Summary
Background
Prune Belly Syndrome (PBS) is a rare entity, usually found in male neonates. It comprises complex urinary tract anomalies, bilateral undescended testis and absence of anterior abdominal wall muscles. Patients with unilateral abdominal wall deficiency, unilateral undescended testis and female neonates with abdominal wall laxity are classified as Pseudo Prune Belly syndrome (PPBS). Reports on PPBS do not highlight the radiological and imaging characteristics of this syndrome and the current literature on the role of newer imaging modalities, such as Magnetic Resonance Imaging (MRI), remains relatively sparse. We describe a new case of PPBS and emphasize the role of imaging, especially ultrasound and MRI in the process of diagnosis and briefly review the subject.
Case Report
A male infant of four months of age was referred for evaluation of left-sided cryptorchidism. Clinical examination revealed laxity of the left abdominal wall. Ultrasound examination of the abdomen, pelvis and scrotum was performed together with routine laboratory tests. Ultrasound examination was followed by intravenous urography, voiding cysto-urethrography and MRI of the abdomen.
On ultrasound, the left testis was located in the inguinal canal, the right kidney was slightly enlarged and the left kidney could not be localized. Ultrasound appearances suggested chronic obstruction in the urinary bladder. Intravenous urography, voiding cysto-urethrography and MRI confirmed the ultrasound diagnosis and also revealed a left dysplastic kidney with a dilated, tortuous ureter. Clinical and imaging features were consistent with pseudo prune belly syndrome (PPBS).
Conclusions
We report a new occurrence of PPBS, a rare entity. The imaging approach for a comprehensive evaluation of the renal system in PPBS, especially with MRI, is emphasized.
MeSH Keywords: Cryptorchidism, Magnetic Resonance Imaging, Prune Belly Syndrome, Pseudo Prune Belly Syndrome, Ultrasound
Background
Prune Belly Syndrome (PBS) or the triad syndrome, is a rare entity, usually described in male neonates. It comprises complex urinary tract anomalies, bilateral undescended testis and absence of anterior abdominal wall muscles [1–3]. The anomaly, which is named after the wrinkled appearance of the abdominal wall resembling a dried prune, is believed to occur in 1 in 40,000 live births, with >95% occurring in males [1,4]. Patients with partial or unilateral abdominal wall deficiency, or with only unilateral undescended testis, as well as female neonates with abdominal wall laxity are classified as pseudo prune belly syndrome (PPBS) or partial prune belly syndrome [1,2,5]. The group of pseudo prune belly syndrome patients comprise only 3–5% of all patients with PBS [1].
PBS is a clinical diagnosis and radiological investigations show only the extent of renal dysplasia and/or dysfunction. Most of the previous reports have described conventional radiographic techniques and ultrasound [1–5]. PPBS is very rare with very few available reports, that do not specifically highlight the radiological and imaging features. So far, reports on the role of newer imaging modalities such as MRI remain relatively sparse. We describe a new case of PPBS and emphasize the role of imaging, especially ultrasound and MRI, not only in prompting the diagnosis but also in confirming it. The role of imaging modalities in arriving at the diagnosis of PPBS is even more significant as clinical signs are subtle in comparison to full blown PBS. The literature on both PBS and PPBS is briefly reviewed.
Case Report
A male infant of four months was brought to our hospital for evaluation of left-sided cryptorchidism. The child had been born by normal vaginal delivery to a non-consanguineous couple. The neonatal and infancy period had been uneventful and no major medical event was recalled by the parents. Antenatal ultrasound evaluation records of the mother were not available. The elder male sibling of four years was normal. Clinical examination of the infant was normal except for a laxity of the left abdominal wall (Figure 1). Routine laboratory investigations were performed. Ultrasound examination of the abdomen, pelvis and scrotum was done. Ultrasound examination was followed by intravenous urography, voiding cysto-urethrography and MRI of the abdomen.
