Abstract
Purpose
To estimate serum vitamin D (25 OH D) level in patients of retinal vein occlusion (RVO) and compare it with age-matched controls.
Methods and material
Forty patients above 18 years of age with retinal vein occlusion and forty age-matched controls underwent serum vitamin D (Vit D) level estimation using a standard protocol. Student’s t test was used to analyse differences between the mean of two groups.
Results
The mean age in RVO and control group was 60.25 and 60.73 years respectively. The mean (±SD) level of vitamin D in RVO patients was 13.68 (±4.58) ng/mL (range 5.5–24.8), and the 95% CI of mean was 12.21–15.14 with SD 4.58 while in control group it was 23.03 (±2.89) ng/ml (range 18.4–30.1) with 95% CI of mean being 22.11–23.96 with SD 2.89 (p value of <0.005). While comparing the level of Vitamin D based on type of occlusion the mean level of Vit D in CRVO patients was 15.36 (SD 5.30) and in BRVO it was 12.77 (SD 3.96) which was statistically not significant (p = 0.08). The odds ratio calculated for RVO cases versus controls was 133.33 which was statistically significant (P < 0.05).
Conclusions
There is a paucity of published literature on level of Vit D in RVO. This study shows significantly lower levels of serum vitamin D in Indian patients with retinal vein occlusion as compared to age matched controls. Establishment of this correlation has possible implications for prophylaxis or treatment of RVOs.
Keywords: Vitamin D, Retinal vein occlusion, Cardiovascular disease
Introduction
Retinal vein occlusion (RVO) is an important retinal vascular cause of reduced vision. RVO can be classified into various groups based on the location of occlusion of the vein like central retinal vein occlusion (CRVO), hemi central retinal vein occlusion (HCRVO) and branch retinal vein occlusion (BRVO).1 Various risk factors have been identified in causation of RVO such as hypertension, diabetes mellitus, abnormal lipid profile, and prothrombotic states like hyperhomocysteinaemia which are common to other vascular diseases.2
Studies1 have shown that cardiovascular risk factors are seen in a significant number of patients with RVO and they also share common biochemical and haematological abnormalities.
Vitamin D is known as the sunlight hormone as it is synthesized by the conversion of 7-dehydrocholesterol present in subcut fat to pro-vitamin D in the presence of ultraviolet rays. This is isomerized to 25 (OH) D which is subsequently metabolized to 1.25 (OH) D in the liver and kidneys.
The role of vitamin D in maintaining the vascular system is now being increasingly understood. The Vitamin D receptors are extensively distributed in several tissues not involved in calcium metabolism like lymphocytes, hepatocytes, cardiac and vascular myocytes.3 This vitamin was considered only essential for bone growth. Almost two decades ago animal studies on rats pointed towards possible association of Vit D with cardiovascular diseases.4Some of the studies published recently5 have shown a positive correlation between vascular diseases like hypertension, coronary heart disease and cerebro - vascular accidents and vitamin D deficiency.6 In large population based studies, vitamin D deficiency has been linked to high mortality rate due to vascular events involving coronary and cerebral circulation.7
Large epidemiological studies have found an association between the lack of sunlight exposure and vitamin D deficiency with renin angiotensin metabolism.8
The role of Vit. D has been studied in the functioning of endothelium in diabetic patients.9 The vascular endothelial function improved after supplementation of vitamin D. The effect of Vit D deficiency on vascular endothelium could possibly have some role to play in the causation of RVO which further needs to be validated. Results of a large study on Vitamin D and heart failure patients suggest that Vitamin D supplementation improves outcomes.10
Vitamin D deficiency has also been implicated in various types of vascular diseases including peripheral arterial disease where a small difference in serum vitamin D has greatly affected vascular disease risk.11 This study was designed considering the facts that other systemic vascular diseases and RVO share common risk factors.
Subjects and methods
In this study forty retinal vein occlusion (CRVO/BRVO) and forty age matched controls were enrolled. The study was conducted at a tertiary care centre in Bangalore (South India) between May 2012 and Apr 2013. Institutional ethical clearance was taken. Informed written consent in the local language was taken. Declaration of Helsinki was adhered to.
A total of 80 subjects formed the study group out of which 40 were RVO patients and forty were controls.
Over a period of the next eight months, the first five patients reporting every month as a case of RVO of less than three months duration were recruited in the study. Similarly five controls were recruited every month to avoid any seasonal variation of Vitamin D levels.
Inclusion criteria: Cases of RVO willing to be part of study with onset less than three months were enrolled as cases and accompanying relatives of these patients willing to be part of study were taken as controls.
Exclusion criteria: Patients on vitamin D supplementation, age less than 18 years, therapeutic diets, renal, hepatic and skin disease and chronic alcoholics were excluded from the study.
