Abstract
Purpose
The purpose of this study was to investigate whether the severity of diabetic disease in the retina is paralleled by changes in the photoreceptor layer.
Methods
This cross-sectional study included healthy volunteers (30 volunteers, 60 eyes) and patients with diabetes (48 patients, 96 eyes). Each patient underwent a single session of spectral domain optical coherence tomography (OCT) in which each retina was imaged twice. On each OCT image, the thickness of the PROS layer was measured at the foveal center and at points 750 μm temporal to and nasal to the center. For statistical analyses, OCT images were assigned to one of the following groups: healthy, diabetes without retinopathy (DM), diabetic retinopathy (DR), or diabetic retinopathy with macular edema (DME).
Results
The mean PROS thickness at the foveal center in the first and second-obtained OCT images was as follows: healthy, 38.5 μm and 38.6 μm; DM, 38.2 μm and 38.2 μm; DR, 35.6 μm and 36.1 μm; DME, 32.6 μm and 32.6 μm. In the first and second-obtained images, significant differences were found between the healthy group and DR and DME (p < 0.05 for all), between the DM group and the DME (p < 0.05 for all), and between the DR group and the DME group (p < 0.05 for all). No significant differences between groups were found at the nasal and temporal locations.
Conclusion
The PROS layer at the foveal center was thinner in patients who had diabetic retinopathy or diabetic macular edema than both the healthy volunteers and diabetic patients without retinopathy.
Keywords: Diabetes mellitus, Diabetic retinopathy, Fovea centralis, Macular edema, Photoreceptor cells
Introduction
Retinal abnormalities in patients with diabetes mellitus have been a focus of research since the late 1800s and have comprised an increasingly diverse range of phenomena.1, 2, 3, 4, 5, 6, 7 Recent findings have extended this range to include abnormalities in the structure of the retina's layers, particularly the photoreceptor layer.8, 9, 10
One finding that suggests photoreceptor dysfunction in diabetes is the abnormally weak Stiles-Crawford effect – the sensitivity of the retina to the angle of light falling on it – that has been reported for patients with diabetes.11, 12 Further evidence comes from retinal abnormalities detected on optical coherence tomography (OCT). The line taken as representing the junction between the photoreceptor inner and outer segments (the IS/OS line) has been found to be disrupted in patients with diabetic macular edema.13, 14, 15 The external limiting membrane has similarly appeared disrupted.14, 16 More recently, an adaptive optics method has been used to measure the population density of cone cells in the retina, and in patients with diabetes the method appears to show a reduction in this density.17
Photoreceptor structure in patients with diabetes has also been investigated in terms of OCT-based measurements of retinal layer thickness.8, 9, 10, 19 In patients with diabetes but no retinopathy, the layer representing photoreceptors at the fovea was reported to be thinner than the corresponding layer in healthy volunteers.8 In patients with diabetic macular edema, visual acuity was found to vary according to thickness of the photoreceptor outer segment layer (PROS).9, 10 particularly when this thickness was measured at or near the center of the fovea.9 Therefore, the purpose of the present study was to use OCT to measure the PROS layer thickness and total length of the photoreceptors in 4 groups of eyes: healthy group, diabetics without retinopathy (DM), diabetics with diabetic retinopathy but no diabetic macular edema (DR), and diabetics with diabetic macular edema (DME).
Methods
Participants
In this cross-sectional study, demographic and clinical data were collected prospectively from healthy group (30 volunteers, 60 eyes) and from patients with DM (48 patients, 96 eyes), with retinal findings in the latter group ranging from normal to diabetic retinopathy plus macular edema. For the purpose of this study, the diagnosis of diabetes mellitus was based on the medical records available at the time of each patient's referral for ophthalmologic evaluation. Diabetic retinopathy was diagnosed according to ETDRS criteria and patients with a CRT of >300 μm were considered to have DME.20
Patients were not included in the study if they had macular ischemia on fluorescein angiography, or signs of other retinal diseases such as age related macular degeneration, and retinal vein occlusion. Data collected from each participant included visual acuities measured via the Early Treatment Diabetic Retinopathy Study (ETDRS) chart at 4 meters, and retinal layer thicknesses measured via OCT. Details of OCT procedures and retinal layer thickness measurements are described below.
