Abstract
In order to evaluate the effect of topical and subconjunctival anti-vascular endothelial growth factor (anti-VEGF) therapy, Ranibizumab, Bevacizumab and Aflibercept as a therapy for corneal neovascularization (NV) treatment, the aim of this study was to review all data related to some of anti-VEGF as a promising therapies for corneal NV treatment.
Corneal NV is a dangerous condition leading to a marked reduction in vision due to angiogenesis of abnormal vessels that block light. During the recent years, we have recognized new drug proliferation for corneal NV treatment. Recently, anti-VEGF therapies are one of the most important drugs used for corneal NV treatment.
Several growth factors are involved in angiogenesis. The most important growth factor in corneal angiogenesis is VEGF. VEGF can be considered as key mediators in corneal angiogenesis. It is upregulated during corneal NV. In fact, anti-VEGF therapies have shown efficacy in attenuation of corneal NV in both animal models and clinical trials. A promising therapeutic success has been achieved using antibodies directed against VEGF. Bevacizumab has demonstrated efficacy and efficiency in the treatment of different neo-vascular ocular diseases and it has partially reduced corneal NV through different routes of administrations: topical, subconjunctival, and intraocular application. A similar efficacy to bevacizumab profiles in the treatment of neo-vascular age-related macular degeneration was induced by ranibizumab. Moreover, at worse levels of initial visual acuity of diabetic macular edema, aflibercept was more effective at improving vision.
Anti-VEGF agents (Bevacizumab, Ranibizumab and Aflibercept) seem to have a higher efficiency and efficacy for corneal NV treatment. Both subconjunctival therapy and topical therapy of bevacizumab prohibit corneal NV, while early treatment with subconjunctival administration of ranibizumab may successfully reduce corneal NV. Therefore, establishment of safe doses is highly important before these drugs can be involved in the clinical setting. Further investigations and studies are highly warranted to adjust the dose and route of administration for the antibodies directed against VEGF to be the key therapeutic agents in the corneal NV treatment.
Keywords: Anti-VEGF, Bevacizumab, Ranibizumab, Aflibercept, Cornea
Introduction
The cornea is considered transparent connective tissue as it is avascular and acts as a mechanical barrier of the eye especially the anterior refractive surface. There are several arteries supplying cornea. The main arteries are ciliary arteries, ophthalmic artery branches and pericorneal plexus end near the limbus. The clarity of the corneal is related to anatomical factors including a vascularity of the cornea, regular arrangement of the collagen fibers and physiological factors which may form a precise intricate balance between its different layers and cellular components.1 The major protective feature of the cornea is also immune-privileged, which is a well-organized structure of the eye contributes to corneal transplants high success rates.2
The exposure of the eyes to chemical injuries may induce extensive damage especially to the epithelium of the ocular surface, cornea and anterior segment, resulting in permanent visual impairment, unilateral or bilateral.3 The most serious injuries to corneal stroma induced by alkali burns as a chemical burns are persistent corneal epithelial defect, ulceration, NV, corneal scar as well as damage to the intraocular structures leading to the visual impairment. There are several cells involved in the pathogenesis of alkali burn-related complications in cornea especially mesenchymal cells as (myofibroblasts and activated keratocytes), macrophages and neo-vascularization mediators.4, 5, 6 A wide range of infectious, inflammatory mediators or traumatic disorders may cause corneal NV.7 Corneal NV can lead to lipid deposition, scar formation, immune rejection of corneal grafts, which leads to a significant visual impairment.8 Corneal NV always represents a state of disease which is generally associated with an inflammatory response.
A new blood vessel growth from pre-existing vascular structures is known as angiogenesis. Numerous pathological conditions are accompanied with corneal angiogenesis that brings about several unwanted consequences. In the cornea, angiogenesis, formation of new blood vessels originating from pericorneal plexus capillaries and venules, may induce light block, compromising visual acuity, leading to edema, corneal scarring and inflammation.7 The main cause of corneal NV may be due to disequilibrium between angiogenic and antiangiogenic stimuli. The overflow of pro-angiogenic factors (such as VEGF, different matrix metalloproteinases and basic fibroblast growth factor [b-FGF]) and the lower production in antiangiogenic factors such as angiostatin, endostatin and soluble VEGF Receptor-2 [sVEGFR-2 or sflt-1], may be behind the vascularity of a normally a vascular cornea.9
VEGF is a signal protein secreted by cells that enhance angiogenesis. The VEGF family composed of five members in mammals: VEGF-A, placenta growth factor (PGF), VEGF-B, VEGF-C and VEGF-D. VEGF-A as a growth factor interacts with its receptors; tyrosine kinase receptors family; VEGFR-1 and VEGF-2 while VEGF-C and VEGF-D bind to VEGFR-3. VEGFR-2 seems to be the key receptor that mediates almost all of well-known cellular responses to VEGF through normal consequence of growth factors-receptor interactions in two sequential steps: dimerization with subsequent activation of the kinase domains located intracellular followed by autophosphorylation of receptors, which initiates the proliferative signal and angiogenesis.10 Therefore, VEGFR-2 is considered the key receptor that initiates the proliferation of vascular endothelial cells induced VEGF.