Figure 1.
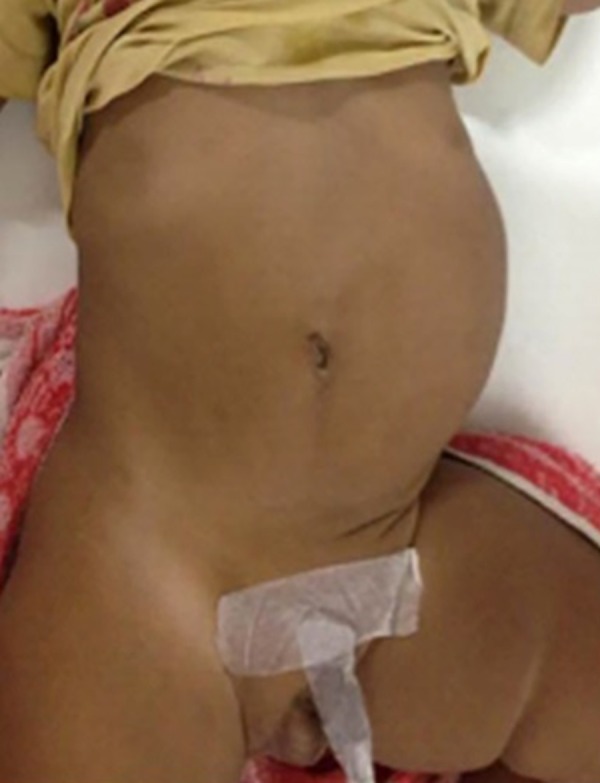
Shows the clinical appearance of the infant, unilateral laxity of left abdominal musculature, with an empty left scrotum.
Results
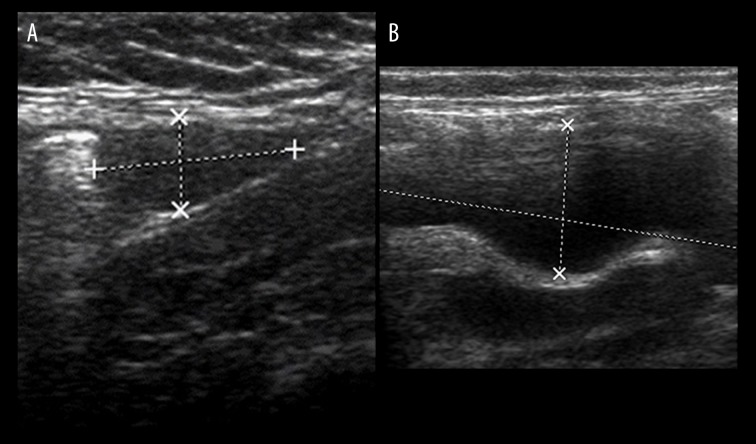
Routine laboratory parameters including renal function were all within normal range. On ultrasound, the left testis was located in the left inguinal canal (Figure 2A). The right kidney was normal in location and echo-texture but appeared slightly enlarged (6.2 cm in length) for the patient’s age. The left kidney was not localized either in the left renal fossa or elsewhere in the abdominal cavity. However, a dilated tubular cystic structure was seen in the left lumbar region, reminiscent of a dilated ureter (Figure 2B). The urinary bladder showed multiple trabeculations and sacculations consistent with outlet obstruction. Ascites was observed in the pelvis and in the left lower lumbar region. The clinical and sonographic features were consistent with incomplete prune belly syndrome also known as pseudo prune belly syndrome (PPBS).
Figure 2.
(A) Ultrasound of the left suprapubic region shows the left testis located in the left inguinal canal. (B) Ultrasound of the left lumbar region shows a tubular cystic structure.