OCT procedure
Pupils were not dilated before retinas were imaged. Each participant underwent a single imaging session during which two successive images were obtained from the left eye and two successive images from the right eye, with the use of a spectral domain OCT device (Spectralis, Heidelberg Engineering, Germany). The acquisition of two images from each eye provided two sets of data by which groups of eyes could be compared, and also provided data for repeatability analysis. Throughout the two imaging procedures for a given eye, the participant’s chin remained in place on the frame of the device, and the participant was instructed to move as little as possible. According to the manufacturer, the device has a transverse resolution of 14 μm and an axial resolution of 3.9 μm. Another feature of the device is an automatic real-time eye tracking system. During our participants' imaging sessions, this system was set at its maximum value of 100 to provide for the highest image quality attainable with the device.
The images were horizontal cross sections through the foveolar depression, with the width of the image corresponding to a length of 6 mm along the surface of the retina. Central retinal thickness, although not investigated specifically in this study, was determined from the images with the semi-automated procedure routinely used with this device, in which the software detects the inner and outer boundaries of the retina and then the measurement line intersecting the retina perpendicularly can be moved sideways if necessary so as to pass through the lowest point of the fovea.
Photoreceptor outer segment layer measurements
The manual measurement of the PROS layer was performed as previously described.18 The OCT images were digitally enlarged to 4 times their original size and the OCT device’s calipers application was used to mark the boundaries of the layer and measure the distance between them. We defined this layer by marking its boundaries at the inner border of the inner segment – outer segment junction and at the inner border of the retinal pigment epithelium layer. We also defined a thicker layer that was assumed to include the total length of the photoreceptors and we measured it similarly, with boundaries at the outer border of the external limiting membrane layer and at the inner border of the retinal pigment epithelium layer.
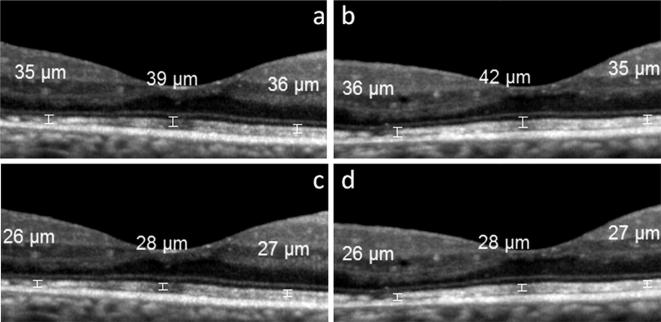
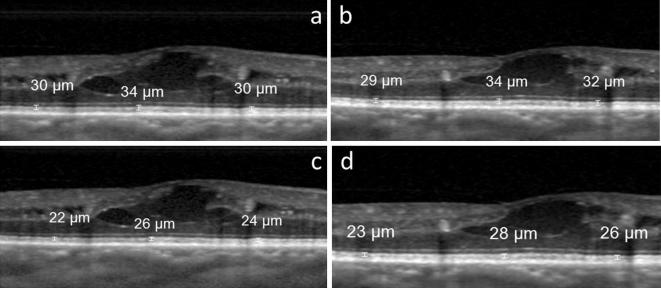
For each eye in the study, in each of the 2 OCT images, measurements with the manual calipers were made at 3 different locations: at the lowest point of the fovea, at 750 μm nasal to this point, and at 750 μm temporal to this point (Fig. 1). The fovea is known to have 1500 μm diameter; therefore, the locations that were 750 μm away from the fovea were chosen for making the measurements from the edges of the fovea. All of the measurements were made by the same physician (YK) who was blinded to the clinical data of the patients (see Fig. 2).
Fig. 1.
OCT images from the right eye of a 55-year-old male with diabetic retinopathy and without macular edema. Images a and c are copies of the same image that was the first of the two taken in this eye. The copies were marked separately with the calipers to measure the thickness, respectively, of the total photoreceptor layer (image a) and the photoreceptor outer segment layer (image c). The second OCT image taken in this eye is shown in b and d, which are likewise copies that were separately marked for measurement of the layers. The central measurements were taken from the foveal center; the other two measurements are taken from 750 μm away from the central fovea.