Recently, the efficacy and safety of anti-VEGF therapies (aflibercept, bevacizumab, and ranibizumab) in the diabetic macular edema treatment have been investigated. Intravitreous administration of bevacizumab, ranibizumab, and aflibercept, can induce an improvement in the vision of eyes with center-involved diabetic macular edema. However, the relative therapeutic effect depends on visual acuity baseline. No significant differences, on average were detected among study groups when the initial visual-acuity loss was mild. However, the more effective anti-VEGF was aflibercept in improving vision when the initial visual acuity was at worse levels.
VEGF has been shown to be upregulated in corneal NV and has been implicated as a significant angiogenic factor in vascularized and inflamed corneas in human and animal models.11, 12, 13 The eye’s response to injury and inflammation is normally associated with formation of new blood vessels in the normally avascular cornea.14 As a consequence of the corneal NV, lipid deposition, inflammation, corneal scarring, and edema will be occurred, which worsen the prognosis of subsequent penetrating keratoplasty and significantly alter visual acuity. It is therefore important to inhibit corneal NV and to prevent the inflammatory response of the eye from injury and disease. Previously, the main therapy used for suppressing actively corneal blood vessel proliferation was topical corticosteroids. Rapamycin, cyclosporine A, non-steroidal anti-inflammatory agents, and methotrexate have been used in other studies.16, 17, 18 In addition, there were other several current treatments for corneal NV include fine needle diathermy, photodynamic therapy, amniotic membrane transplantation and laser photocoagulation.19, 20 Unfortunately, all these methods have a lower efficiency and a limited clinical efficacy; also, they induce multiple unwanted side effects, especially posterior sub-capsular cataracts and elevated intraocular pressure subsequent to corticosteroid use. However, all of these treatments are not specific inhibitor or antagonize the growth factors inducing angiogenesis.
Therefore, the requirement of anti-VEGF for treatment of corneal NV was highly warranted and urgent. The involvement of VEGF in the corneal NV was first studied in animal model.8 Anti-VEGF antibodies block VEGF up-regulated during corneal injury, and neovascularization. A later study showed that amelioration of the corneal angiogenesis secondary to herpes simplex virus has been achieved by blocking VEGF through treatment with mFlt immunoglobulin G protein directed against murine soluble VEGF receptor.21, 22, 23 This may confirm role of VEGF in corneal NV and led to the hypothesis confirming the role of anti-VEGF antibodies as effective treatment for corneal NV.24 Indeed, diabetic retinopathy, neo-vascular age-related macular degeneration, and neo-vascular glaucoma as other ocular diseases can be effectively treated by anti-VEGF drugs.25
Three drugs, bevacizumab, ranibizumab, and aflibercept represent the most promising of anti-VEGF therapy, ranibizumab and aflibercept were designed specifically for intravitreal injection, and they received Food and Drug Administration (FDA) approval for neovascular AMD treatment.
Bevacizumab, ranibizumab, and aflibercept differ in their molecular weight, structure and pharmacokinetics. Bevacizumab is a humanized monoclonal antibody (mAb) immunoglobulinG1 (IgG1) against VEGF that selectively antagonizes all VEGF-A isoforms. 93% of amino acid sequences of bevacizumab are similar to human IgG1 and 7% murine antibody.26 Bevacizumab is a 149 KDa full length, bivalent monoclonal antibody developed against VEGF-A, designed to prevent blood supply to solid tumors and therefore starve them by systemically inhibiting angiogenesis, while ranibizumab is a 48 KDa monovalent monoclonal antibody fragment, the antigen-binding Fab without the Fc domain.27, 28 This structure was developed and designed to inhibit FcRn binding and therefore, dramatically lowers its elimination half-life to around two h after absorption from eye tissues to blood circulation.29, 30
Ranibizumab, is 5–20-fold more potent than full-length bevacizumab based on molar basis, although it has only a single antigen binding site and therefore lacked the power for avidity effects. Ranibizumab has the power to antagonize the biologic activities of all human spliced VEGF-A isoforms and the proteolytic cleavage product VEGF110 similar to the parent antibody (Bevacizumab).31, 32, 33 Aflibercept, by contrast, is a 115 KDa Fc fusion protein connecting the binding domains of VEGF receptors 1 and 2 with an Fc fragment antibody, and was designed for intraocular injection and a systemic infusion.34
Early intervention using anti-VEGF therapy for treatment corneal neovascularization
Topical anti-VEGF: bevacizumab, ranibizumab and aflibercept
Anti-VEGF therapy used for corneal NV treatment has achieved successful results in many preliminary in vivo animal experiments. In rat models, topical bevacizumab (4 mg/ml) applied twice daily for 1 week attenuates chemically induced corneal NV and anti-VEGF antibody implanted in neo-vascularized corneal stroma reduces corneal NV.22, 35 However, such findings indicate only the potential usefulness of topical anti-VEGF therapy for controlling NV. After chemical injury, topically administered bevacizumab was found to inhibit NV and therefore, reduces corneal NV in an experimental rat model.35 Other studies confirmed previous results and reported that topical bevacizumab administration partially decreases corneal NV in experimental animal models.36, 37, 35
