Breunig JJ, Vaccarino FM. FGF signaling expands embryonic cortical surface area by regulating Notch-dependent neurogenesis. J Neurosci. 2011;31:15604–15617. doi: 10.1523/JNEUROSCI.4439-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savignat M, De-Doncker L, Vodouhe C, Garza J, Lavalle P, Libersa P. Rat nerve regeneration with the use of a polymeric membrane loaded with NGF. J Dent Res. 2007;86:1051–1056. doi: 10.1177/154405910708601106. [DOI] [PubMed] [Google Scholar]
- Savignat M, Vodouhe C, Ackermann A, Haikel Y, Lavalle P, Libersa P. Evaluation of early nerve regeneration using a polymeric membrane functionalized with nerve growth factor (NGF) after a crush lesion of the rat mental nerve. J Oral Maxillofac Surg. 2008;66:711–717. doi: 10.1016/j.joms.2007.06.654. [DOI] [PubMed] [Google Scholar]
- Seino H, Seo K, Maeda T, Someya G. Behavioural and histological observations of sensory impairment caused by tight ligation of the trigeminal nerve in mice. J Neurosci Methods. 2009;181:67–72. doi: 10.1016/j.jneumeth.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Shin DM, Korada S, Raballo R, Shashikant CS, Simeone A, Taylor JR, Vaccarino F. Loss of glutamatergic pyramidal neurons in frontal and temporal cortex resulting from attenuation of FGFR1 signaling is associated with spontaneous hyperactivity in mice. J Neurosci. 2004;24:2247–2258. doi: 10.1523/JNEUROSCI.5285-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Dailey T, Duncan K, Abel N, Borlongan CV. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int J Mol Sci. 2016;17:2101. doi: 10.3390/ijms17122101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Sun C, Lin H, Zhao H, Wang J, Ma H, Chen B, Xiao Z, Dai J. The effect of collagen-binding NGF-β on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials. 2009;30:4649–4656. doi: 10.1016/j.biomaterials.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin JD, Wyland JJ, Hung Y-TE. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci. 1999;2:246–253. doi: 10.1038/6350. [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- Werner S, Unsicker K, von Bohlen und Halbach O. Fibroblast growth factor-2 deficiency causes defects in adult hippocampal neurogenesis, which are not rescued by exogenous fibroblast growth factor-2. J Neurosci Res. 2011;89:1605–1617. doi: 10.1002/jnr.22680. [DOI] [PubMed] [Google Scholar]
- Yoshimura S, Takagi Y, Harada J, Teramoto T, Thomas SS, Waeber C, Bakowska JC, Breakefield XO, Moskowitz MA. FGF-2 regulation of neurogenesis in adult hippocampus after brain injury. Proc Natl Acad Sci U S A. 2001;98:5874–5879. doi: 10.1073/pnas.101034998. [DOI] [PMC free article] [PubMed] [Google Scholar]