Fig. 2.
OCT images from the right eye of a 58-year-old male with diabetic retinopathy and diabetic macular edema. Images a and c are copies of the same image that was the first of the two taken in this eye. The copies were marked separately with the calipers to measure the thickness, respectively, of the total photoreceptor layer (image a) and the photoreceptor outer segment layer (image c). The second OCT image taken in this eye is shown in b and d, which are likewise copies that were separately marked for measurement of the layers. The central measurements were taken from the foveal center; the other two measurements are taken from 750 μm away from the central fovea.
Data analysis
For statistical analyses, eyes were assigned to one of 4 groups: healthy group, DM, DR, and DME. First one-way ANOVA test was used to evaluate intergroup difference among the four groups; then, if there was a significant difference, between-groups differences were then evaluated pairwise with the use of Student's unpaired t test. In each set of pairwise tests, in which each group was compared in turn with each other group, the resulting six p values were then corrected with the Bonferroni method, i.e. by multiplying each p value by the total number of times the test was performed21 - in this case six - so as to take into account the effect of performing multiple tests. Pearson’s test was used for correlation analysis. A p value of less than 0.05 was considered to be statistically significant. Statistical calculations were performed with the use of SPSS version (Version 15.0, SPSS Inc., Chicago, IL, USA) and Microsoft Excel.
From each eye in the study, 2 OCT images were obtained for the purpose of repeatability analysis. However, this method of data collection also made it possible to perform the pairwise testing procedure by using the first-obtained images alone, and then again by using the values from the second-obtained images.
Repeatability was evaluated with the Bland-Altman repeatability coefficient, which was calculated as follows. For each eye in the study, the thickness of the PROS layer and total photoreceptor length was measured on the first image of that eye and again on the second image of that eye. Then repeatability analysis was done as defined by Bland and Altman.22, 23 The difference between the two measurements in a given eye was calculated, and then within each of the four groups in the study the standard deviation of these differences was recorded. The standard deviation was then multiplied by 1.96–give the value of the difference that would be expected to be exceeded in only 5% of cases.
Results
Groups defined by retinal findings
A total of 156 eyes of 78 patients and healthy volunteers were enrolled. Of the 60 healthy volunteer eyes, none had signs of retinopathy in either eye. Of the eyes with diabetes, the counts of eyes by category were as follows: 36 eyes with DM, 27 eyes with DR, and 33 eyes with DME. Since retinal involvement was not the same in both eyes in some patients, statistical analyses were performed on groups of eyes rather than on groups of patients.
Table 1 summarizes the general characteristics of the study groups.
Table 1.
General characteristics of the patients in healthy volunteers and in patients with diabetes, diabetic retinopathy, or diabetic retinopathy with macular edema.
| Age | Gender F/M | Mean visual acuity | |
|---|---|---|---|
| Healthy volunteers (60 eyes) | 42.4 ± 12.9 | 30/30 | 1.00 Decimals |
| DM without DR (35 eyes) | 54.4 ± 9.07 | 13/22 | 1.00 Decimals |
| DR (30 eyes) | 57.2 ± 6.81 | 11/19 | 0.88 ± 0.25 Decimals |
| DME (34 eyes) | 56.1 ± 9.84 | 9/24 | 0.56 ± 0.26 Decimals |
Abbreviations: OCT; optical coherence tomography; DM, diabetes mellitus; DR, diabetic retinopathy; DME, diabetic macular edema; F, female; M, male.
Table 2 summarizes the PROS findings for the 4 groups of eyes: healthy, DM, DR, and DME. Table 3 summarizes the results for the total length of the photoreceptors for the 4 groups of eyes.
Table 2.