The first report studied the effect of topical bevacizumab therapy for human corneal NV treatment showed a significant reduction in superficial and deep stromal NV in two patients used topical bevacizumab 1% four times a day.38 Using topical bevacizumab on several patients (30 eyes of 27 patients) who did not response to the traditional anti-inflammatory drugs demonstrated that the mean vascularized area and vessel diameter were significantly reduced 61% and 24% respectively, P > 0.05.39 They also demonstrated that maximal effects were noticed in early administration of topical bevacizumab in the corneal NV course, which is parallel with animal results studies.40, 41 The efficiency of topical bevacizumab in the treatment of corneal NV within the first month of treatment has reported and has been confirmed by other human studies 42, 43, 44 (Fig. 1). In a short-term follow-up study, it has been demonstrated that topical bevacizumab can attenuate and mitigate corneal NV in patients with significant corneal NV.38 Topically delivered small doses of bevacizumab would not produce serious systemic effects.45 In addition, systemic administration of bevacizumab has a low incidence of side effects such as thrombosis and hypertension.46
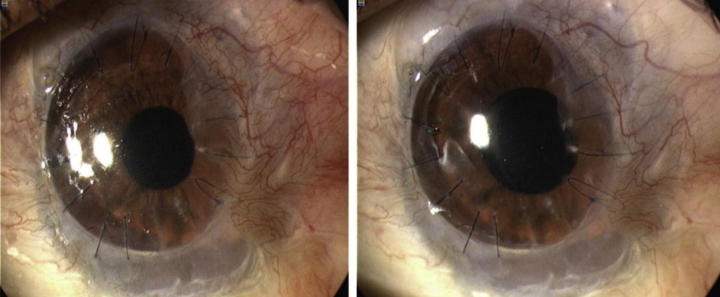
Fig. 1.
Treatment of corneal NV by topical bevacizumab. The baseline photograph shows active NV reaching donor graft (left). Treatment with topical bevacizumab for three months, NV decreased and is held on corneal graft border (right).48
The role of ranibizumab in the treatment of corneal NV has been investigated. Several studies indicated that topical ranibizumab can be used also for corneal NV. The efficacy and safety of ranibizumab used topically in the corneal NV treatment as novel evidence have been declared.47 Topical administration of ranibizumab 1% is a powerful and effective in down-regulation corneal NV parameters. However, due to its cost that should be taken in our consideration and should be balanced against its therapeutic efficacy, it is important to realize that the cost difference between bevacizumab and ranibizumab is significant. Thus, topical ranibizumab used for corneal NV treatment may be limited due to the current drug pricing.
Based on the dose and duration of treatment of topical bevacizumab, it seems to be accompanied with increased risk of corneal epithelial defects. Topically delivered bevacizumab 1.25% for several months can induce in the second month of treatment epitheliopathy in six eyes of the 10 eyes of 7 patients, suggesting that there were a disturbance in adhesion between the basement membrane and epithelium or in the process of wound healing.44 However, no side effects were detected using a slightly lower dose (1.0%) for three weeks.43 Furthermore, another study demonstrated a low incidence of new corneal epithelial defects during topical administration of an even lower dose (0.5%) of bevacizumab in their patients.39
Bevacizumab used topically induced not only vascular suppression but also caused loss of epithelial integrity spontaneously and stromal thinning progression.44 In this study they investigated the patients before treatment and all patients were with a stable state of corneal NV and epithelium. Bevacizumab delivered topically can induce corneal epithelial defects which can suggest that anti-VEGF drugs appear to be effective to reduce formation of new vessel and vascular leakage, leading to improvement in the visual function. However, blocking VEGF function may also disrupt wound healing, causing ischemia and increasing overall tissue damage. The effect of topical bevacizumab treatment on corneal NV of various etiologies was investigated and concluded that during the first month, topical application of bevacizumab was effective in reducing corneal NV.44. However, by the second month there was an increased risk of adverse effects.44
Subconjunctival anti-VEGF: bevacizumab, ranibizumab and aflibercept
Promising results have been achieved up on using sub-conjunctival bevacizumab therapy for treatment of NV of the cornea. Several studies showed that subconjunctival treatment with bevacizumab is able to prohibit corneal NV.49 In addition, subconjunctival bevacizumab administration in rabbits, mice and rats can induce consistent profound amelioration in corneal NV and it can attenuate VEGF levels in ocular tissue. Monthly treatment of lipid keratopathy secondary to corneal NV of 18 patients using sub-conjunctival injections of bevacizumab was investigated.50 The percentage of corneal NV extent was measured. A profound improvement has been reported in all corneal NV parameters and lipid deposition associated with sub-conjunctival injections of bevacizumab (p < 0.05). Another study using sub-conjunctival of 2.5 mg bevacizumab up to 3 months for treatment of major and minor corneal vessel NV of 10 patients declared that there was a marked decrease in the total area and extent of NV.51 It was determined that there was a profound reduction in corneal NV in the sub-conjunctival group.52 Further human studies continue to confirm the role of bevacizumab in the treatment of corneal NV. Regular subconjunctival administration of bevacizumab decreases corneal NV and also the elevation of therapeutic efficacy of bevacizumab was associated with higher doses53, 54, 55 (Fig. 2).