The selected willing patients were then subjected to ophthalmic evaluation consisting of vision assessment, pupillary reaction, fundus picture, Fluorescein angiography (Carl Zeiss) and Optical Coherence Tomography (Carl Zeiss Cirrus HD OCT). Relevant history of diabetes mellitus (DM), hypertension (HTN), angina (CAD) and stroke (CVA) was taken with confirmation from medical records. The patients also underwent systemic evaluation which included blood pressure recording and haematological evaluation in the form of blood sugar fasting and post prandial, lipid profile and ECG. Forty age, sex matched attendants of patients were concurrently enrolled as controls, as they had comparable dietary and socio-economic status.
After fasting for 12 h the blood sample was collected from each participant. The serum was separated and was frozen at minus 20 C before further analysis using tandem mass spectrometry (Waters India Pvt Ltd.) for total vitamin D (25 OH D). A total of 80 subjects with their vitamin D levels were included for analysis.
Levels <20 ng/mL were taken as Vit D deficiency. The results were collected on Microsoft Excel and analysed using SPSS (Version 17) software. The mean levels of vitamin D were compared using Student’s t test and a p value < 0.05 was considered significant.
Results
A total of eighty subjects were enrolled in the study out of which forty were cases of RVO and forty were control subjects. The RVO patients were well matched to controls. Out of 40 patients of RVO 35% were CRVO and 65% were BRVO (Table 1).
Table 1.
Profile of cases and controls.
| Parameter | Cases | Controls |
|---|---|---|
| Number | 40 | 40 |
| Age | ||
| Mean ± SD | 60.25 ± 9.67 | 60.73 ± 9.89 |
| Gender | ||
| M | 75% | 65% |
| F | 25% | 35% |
| HTN | 35% | 30% |
| DM | 10% | 7.5% |
| CVA/CAD | 10% | 12.5% |
| Inhabitation | ||
| Rural | 47.5% | 52.5% |
| Urban | 55% | 45% |
| Smokers | 25% | 22.5% |
The mean age of RVO and control patients was 60.25 ± 9.67 years and 60.73 ± 9.89 years. No significant difference between cases and controls was noted based on age (p = 0.605), gender (p = 0.328), comorbid condition (p = 0.303), inhabitation (p = 0.4) and smoking status (p = 0.56).
Serum vitamin D levels were normally distributed. The mean (±SD) level of vitamin D in RVO patients was 13.68 (±4.58) ng/mL (range 5.5–24.8) with 95% CI of mean that was 12.21–15.14 with SD 4.58 and in control group was 23.03 (±2.89) ng/ml (range 18.4–30.1) with 95% CI of mean that was 22.11–23.96 with SD 2.89 with a p value of <0.005 which was statistically significant. 95% CI of difference was −11.06 and −7.
On percentage wise analysis of cases 95% (38/40) of RVO patients had a Vit D level of less than 20 ng/ml whereas 8% (5/40) of controls had Vit D level less than 20 ng/ml. Only 5% (2/40) of RVO patients had Vit D level > 20 ng/ml (Table 2).
Table 2.
Serum Vit D levels in cases and controls.
| Vit D level ng/ml | RVO No. (%) | Controls No. (%) |
|---|---|---|
| <10 | 10 (25%) | 0 (0%) |
| 10–15 | 14 (35%) | 0 (0%) |
| 15–20 | 14 (35%) | 5 (12.5%) |
| 20–25 | 2 (5%) | 27 (67.5%) |
| 25–30 | 0 (0%) | 7 (17.5%) |
| >30 | 0 (0%) | 1 (2.5%) |
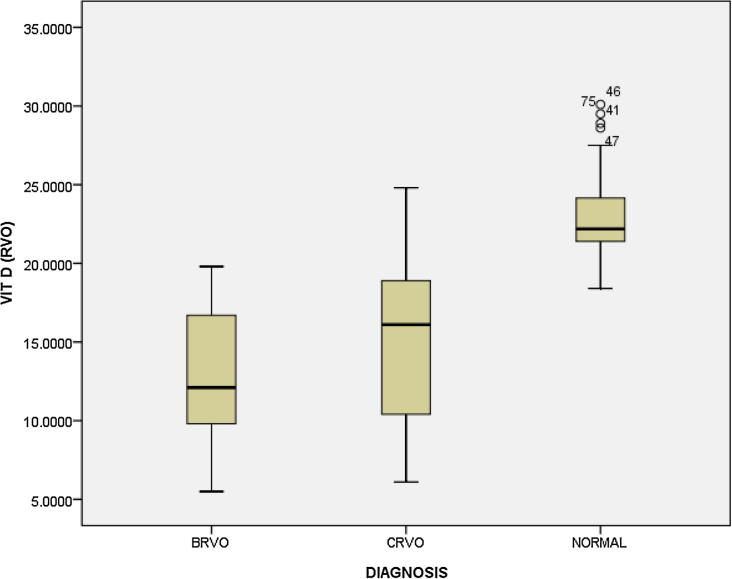
While comparing the level of Vitamin D based on type of occlusion the mean level in CRVO patients was 15.36 (SD 5.30) and in BRVO it was 12.77 (SD 3.96) which was statistically not significant (p = 0.08) (Fig. 1).