Photoreceptor outer segment layer thickness at different locations in healthy volunteers and in patients with diabetes, diabetic retinopathy, or diabetic retinopathy with macular edema.
| Groups | Mean subfoveal PROS thickness (μm) (range) | Mean nasal PROS thickness (μm) (range) | Mean temporal PROS thickness (μm) (range) |
|---|---|---|---|
| Healthy(60 eyes) | |||
| 1st OCT image | 38.5 ± 2.5 (29–43) | 27.3 ± 2.0 (24–34) | 27.4 ± 1.9 (23–32) |
| 2nd OCT image | 38.6 ± 5.5 (30–43) | 27.0 ± 1.7 (24–32) | 27.1 ± 2.2 (21–34) |
| DM without DR (36 eyes) | |||
| 1st OCT image | 38.2 ± 3.1 (28–43) | 26.9 ± 1.9 (23–31) | 27.1 ± 2.2 (23–25) |
| 2nd OCT image | 38.2 ± 4.6 (17–42) | 26.9 ± 2.5 (23–32) | 27.2 ± 2.2 (23–33) |
| DR (27 eyes) | |||
| 1st OCT image | 35.6 ± 4.4 (22–41) | 26.8 ± 3.4 (16–31) | 27.0 ± 2.5 (22–32) |
| 2nd OCT image | 36.1 ± 4.4 (28–47) | 26.3 ± 3.5 (12–31) | 26.8 ± 2.5 (22–31) |
| DR with DME (33 eyes) | |||
| 1st OCT image | 32.6 ± 6.9 (19–50) | 27.2 ± 4.2 (18–40) | 26.7 ± 6.0 (15–39) |
| 2nd OCT image | 32.6 ± 5.7 (17–44) | 26.7 ± 3.2 (22–35) | 28.0 ± 4.7 (19–37) |
| p value for ANOVA test | |||
| 1st OCT image | < 0.001* | 0.3 | 0.7 |
| 2nd OCT image | < 0.001* | 0.7 | 0.5 |
Abbreviations: OCT; optical coherence tomography; DM, diabetes mellitus; DR, diabetic retinopathy; DME, diabetic macular edema; PROS: photoreceptor retinal photoreceptor outer segment; μm, micrometer.
Bold values indicate statistical significance.
Table 3.
The total length of the photoreceptors at different locations in healthy volunteers and in patients with diabetes, diabetic retinopathy, or diabetic retinopathy with macular edema.
| Groups | Mean subfoveal TLP (μm) (range) | Mean nasal TLP (μm) (range) | Mean temporal TLP (μm) (range) |
|---|---|---|---|
| Healthy(60 eyes) | |||
| 1st OCT image | 50.7 ± 2.5 (43–57) | 36.1 ± 3.0 (27–43) | 37.0 ± 2.1 (32–43) |
| 2nd OCT image | 50.6 ± 2.7 (44–56) | 36.7 ± 2.2 (31–42) | 36.2 ± 2.4 (30–41) |
| DM without DR (36 eyes) | |||
| 1st OCT image | 50.2 ± 3.0 (40–55) | 36.2 ± 2.5 (29–41) | 36.7 ± 2.7 (32–43) |
| 2nd OCT image | 49.5 ± 4.8 (26–53) | 36.5 ± 3.0 (30–42) | 37.1 ± 2.9 (33–45) |
| DR (27 eyes) | |||
| 1st OCT image | 48.2 ± 5.7 (32–56) | 36.5 ± 3.8 (26–43) | 36.6 ± 3.0 (30–45) |
| 2nd OCT image | 48.6 ± 6.2 (34–58) | 35.8 ± 3.8 (22–41) | 36.8 ± 2.7 (33–42) |
| DR with DME (33 eyes) | |||
| 1st OCT image | 44.1 ± 7.4 (19–50) | 36.7 ± 5.4 (26–52) | 36.5 ± 6.9 (23–52) |
| 2nd OCT image | 43.0 ± 7.4 (26–54) | 36.6 ± 4.1 (28–47) | 37.9 ± 5.1 (27–48) |
| p value for ANOVA test | |||
| 1st OCT image | < 0.001* | 0.9 | 0.9 |
| 2nd OCT image | < 0.001* | 0.6 | 0.1 |
Abbreviations: OCT; optical coherence tomography; DM, diabetes mellitus; DR, diabetic retinopathy; DME, diabetic macular edema; TLP: the total length of the photoreceptors; μm, micrometer.