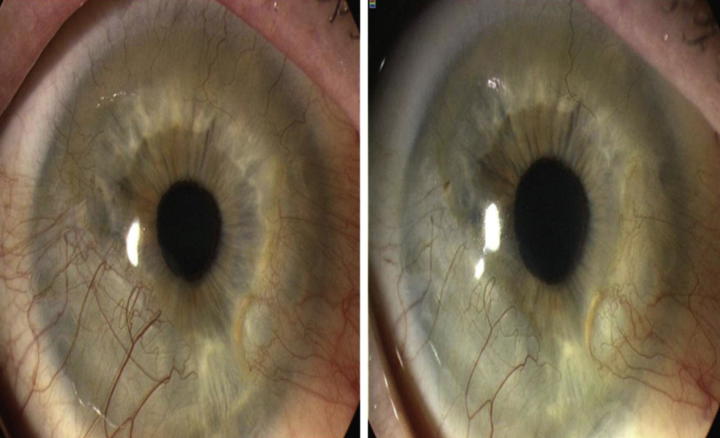
Fig. 2.
Corneal NV treated with subconjunctival injection of bevacizumab. Six months after subconjunctival bevacizumab treatment, NV decreased significantly (right).48
Regarding the effect of bevacizumab on corneal graft models, it has been shown that corneal graft survival rate can be increased when bevacizumab used either topically or sub-conjunctivally.56 However, both models of experiments showed that the two models are succeeded in decreasing corneal NV, but the efficacy was different, as a lower dose of subconjunctival injection is similar to the higher dose of topical administration.57 Recently, efficacy of both subconjunctival administration and topical applications of bevacizumab, was compared and concluded that both effectively inhibit corneal NV and reduce inflammation.58 These results may indicate that different types of treatment prohibit corneal NV development. However, after careful examination of all reported data they found that, the therapeutic efficacy of subconjunctival administration seems to be more efficient than topical, although the difference was not statistically significant. The difference in the effect between both methods may be due to the penetration of topical bevacizumab through the corneal epithelium may be limited and/or its rapid clearance by tears.59, 60 However, it remains unclear why subconjunctival injection is more safe and effective than topical administration. Therefore, more studies that investigate and compare the efficacy and safety of both methods are required before any conclusions can be drawn.
In a comparative study using several anti-VEGF, bevacizumab, ranibizumab, pegaptanib and human epidermal growth factor receptor 2 antibody trastuzumab delivered subconjunctivally, the inhibitory effect on experimental corneal NV was investigated in a rat model. Bevacizumab was reported to be the most effective agent.61 In addition, many studies demonstrated the clinical benefit of bevacizumab for treatment of corneal NV. Treatment of NV of the cornea in two cases with filamentary keratitis and corneal graft failure by subconjunctival application of bevacizumab has been reported.62 Subconjunctival injection of bevacizumab is an easy and simple application for inhibiting corneal NV. However, uncertainty regarding systemic absorption and the duration of treatment should be considered.
It has been demonstrated that bevacizumab and etanercept as a combination therapy is more efficient in the treatment of corneal NV.63 However, there is still no clear consensus about an ideal antiangiogenic treatment against actively growing and established corneal NV. A combination therapy of VEGF inhibitors and anti-TNF mAbs is expected to have better therapeutic outcome. Several studies on combination therapy in the corneal NV treatment were published in recent years, especially bevacizumab with steroids or verteporfin.64, 65 The effects of a combination therapy of different doses of bevacizumab with both saline and dexamethasone were investigated on inflammatory angiogenesis induced by small chemical lesions in rat cornea. The therapeutic outcome of both bevacizumab and dexamethasone as a combination therapy may be effective than monotherapy approaches.66 VEGF and may other cytokines contributing to the pathology of corneal NV may be inhibited only by bevacizumab. Therefore, using a combination therapy, both the antiangiogenic and anti-inflammatory agents may be better than monotherapy approaches. The maximal inhibition of corneal NV was achieved by the combination of bevacizumab and etanercept, as expected.