Fig. 1.
Serum Vitamin D levels in controls and cases.
The odds ratio calculated for RVO cases versus controls was 133.33 which was statistically significant (P < 0.05).
Discussion
Studies have indicated association of lower serum Vitamin D levels with cardiovascular diseases. These studies have taken different cut-off levels of Vitamin D but most agree to 20 ng/ml as deficiency.12 Levels between 20 and 30 ng/ml have been taken as Vit D insufficiency in large prospective studies.13
Vitamin D deficiency can be caused by various endogenous and exogenous factors like inadequate dietary intake, less sunlight exposure, indoor confinement, use of full body covered clothes like burkha,14 pollution reducing UV rays, use of sunscreen lotions,15 and various chronic diseases like liver, kidney and skin diseases.16
Vitamin D was considered a nutritional supplement till recently, but studies have shown it actually acts like a hormone which has multiple roles to play.17 It is actively involved in synthesis of many proteins and body enzymes which are required in various crucial steps of metabolism.
Large prospective studies have shown that 25 (OH) D is an appropriate indicator of cardiovascular diseases.18 Although 1.25 (OH) D is the active form of Vit D it is not the ideal marker of body Vit D stores as it has shown a weaker correlation with disease status.19
Some of the studies have also tried to establish an association between various chronic inflammatory diseases20 which could also play a role in RVO in certain subgroup of young patients. In this study only one patient was younger than 30 years with Vit D deficiency.
The ideal regimen for Vit D supplementation is also not very well established. Sun exposure required to avoid deficiency of Vit D is thirty minutes between 10 AM and 2 PM as per WHO recommendations.21 For treatment of Vit D deficiency Cholecalciferol21 or ergocalciferol22 has been used successfully. The actual dose required may depend on various factors like sun exposure, skin colour, and outdoor activity. Close monitoring of dose, duration and serum levels of Vitamin D is required to be done.23
Our study has a few limitations like nonestimation of Vit D at the time of onset of RVO. Accordingly the possibility of late onset Vit D deficiency cannot be ruled out but three month cut-off from the onset of event was followed which seems reasonable.
Levels of Vit D may get affected by fasting status of patient. Any noncompliance on this account could change the results and therefore a 12 h fasting period before blood collection was followed. The estimation of serum calcium and parathormone could have further qualified the type of Vit D deficiency.
Age could be one confounding factor for Vit D levels but no significant difference was found between the average age of the two groups (p = 0.605). Vitamin D being sunlight dependent hormone, the recruitment of cases and controls was done in equal number every month to avoid the effect of seasonal variation.
In a recently published study by Epstein D et al.24 Vitamin D was reported to be deficient in 50% of CRVO patients. Elderly patients more than 75 years had severe Vitamin D deficiency. Talcott KE, Eliott D in a case report also found vitamin D deficiency in a case of CRVO.25 These studies also establish correlation of seasonal variation in occurrence of RVO and Vitamin D levels. In our study we found that levels of Vitamin D in BRVO were numerically lower than CRVO patients but it did not reach statistical significance (p = 0.08).
The results of our study point towards the role of vitamin D in vascular health of the eye in this subset of patients. This study may act as a pilot study for establishing a possible correlation of RVO and Vitamin D deficiency. Large randomized controlled trials are required to study the effect of Vitamin D supplementation in prevention of RVO.
The correction of Vitamin D deficiency is technically simple, which may also help in further prevention of cardiovascular diseases as they share common risk factors.
Conclusions: Several disease entities like hypertension, coronary heart disease and cerebro-vascular accidents are associated with Vitamin D deficiency. There is paucity of published literature on the level of Vit. D in RVO. This study on Indian patients shows significantly lower levels of serum vitamin D in patients with retinal vein occlusion as compared to age matched controls. Establishment of this correlation has possible implications for prophylaxis or treatment of RVOs.