Bold values indicate statistical significance.
Between-groups differences in PROS thickness and total photoreceptor length
The mean PROS thickness and total photoreceptor length values in the four groups are summarized in Table 2, Table 3. The mean PROS thickness at the foveal center in the first and second-obtained OCT images was as follows: healthy, 38.5 ± 2.5 μm and 38.6 ± 5.5 μm; DM, 38.2 ± 3.1 μm and 38.2 ± 4.6 μm; DR, 35.6 ± 4.4 μm and 36.1 ± 4.4 μm; DME, 32.6 ± 6.9 μm and 32.6 ± 5.7 μm. Between the groups of eyes, significant differences in PROS length were found only with respect to the measurements made at the foveal center (p < 0.001 for the first measurement and p < 0.001 for the second measurement), and not those made at the nasal (p = 0.3 for the first measurement and p = 0.7 for the second measurement) or temporal locations (p = 0.7 for the first measurement and p = 0.5 for the second measurement) among the four groups via one way ANOVA test (Table 2). Therefore Student’s t test was used for each group for only PROS length at the foveal center. There was a statistically significant difference in PROS thickness at the foveal center between the healthy volunteers and DR and between healthy volunteers and DME group (p < 0.05 for all, Table 4). Also a statistical significant difference was demonstrated between the DM group and DME group (p < 0.05 for all, Table 4) and between the DR and DME group (p < 0.05 for all, Table 4). However, there was not a statistically difference between the DM and DR group (p > 0.05 for all, Table 4).
Table 4.
Between-groups differences in subfoveal photoreceptor outer segment thickness and total photoreceptor length.
| Groups | p values for PROS | p values for TLP | |
|---|---|---|---|
| t test Bonferroni-corrected | |||
| Healthy vs DM | 1st OCT image | 0.9 | 0.9 |
| 2nd OCT image | 0.9 | 0.8 | |
| Healthy vs DR | 1st OCT image | <0.001* | 0.03* |
| 2nd OCT image | 0.004* | 0.04* | |
| Healthy vs DME | 1st OCT image | <0.001* | <0.001* |
| 2nd OCT image | <0.001* | <0.001* | |
| DM vs DR | 1st OCT image | 0.06 | 0.3 |
| 2nd OCT image | 0.3 | 0.9 | |
| DM vs DME | 1st OCT image | <0.001* | <0.001* |
| 2nd OCT image | <0.001* | <0.001* | |
| DR vs DME | 1st OCT image | 0.04* | 0.04* |
| 2nd OCT image | 0.01* | 0.01* | |
Abbreviations: vs, versus; DM, diabetes mellitus; DR, diabetic retinopathy; DME, diabetic macular edema.
Bold values indicate statistical significance.
The mean total photoreceptor length at the foveal center in the first and second-obtained OCT images was as follows: healthy, 50.7 ± 2.5 μm and 50.6 ± 2.7 μm; DM, 50.2 ± 3.0 μm and 49.5 ± 4.8 μm; DR, 48.2 ± 5.7 μm and 48.6 ± 6.2 μm; DME, 44.1 ± 7.2 μm and 43.0 ± 7.4 μm. Between the groups of eyes, significant differences in the total photoreceptor length were found only with respect to both of the measurements made at the foveal center (p < 0.001 for the first measurement and p < 0.001 for the second measurement), and not those made at the nasal (p = 0.9 for the first measurement and p = 0.6 for the second measurement) or temporal locations (p = 0.9 for the first measurement and p = 0.1 for the second measurement) among the four groups via one way ANOVA test (Table 3). Therefore Student’s t test was used for each group for only the total photoreceptor length at the foveal center. There was a statistically significant difference in the total photoreceptor length at the foveal center between the healthy volunteers and DR and between healthy volunteers and DME group (p < 0.05 for all, Table 4). Also a statistical significant difference was demonstrated between the DM group and DME group (p < 0.05 for all, Table 4) and between the DR and DME group (p < 0.05 for all, Table 4). However, there was not a statistically difference between the DM and DR group (p > 0.05 for all, Table 4).