The mechanism of action of early and delayed subconjunctival administration of bevacizumab was evaluated recently. Early treatment with bevacizumab injection reduced corneal NV significantly and a better therapeutic effect was the outcome of early treatment than delayed treatment.67 The apoptosis of vascular endothelium cells is not involved in early treatment of corneal NV, although bevacizumab was detected in the corneal stroma tissues, one week after subconjunctival injection, it can inhibit macrophage infiltrate. The expression of VEGF, VEGFR1, and VEGFR2 on corneal vessels and macrophage infiltration was not changed by delayed administration of bevacizumab.67
Age-related macular degeneration neovascular diseases have been treated with both ranibizumab and bevacizumab. A similar efficacy profiles have been reported using both drugs. An early subconjunctival administration of ranibizumab appears to be successfully inhibiting alkali-induced corneal NV in an animal model.68 VEGF levels reduced significantly not only in the cornea and the bulbar conjunctiva but also in the aqueous humor and the iris by subconjunctival ranibizumab administration, suggesting a possible treatment for secondary neovascularization of the anterior segment of the eye.
Aflibercept is the recent addition to the therapeutic arsenal against corneal NV. It antagonizes VEGF similar to bevacizumab and ranibizumab, but it has additional advantage as it also interacts with platelet-derived growth factor (PDGF).69 The structure of Aflibercept is dissimilar to the other drugs, and it has been suggested that aflibercept can be used for treatment of corneal NV to patients previously treated with either ranibizumab or bevacizumab. Comparing the binding affinity for Aflibercept, ranibizumab and bevacizumab for VEGF, a greater binding affinity for VEGF between 10 and 12 weeks after injection has been reported to aflibercept than either bevacizumab or ranibizumab.70 Patients with age-related macular degeneration with increasingly unsatisfactory response to long-term ranibizumab treatment, conversion to aflibercept can improve visual acuity significantly and reduce retinal thickness. Therefore, the best choice of action when bevacizumab injections cannot be tolerated is to use aflibercept as a substitution medication.
Conclusion
Enormous hope for the treatment of corneal NV has been generated using anti-VEGF agents (Bevacizumab, Ranibizumab and Aflibercept). Both subconjunctival therapy and topical therapy of bevacizumab ranibizumab and aflibercept inhibit cornea NV. Therefore, safe doses and duration of action establishment are highly warranted before introducing these drugs in the clinical setting. Further studies are required to adjust the dose and route of administration for anti-VEGF drugs to be the key therapeutic agents in the treatment of corneal NV.
Conflict of interest
There are no financial or other interests with regard to this manuscript that might be construed as a conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.DelMonte D.W., Kim T. Anatomy and physiology of the cornea. J Cataract Refract Surg. 2011;37:588–598. doi: 10.1016/j.jcrs.2010.12.037. [DOI] [PubMed] [Google Scholar]
- 2.Streilein J.W. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol. 2003;3:879–889. doi: 10.1038/nri1224. [DOI] [PubMed] [Google Scholar]
- 3.Wagoner M.D. Chemical injuries of the eye: current concepts in pathophysiology and therapy. Surv Ophthalmol. 1997;41:275–313. doi: 10.1016/s0039-6257(96)00007-0. [DOI] [PubMed] [Google Scholar]
- 4.Brodovsky S.C., McCarty C.A., Snibson G. Management of alkali burns: an 11 year retrospective review. Ophthalmology. 2000;107:1829–1835. doi: 10.1016/s0161-6420(00)00289-x. [DOI] [PubMed] [Google Scholar]
- 5.Saika S., Kobata S., Hashizume N. Epithelial basement membrane in alkali-burned corneas in rats. Immunohistochemical study. Cornea. 1993;12:383–390. doi: 10.1097/00003226-199309000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Ishizaki M., Zhu G., Haseba T. Expression of collagen I, smooth musclea-actin, and vimentin during the healing of alkali-burned and lacerated corneas. Invest Ophthalmol Vis Sci. 1993;34:3320–3328. [PubMed] [Google Scholar]