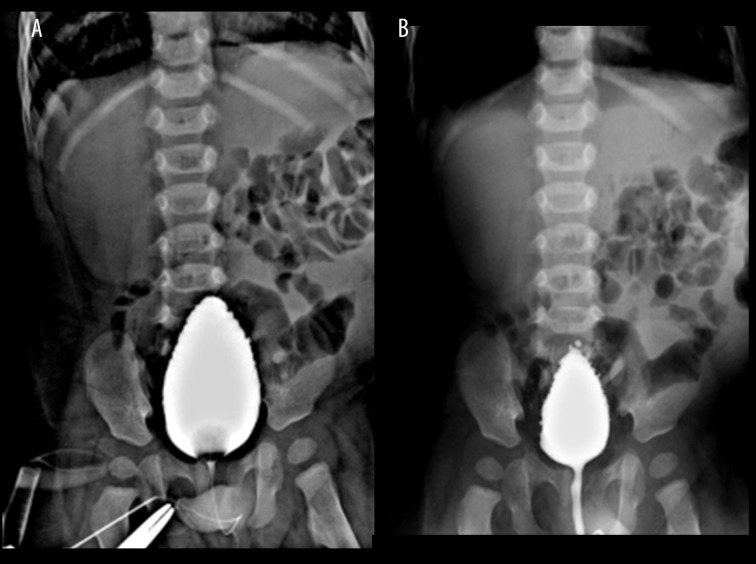
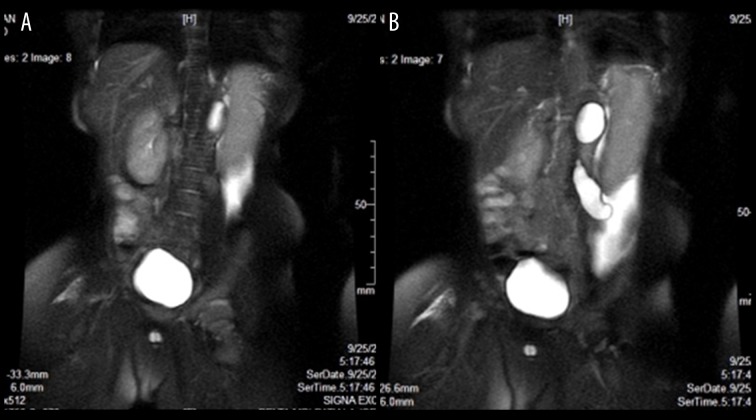
Further evaluation by radiographs and intravenous urography revealed bowel loops displaced to the left side of the abdomen under the lax left abdominal wall (Figure 3). There was no functional left renal moiety or renal tissue seen (Figure 3). Compensatory hypertrophy of the normally functioning right kidney was seen. The right ureter was normal (Figure 3). Cystogram and voiding cysto-urethrography revealed features of chronic bladder obstruction (Figure 4A, 4B). The posterior urethra appeared to be mildly dilated, reflux was however absent (Figure 4A, 4B). MRI showed an extremely small, dysplastic left kidney, with a dilated and tortuous upper left ureter (Figure 5A, 5B). Free fluid was seen in the left lumbar region as was seen on the ultrasound study. Barium study was performed which excluded malrotation and other structural anomalies and motility disorders of the bowel. There were no clinical signs to suggest skeletal anomalies. Echocardiography was normal. The entire spectrum of radiological and imaging features confirmed PPBS with unilateral abdominal wall laxity, ipsilateral undescended testis, ipsilateral renal dysplasia, bladder neck obstruction and dilatation of posterior urethra. No clinical or radiological evidence for any known associated congenital anomalies was found.
Figure 3.