Correlation analysis
The mean visual acuity of the patients is summarized in Table 1. The mean visual acuity was positively correlated with both PROS thickness and total photoreceptor length at the foveal center in the whole group (r = 0.51, p < 0.0001; and r = 0.48, p < 0.0001, respectively). There was not any correlation between the visual acuity and the PROS thickness and total photoreceptor length in the nasal or temporal locations (p > 0.05 for all). On the other hand, there was a negative correlation between the visual acuity and central retinal thickness in the whole group (r = −0.56, p < 0.0001).
Intra-session, intra-observer repeatability
In the healthy eyes group and diabetes groups, measurement of photoreceptor outer segment layer thickness was possible on all first-obtained and second-obtained OCT images. Repeatability findings were acceptable for all of the measurements. As an example, repeatability results of the PROS thickness at the foveal center are shown in Table 5. Compared to the healthy eyes, the eyes in the diabetes groups had higher coefficients of repeatability. This means that for a given eye measured twice by the same observer, measurements of the PROS thickness were less reproducible in the groups with diabetes. The DME group had the highest coefficient, indicating the lowest degree of repeatability among the groups.
Table 5.
Repeatability of measurements of the subfoveal photoreceptor outer segment layer made manually on images from spectral domain OCT in healthy volunteers and in patients with diabetes, diabetic retinopathy, or diabetic retinopathy with macular edema.
| Group | Number of eyes with measurable 1st and 2nd images | SD of differences between 1st and 2nd images (μm) | Repeatability coefficient (μm) |
|---|---|---|---|
| Healthy | 60 | 0.8 | 1.6 |
| Diabetes without retinopathy | 36 | 2.0 | 3.9 |
| Diabetic retinopathy | 27 | 1.6 | 3.1 |
| Diabetic macular edema | 33 | 2.9 | 5.7 |
Abbreviations: SD, standard deviation; μm, micrometer.
Discussion
The retinal abnormalities reported to occur in patients with diabetes mellitus have included vascular changes noticed in the historically early studies7 and a diversity of other phenomena described more recently, including changes in visual fields,1 dark adaptation,2 contrast sensitivity,3 color vision,4, 5 photopigment bleaching,6 and photoreceptor subsets.24 Technological improvements in OCT have revealed abnormalities in the retina’s layered structure, particularly in boundary lines15 and in the thickness of layers involving the photoreceptors.8, 9, 10
We evaluated the PROS layer- and total photoreceptor thickness in four different groups which were healthy volunteers, DM group, DR group, and DME group. We showed a statistically difference in both PROS thickness and total photoreceptor length between healthy volunteers and DR- and DME patients, respectively. Also both PROS thickness and total photoreceptor length were found to be decreased in DME group as compared to the three other groups. Correlation analysis of the whole group revealed a significant positive relationship of visual acuity with PROS thickness and total photoreceptor length, respectively. Verma et al.8 in a study of 39 healthy volunteers and 39 patients with diabetes mellitus unaccompanied by diabetic retinopathy, reported that the thickness of the retinal photoreceptor layer was significantly less in the patients with diabetes (68.79 ± 7.84 μm healthy vs. 61.62 ± 4.48 μm diabetes). Our measurements of the thickness of the PROS layer showed no statistical difference between healthy volunteers and patients with DM. However, the overall pattern across the participants in our study is consistent with the findings of Verma et al., in that the mean thickness of the PROS layer decreases across our groups from DM to DR to DME. This pattern suggests that at some point, in the presence of diabetes, the PROS layer becomes thinner as the disease progresses toward DME. This raises the question of disease progression in individual patients, which would require a study with a longitudinal design. Forooghian et al.9 described a series of 27 patients (30 eyes) with diabetic macular edema in which OCT images of retina were analyzed for thicknesses of the PROS layer. In each retina, PROS layer thickness was measured for three successively smaller zones centered on the fovea. Mean thicknesses of these zones ranged from 30 to 32 μm. The authors, using a linear regression method, found that greater PROS layer thickness was associated with better visual acuity, particularly at the center of the fovea. Our findings are complementary to those of Forooghian et al., in that our groups of eyes differed significantly from each other in both PROS layer thickness and total photoreceptor length only at the fovea. The measurements that were made from nasal and temporal locations did not show any significant differences.