- 7.Chang J.H., Gabison E.E., Kato T. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Epstein R.J., Stulting R.D., Hendricks R.L. Corneal neovascularization: pathogenesis and inhibition. Cornea. 1987;6:250–257. doi: 10.1097/00003226-198706040-00004. [DOI] [PubMed] [Google Scholar]
- 9.Ambati B.K., Nozaki M., Singh N. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klettner A., Roider J. Treating age-related macular degeneration-interaction of VEGF-antagonists with their target. Mini Rev Med Chem. 2009;9:1127–1135. doi: 10.2174/138955709788922665. [DOI] [PubMed] [Google Scholar]
- 11.Philipp W., Speicher L., Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41:2514–2522. [PubMed] [Google Scholar]
- 12.Phillips G.D., Stone A.M., Jones B.D. Vascular endothelial growth factor (rhVEGF165) stimulates direct angiogenesis in the rabbit cornea. In Vivo. 1994;8:961–965. [PubMed] [Google Scholar]
- 13.Cursiefen C., Rummelt C., Küchle M. Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor alpha, and transforming growth factor beta1in human corneas with neovascularization. Cornea. 2000;19:526–533. doi: 10.1097/00003226-200007000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Smith J.R., Levinson R.D., Holland G.N. Differential efficacy of tumor necrosis factor inhibition in the management of inflammatory eye disease and associated rheumatic disease. Arthritis Rheum. 2001;45:252–257. doi: 10.1002/1529-0131(200106)45:3<252::AID-ART257>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Mahoney J.M., Waterbury L.D. Drug effects on the neovascularization response to silver nitrate cauterization of the rat cornea. Curr Eye Res. 1985;4:531–535. doi: 10.3109/02713688508999984. [DOI] [PubMed] [Google Scholar]
- 17.Kwon Y.S., Kim J.C. Inhibition of corneal neovascularization by rapamycin. Exp Mol Med. 2006;38:173–179. doi: 10.1038/emm.2006.21. [DOI] [PubMed] [Google Scholar]
- 18.Lipman R.M., Epstein R.J., Hendricks R.L. Suppression of corneal neovascularization with cyclosporine. Arch Ophthalmol. 1992;110:405–407. doi: 10.1001/archopht.1992.01080150103037. [DOI] [PubMed] [Google Scholar]
- 19.Lee P., Wang C.C., Adamis A.P. Ocular neovascularization: an epidemiologic review. Surv Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 20.Shakiba Y., Mansouri K., Arshadi D. Corneal neovascularization: molecular events and therapeutic options. Recent Pat Inflamm Allergy Drug Discov. 2009;3:221–231. doi: 10.2174/187221309789257450. [DOI] [PubMed] [Google Scholar]
- 21.Zheng M., Deshpande S., Lee S. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol. 2001;75:9828–9835. doi: 10.1128/JVI.75.20.9828-9835.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmed A., Berati H., Nalan A. Effect of bevacizumab on corneal neovascularization in experimental rabbit model. Clin Experiment Ophthalmol. 2009;37:730–736. doi: 10.1111/j.1442-9071.2009.02112.x. [DOI] [PubMed] [Google Scholar]
- 23.Amano S., Rohan R., Kuroki M. Requirement for vascular endothelial growth factor in woundand inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998;39:18–22. [PubMed] [Google Scholar]
- 24.Hosseini H., Nejabat M. A potential therapeutic strategy for inhibition of corneal neovascularization with new anti-VEGF agents. Med Hypotheses. 2007;68:799–801. doi: 10.1016/j.mehy.2006.06.063. [DOI] [PubMed] [Google Scholar]
- 25.Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011;56:95–113. doi: 10.1016/j.survophthal.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Rodrigues E.B., Farah M.E., Maia M. Therapeutic monoclonal antibodies in ophthalmology. Prog Retin Eye Res. 2009;28:117–144. doi: 10.1016/j.preteyeres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Avastin (bevacizumab) solution for intravenous infusion prescribing information. <http://www.gene.com/download/pdf/avastin_prescribing.pdf> [accessed 11 March 2014].
- 28.Lucentis (ranibizumab injection) intravitreal injection prescribing information. <http://www.gene.com/download/pdf/lucentis_prescribing.pdf> [accessed 11 March 2014].