Conflict of interest
Authors declare that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Hayreh S. Retinal vein occlusion. Indian J Ophthalmol. 1994;42(3):109–132. [PubMed] [Google Scholar]
- 2.Hayreh S.S., Zimmerman M.B., Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1999;117:429–441. doi: 10.1016/s0002-9394(14)70001-7. [DOI] [PubMed] [Google Scholar]
- 3.DeLuca H.F. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80:1689–1696. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 4.Weishaar R.E., Simpson R.U. Vitamin D3 and cardiovascular function in rats. J Clin Invest. 1987;79:1706–1712. doi: 10.1172/JCI113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akin F., Ayca B., Kose N., Duran M., Sari M., Uysal O.K. Serum vitamin D levels are independently associated with severity of coronary artery disease. J Investig Med. 2012;60:869–873. doi: 10.2310/JIM.0b013e31825457cb. [DOI] [PubMed] [Google Scholar]
- 6.Snijder M.B., Lips P., Seidell J.C. Vitamin D status and parathyroid hormone levels in relation to blood pressure: a population-based study in older men and women. J Intern Med. 2007;261:558–565. doi: 10.1111/j.1365-2796.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 7.Schleithoff S.S., Zittermann A., Tenderich G., Berthold H.K., Stehle P., Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–759. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 8.Zittermann A. Vitamin D in preventive medicine: are we ignoring the evidence? Br J Nutr. 2003;89:552–572. doi: 10.1079/BJN2003837. [DOI] [PubMed] [Google Scholar]
- 9.Sugden J.A., Davies J.I., Witham M.D., Morris A.D., Struthers A.D. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–325. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 10.Gotsman I., Shauer A., Zwas D.R., Hellman Y., Keren A., Lotan C. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Eur J Heart Fail. 2012;14:357–366. doi: 10.1093/eurjhf/hfr175. [DOI] [PubMed] [Google Scholar]
- 11.Targher G., Bertolini L., Padovani R. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima media thickness among type 2 diabetic patients. Clin Endocrinol. 2006;65:593–597. doi: 10.1111/j.1365-2265.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 12.Forman J.P., Giovannucci E., Holmes M.D. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E., Liu Y., Hollis B.W., Rimm E.B. 25-Hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goswami R., Gupta N., Goswami D., Marwaha R.K., Tandon N., Kochupillai N. Prevalence and significance of low 25-hydroxyvitamin D concentrations in healthy subjects in Delhi. Am J Clin Nutr. 2000;72:472–475. doi: 10.1093/ajcn/72.2.472. [DOI] [PubMed] [Google Scholar]
- 15.Harinarayan C.V., Joshi S.R. Vitamin D status in India-Its implications and Remedial Measures. J Assoc Physicians India. 2009;57:40–48. [PubMed] [Google Scholar]
- 16.Li Y.C., Qiao G., Uskokovic M., Xiang W., Zheng W., Kong J. Vitamin D: a negative endocrine regulator of the renin angiotensin system and blood pressure. J Steroid Biochem Mol Biol. 2004;90:387–392. doi: 10.1016/j.jsbmb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Zittermann A., Schleithoff S.S., Gotting C. Poor outcome in end-stage heart failure patients with low circulating calcitriol levels. Eur J Heart Fail. 2008;10:321–327. doi: 10.1016/j.ejheart.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Holick M.F. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sokol S.I., Srinivas V., Crandall J.P.V., Kim M., Tellides P., Lebastchi A. The effects of vitamin D repletion on endothelial function and inflammation in patients with coronary artery disease. Vasc Med. 2012;17:394–404. doi: 10.1177/1358863X12466709. [DOI] [PubMed] [Google Scholar]
- 20.Expert Consultation on vitamin and mineral requirement in human nutrition: Second Edition FAO Rome. Available at <http:// whqlibdoc.who.int/ publications/2004/9241546123.pdf>. Report of Joint FAO/WHO; 2004.
- 21.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 22.Pfeifer M., Begerow B., Minne H.W., Nachtigall D., Hansen C. Effects of a short-term vitamin D3 and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–1637. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 23.Joshi R. Hypercalcemia due to hypervitaminosis D: report of seven patients. J Trop Pediatr. 2009;55:396–398. doi: 10.1093/tropej/fmp020. [DOI] [PubMed] [Google Scholar]
- 24.Epstein D., Kvanta A., Lindqvist P.G. Vitamin D deficiency in patients with central retinal vein occlusion: a case control study. Curr Eye Res. 2017;42(3):448–451. doi: 10.1080/02713683.2016.1188117. [DOI] [PubMed] [Google Scholar]
- 25.Talcott K.E., Eliott D. Central retinal vein occlusion associated with severe vitamin D deficiency. Ophthal. Surg. Lasers Imag. Retina. 2016;47(4):372–375. doi: 10.3928/23258160-20160324-13. [DOI] [PubMed] [Google Scholar]