Alasil et al.10 studied 67 patients (67 eyes) with diabetic macular edema and analyzed OCT features of retina in relation to visual acuity. From multivariate analyses, the authors concluded that PROS thickness seemed to be a predictor of visual acuity. Our findings corroborate those of Alasil et al., with mean visual acuity decreasing across the diabetes groups in the same order as disease severity. Also we showed a positive correlation between the visual acuity and both PROS thickness and total photoreceptor length which was also consistent with the findings of Alasil et al.
In this study we evaluated the PROS thickness and total photoreceptor length of four different groups. The three diabetic groups were mainly classified according to having DR and/or DME. The standard DR staging was not taken into account, and also the duration of the disease were not analyzed which might be further limitations. However, the study consisted of relatively good number of eyes in each group and the measurements were made from three different parts of the retina in order to evaluate the different parts of macula.
In conclusion, we found that in patients with diabetes mellitus who underwent OCT, the PROS layer and total photoreceptor length were thinner in patients who had diabetic retinopathy or diabetic macular edema, and this thinning was limited to the central part of the fovea. These findings suggest the possibility of a disease mechanism specific to this part of the retina. A direction for further research would be to explain how the central fovea is preferentially affected in the diabetic diseases of the retina. Factors to be considered include the central fovea’s location in the center of the capillary free zone, which would provide a smaller margin of safety when oxygen levels fall. Another possibility is that the central fovea’s high metabolic rate makes this region sensitive to changes in metabolism. As the thinning of PROS thickness and total photoreceptor length is most prominent in DME patients and there was a significant relationship between visual acuity and both PROS thickness and total photoreceptor length we can hypothesize that these measurements may be used as functional parameters in DME patients. However, this relationship needs to be proven in longitudinal studies.
Conflict of interest
The authors report no conflict of interests in this work.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
Contributor Information
Abdullah Ozkaya, Email: abdozkaya@gmail.com.
Zeynep Alkin, Email: zeynepalkin@gmail.com.
Yalcin Karakucuk, Email: drkarakucuk83@gmail.com.
Gonul Karatas, Email: gnlkaratas@gmail.com.
Korhan Fazil, Email: korhanfazil@hotmail.com.
M. Gurkan Erdogan, Email: erdogangurkan@yahoo.com.
Irfan Perente, Email: perente@gmail.com.
Muhittin Taskapili, Email: mutaskapili@gmail.com.
References
- 1.Roth J.A. Central visual field in diabetes. Br J Ophthalmol. 1969;53:16–25. doi: 10.1136/bjo.53.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henson D.B., North R.V. Dark adaptation in diabetes mellitus. Br J Ophthalmol. 1979;63:539–541. doi: 10.1136/bjo.63.8.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghafour I.M., Foulds W.S., Allan D., McClure E. Contrast sensitivity in diabetic subjects with and without retinopathy. Br J Ophthalmol. 1982;66:492–495. doi: 10.1136/bjo.66.8.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy M.S., McCulloch C., Hanna A.K., Mortimer C. Colour vision in long-standing diabetes mellitus. Br J Ophthalmol. 1984;68:215–217. doi: 10.1136/bjo.68.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green F.D., Ghafour I.M., Allan D., Barrie T., McClure E., Foulds W.S. Colour vision of diabetics. Br J Ophthalmol. 1985;69:533–536. doi: 10.1136/bjo.69.7.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elsner A.E., Burns S.A., Lobes L.A., Jr., Doft B.H. Cone photopigment bleaching abnormalities in diabetes. Invest Ophthalmol Vis Sci. 1987;28:718–724. [PubMed] [Google Scholar]