- 29.Xu L., Lu T., Tuomi L. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci. 2013;54:1616–1624. doi: 10.1167/iovs.12-10260. [DOI] [PubMed] [Google Scholar]
- 30.Ferrara N., Damico L., Shams N. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859–870. doi: 10.1097/01.iae.0000242842.14624.e7. [DOI] [PubMed] [Google Scholar]
- 31.Houck K.A., Leung D.W., Rowland A.M. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992;267:26031–26037. [PubMed] [Google Scholar]
- 32.Chen Y., Wiesmann C., Fuh G. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293:865–881. doi: 10.1006/jmbi.1999.3192. [DOI] [PubMed] [Google Scholar]
- 33.Kim K.J., Li B., Houck K. The vascular endothelial growth factor proteins: identification of biologically relevant regions by neutralizing monoclonal antibodies. Growth Factors. 1992;7:53–64. doi: 10.3109/08977199209023937. [DOI] [PubMed] [Google Scholar]
- 34.Stewart M.W. Aflibercept (VEGF-TRAP): the next anti-VEGF drug. Inflamm Allergy Drug Targets. 2011;10:497–508. doi: 10.2174/187152811798104872. [DOI] [PubMed] [Google Scholar]
- 35.Manzano R.P., Peyman G.A., Khan P. Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin) Br J Ophthalmol. 2007;91:804–807. doi: 10.1136/bjo.2006.107912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bock F., Konig Y., Dietrich T. Inhibition of angiogenesis in the anterior chamber of the eye. Ophthalmologe. 2007;104:336–344. doi: 10.1007/s00347-007-1512-2. [DOI] [PubMed] [Google Scholar]
- 37.Habot-Wilner Z., Barequet I.S., Ivanir Y. The inhibitory effect of different concentrations of topical bevacizumab on corneal neovascularization. Acta Ophthalmol. 2010;88:862–867. doi: 10.1111/j.1755-3768.2009.01571.x. [DOI] [PubMed] [Google Scholar]
- 38.DeStafeno J.J., Kim T. Topical bevacizumab therapy for corneal neovascularization. Arch Ophthalmol. 2007;125:834–836. doi: 10.1001/archopht.125.6.834. [DOI] [PubMed] [Google Scholar]
- 39.Koenig Y., Bock F., Horn F. Short- and long-term safety profile and efficacy of topical bevacizumab (Avastin) eye drops against corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2009;247:1375–1382. doi: 10.1007/s00417-009-1099-1. [DOI] [PubMed] [Google Scholar]
- 40.Lin C.T., Hu F.R., Kuo K.T. The different effects of early and late bevacizumab (Avastin) injection on inhibiting corneal neovascularization and conjunctivalization in rabbit limbal insufficiency. Invest Ophthalmol Vis Sci. 2010;51:6277–6285. doi: 10.1167/iovs.09-4571. [DOI] [PubMed] [Google Scholar]
- 41.Papathanassiou M., Theodossiadis P.G., Liarakos V.S. Inhibition of corneal neovascularization by subconjunctival bevacizumab in an animal model. Am J Ophthalmol. 2008;145:424–431. doi: 10.1016/j.ajo.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Bock F., Konig Y., Kruse F. Bevacizumab (Avastin) eye drops inhibit corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2008;246:281–284. doi: 10.1007/s00417-007-0684-4. [DOI] [PubMed] [Google Scholar]
- 43.Dastjerdi M.H., Al-Arfaj K.M., Nallasamy N. Topical bevacizumab in the treatment of corneal neovascularization: results of a prospective, open-label, noncomparative study. Arch Ophthalmol. 2009;127:381–389. doi: 10.1001/archophthalmol.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S.W., Ha B.J., Kim E.K. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology. 2008;115:e33–e38. doi: 10.1016/j.ophtha.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Fung A.E., Rosenfeld P.J., Reichel E. The International Intravitreal Bevacizumab Safety Study: using the internet to assess drug safety worldwide. Br J Ophthalmol. 2006;90:1344–1349. doi: 10.1136/bjo.2006.099598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernando N.H., Hurwitz H.I. Targeted therapy of colorectal cancer: clinical experience with bevacizumab. Oncologist. 2004;9(suppl):11–18. doi: 10.1634/theoncologist.9-suppl_1-11. [DOI] [PubMed] [Google Scholar]
- 47.Ferrari G., Dastjerdi M., Okanobo A., Sheng-Fu Cheng S.-F., Amparo F., Nallasamy N. Topical ranibizumab as a treatment of corneal neovascularization. Cornea. 2013;32:992–997. doi: 10.1097/ICO.0b013e3182775f8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krizova D., Vokrojova M., Liehneova K., Studeny P. Treatment of corneal neovascularization using anti-VEGF bevacizumab. J Ophthalmol. 2014;2014:1–7. doi: 10.1155/2014/178132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee P., Wang C.C., Adamis A.P. Ocular neovascularization: epidemiologic review. Surv Ophthalmol. 1998;43:245–269. doi: 10.1016/s0039-6257(98)00035-6. [DOI] [PubMed] [Google Scholar]
- 50.Chu H.S., Hu F.R., Yang C.M. Subconjunctival injection of bevacizumab in the treatment of corneal neovascularization associated with lipid deposition. Cornea. 2011;30:60–66. doi: 10.1097/ICO.0b013e3181e458c5. [DOI] [PubMed] [Google Scholar]
- 51.Zaki A.A., Farid S.F. Subconjunctival bevacizumab for corneal neovascularization. Acta Ophthalmol. 2010;88:868–871. doi: 10.1111/j.1755-3768.2009.01585.x. [DOI] [PubMed] [Google Scholar]
- 52.Doctor P.P., Bhat P.V. Subconjunctival bevacizumab for corneal neovascularization. Cornea. 2008;27:992–995. doi: 10.1097/ICO.0b013e31817786ad. [DOI] [PubMed] [Google Scholar]
- 53.Gueudry J., Richez F., Tougeron-Brousseau B. Sub-conjunctival bevacizumab for corneal neovascularization. J Fr Ophtalmol. 2010;33:630–636. doi: 10.1016/j.jfo.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Yeung S.N., Lichtinger A., Kim P. Combined use of subconjunctival and intracorneal bevacizumab injection for corneal neovascularization. Cornea. 2011;30(10):1110–1114. doi: 10.1097/ICO.0b013e31821379aa. [DOI] [PubMed] [Google Scholar]
- 55.You I.C., Kang I.S., Lee S.H. Therapeutic effect of subconjunctival injection of bevacizumab in the treatment of corneal neovascularization. Acta Ophthalmol. 2009;87:653–658. doi: 10.1111/j.1755-3768.2008.01399.x. [DOI] [PubMed] [Google Scholar]
- 56.Dastjerdi M.H., Saban D.R., Okanobo A. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival. Invest Ophthalmol Vis Sci. 2010;51:2411–2417. doi: 10.1167/iovs.09-3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hashemian M.N., Z-Mehrjardi H., Moghimi S. Prevention of corneal neovascularization: comparison of different doses of subconjunctival bevacizumab with its topical form in experimental rats. Ophthalmic Res. 2011;46:50–54. doi: 10.1159/000322061. [DOI] [PubMed] [Google Scholar]
- 58.Ozdemir O., Altintas O., Altintas L., Ozkan B., Akdag C., Yüksel N. Comparison of the effects of subconjunctival and topical anti-VEGF therapy (bevacizumab) on experimental corneal neovascularization. Arq Bras Oftalmol. 2014;77(4):209–213. doi: 10.5935/0004-2749.20140054. [DOI] [PubMed] [Google Scholar]
- 59.Ahmed A., Berati H., Nalan A., Aylin S. Effect of bevacizumab on corneal neovascularization in experimental rabbit model. Clin Exper Ophthalmol. 2009;37(7):730–736. doi: 10.1111/j.1442-9071.2009.02112.x. [DOI] [PubMed] [Google Scholar]
- 60.Dastjerdi MH, Sadrai Z, Saban DR, Zhang Q, Dana R. Corneal penetration of topical and subconjunctival bevacizumab. Invest Ophthalmol Vis Sci 2011;7;52(12):8718–8723. [DOI] [PMC free article] [PubMed]
- 61.Sener E., Yuksel N., Yildiz D.K. The impact of subconjunctivally injected EGF and VEGF inhibitors on experimental corneal neovascularization in rat model. Curr Eye Res. 2011;36:1005–1013. doi: 10.3109/02713683.2011.601840. [DOI] [PubMed] [Google Scholar]
- 62.Erdurmus M., Totan Y. Subconjunctival bevacizumab for corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2007;245:1577–1579. doi: 10.1007/s00417-007-0587-4. [DOI] [PubMed] [Google Scholar]
- 63.Ozdemir O., Ozgul A., Levent A., Demir K., Ender S., Yusuf C. Effects of subconjunctivally injected bevacizumab, etanercept, and the combination of both drugs on experimental corneal neovascularization. Can J Ophthalmol. 2013;48:115–120. doi: 10.1016/j.jcjo.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 64.Veritti D., Vergallo S., Lanzetta P. Triple therapy for corneal neovascularization: a case report. Eur J Ophthalmol. 2012;22(Suppl 7):S126–S128. doi: 10.5301/ejo.5000050. [DOI] [PubMed] [Google Scholar]
- 65.Kang S., Chung S.K. The effect of subconjunctival combined treatment of bevacizumab and triamcinolone acetonide on corneal neovascularization in rabbits. Cornea. 2010;29:192–196. doi: 10.1097/ICO.0b013e3181b1c82f. [DOI] [PubMed] [Google Scholar]
- 66.Hoffart L., Matonti F., Conrath J. Inhibition of corneal neovascularization after alkali burn: comparison of different doses of bevacizumab in monotherapy or associated with dexamethasone. Clin Exp Ophthalmol. 2010;38:346–352. doi: 10.1111/j.1442-9071.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 67.Chen W.-L., Chen Y.-M., Chu H.-S., Lin C.-T., Chow L.-P. Mechanisms controlling the effects of bevacizumab (Avastin) on the inhibition of early but not late formed corneal neovascularization. PLoS ONE. 2014;9(4):e94205. doi: 10.1371/journal.pone.0094205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liarakos V., Papaconstantinou D., Vergados I., Douvali M., Theodossiadis P. The effect of subconjunctival ranibizumab on corneal and anterior segment neovascularization: study on an animal model. Eur J Ophthalmol. 2014;24(3):299–308. doi: 10.5301/ejo.5000391. [DOI] [PubMed] [Google Scholar]
- 69.Rosenfeld PJ. My use of aflibercept in clinical practice. Retina Today 2012; 50.
- 70.Stewart M.W., Rosenfeld P.J. Predicted biological activity of intravitreal VEGF Trap. Br J Ophthalmol. 2008;92:667–668. doi: 10.1136/bjo.2007.134874. [DOI] [PubMed] [Google Scholar]