- 7.Ashton N. Vascular changes in diabetes with particular reference to the retinal vessels: preliminary report. Br J Ophthalmol. 1949;33:407–420. doi: 10.1136/bjo.33.7.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma A., Rani P.K., Raman R., Pal S.S., Laxmi G., Gupta M. Is neuronal dysfunction an early sign of diabetic retinopathy? Microperimetry and spectral domain optical coherence tomography (SD-OCT) study in individuals with diabetes, but no diabetic retinopathy. Eye (Lond) 2009;23:1824–1830. doi: 10.1038/eye.2009.184. [DOI] [PubMed] [Google Scholar]
- 9.Forooghian F., Stetson P.F., Meyer S.A., Chew E.Y., Wong W.T., Cukras C. Relationship between photoreceptor outer segment length and visual acuity in diabetic macular edema. Retina. 2010;30:63–70. doi: 10.1097/IAE.0b013e3181bd2c5a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alasil T., Keane P.A., Updike J.F., Dustin L., Ouyang Y., Walsh A.C. Relationship between optical coherence tomography retinal parameters and visual acuity in diabetic macular edema. Ophthalmology. 2010;117:2379–2386. doi: 10.1016/j.ophtha.2010.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lardenoye C.W., Probst K., DeLint P.J., Rothova A. Photoreceptor function in eyes with macular edema. Invest Ophthalmol Vis Sci. 2000;41:4048–4053. [PubMed] [Google Scholar]
- 12.Zagers N.P.A., Pot M.C.A., van Norren D. Spectral and directional reflectance of the fovea in diabetes mellitus: photoreceptor integrity, macular pigment and lens. Vision Res. 2005;45:1745–1753. doi: 10.1016/j.visres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Maheshwary A.S., Oster S.F., Yuson R.M., Cheng L., Mojana F., Freeman W.R. The association between percent disruption of the photoreceptor inner segment-outer segment junction and visual acuity in diabetic macular edema. Am J Ophthalmol. 2010;150(63–67):e61. doi: 10.1016/j.ajo.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otani T., Yamaguchi Y., Kishi S. Correlation between visual acuity and foveal microstructural changes in diabetic macular edema. Retina. 2010;30:774–780. doi: 10.1097/IAE.0b013e3181c2e0d6. [DOI] [PubMed] [Google Scholar]
- 15.Murakami T., Yoshimura N. Structural changes in individual retinal layers in diabetic macular edema. J Diabetes Res. 2013;2013:11. doi: 10.1155/2013/920713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami T., Nishijima K., Sakamoto A., Ota M., Horii T., Yoshimura N. Association of pathomorphology, photoreceptor status, and retinal thickness with visual acuity in diabetic retinopathy. Am J Ophthalmol. 2011;151:310–317. doi: 10.1016/j.ajo.2010.08.022. [DOI] [PubMed] [Google Scholar]
- 17.Lombardo M., Parravano M., Lombardo G., Varano M., Boccassini B., Stirpe M. Adaptive optics imaging of parafoveal cones in type 1 diabetes. Retina. 2014;34:546–557. doi: 10.1097/IAE.0b013e3182a10850. [DOI] [PubMed] [Google Scholar]
- 18.Ozkaya A., Alkin Z., Karatas G., Karakucuk Y., Perente I., Taylan Yazici A. Photoreceptor outer segment layer thickness measured manually on images from spectral domain optical coherence tomography in healthy volunteers. J Fr Ophtalmol. 2014;37:475–479. doi: 10.1016/j.jfo.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Yuksel K., Karakucuk Y., Ozkaya A., Pekel G., Baz O., Alagoz C. Comparison of photoreceptor outer segment length in diabetic and idiopathic epiretinal membranes. Eye (Lond) 2015;29:1446–1452. doi: 10.1038/eye.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs–an extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 21.Bland J.M., Altman D.G. Multiple significance tests: the Bonferroni method. BMJ. 1995;310:170. doi: 10.1136/bmj.310.6973.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bland J.M., Altman D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 23.Bland J.M., Altman D.G. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol. 2003;22:85–93. doi: 10.1002/uog.122. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto S., Kamiyama M., Nitta K., Yamada T., Hayasaka S. Selective reduction of the S cone electroretinogram in diabetes. Br J Ophthalmol. 1996;80:973–975. doi: 10.1136/bjo.80.11.973. [DOI] [PMC free article] [PubMed] [Google Scholar]