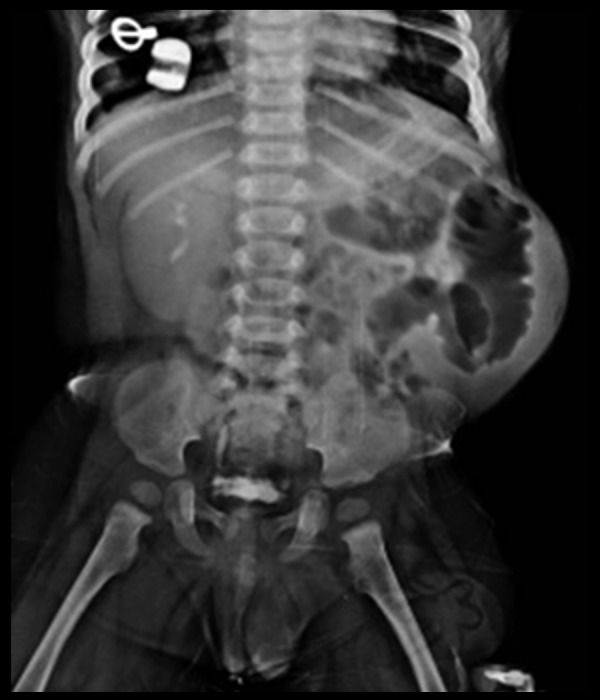
Intravenous urography study shows a single, normally-functioning, but enlarged right kidney, normal right ureter; left kidney cannot be localised in the abdomen or pelvis.
Figure 4.
(A) Cystogram: shows tall peaked urinary bladder with trabeculation, consistent with chronic bladder neck obstruction. (B) Voiding cysto uretherogram: shows trabeculated bladder, mildly dilated posterior urethra, however no reflux is seen.
Figure 5.
(A) MRI abdomen, T2 W, coronal view, shows right kidney is enlarged, but normal in morphology, the left kidney shows cystic dysplasia, ascites is seen in left lower lumbar region. (B) MRI abdomen, T2 W, coronal view, shows right kidney is enlarged, but normal in morphology, the left kidney shows cystic dysplasia, left ureter is dilated and tortuous, (the tubular cystic structure which was seen on ultrasound in the left lumbar region), ascites is seen in left lumbar region.
Surgical management advised for the patient was left uretero-nephrectomy, orchidopexy and repair of the left abdominal wall. At the time of evaluation, the parents requested to delay major surgery as the infant had normal voiding and serum parameters of renal function were also normal. On follow-up, the patient was in good condition at two years of age and had no clinical or laboratory evidence of urinary retention, sepsis or renal derangement. The parents decided to further postpone surgical intervention. At this stage, written informed consent was obtained from them to publish this report.
Discussion
The literature on aetiological and embryogenetic factors of PBS and PPBS is controversial. Some investigators postulate that the disease is due to three possible factors occurring during embryogenesis: severe bladder outlet obstruction, dysgenesis of yolk sac and possibly due to abdominal muscle deficiency secondary to a migrational defect of the lateral mesoblast between 6th to 10th weeks of pregnancy [1,3,4]. The chronic obstruction at the bladder neck is attributed to a dysplastic and dysfunctional posterior urethra [2].
Familial occurrence of the disease had suggested a genetic cause, but Granberg et al. have shown that the implicated HNF1β mutation was detected only in 3% of patients with prune belly syndrome [6]. Associated anomalies are numerous and include those of the VACTERL group, gastrointestinal, orthopaedic and cardiopulmonary systems [1,3,4,7]. We presume that the aetiological and genetic factors and the associated anomalies in PBS and PPBS are similar, as these entities belong to a single group of congenital defects lying at both ends of a spectrum, and only differ in the severity of manifestations.
It has been stressed by Bellah et al. that although the clinical appearance of PBBS is not severe, renal involvement should never be expected to be mild. These authors have documented moderate to severe changes in the urinary tract in most patients [5]. Renal status is of clinical importance not only for the management but also affects the overall prognosis.
As with all congenital anomalies, the radiologist is the primary physician who detects the problem even in the foetal stage, on antenatal ultrasound scan. Byon and Kim have reported antenatal diagnosis of PBS as early as 12 weeks [8].
The clinically obvious varieties PBS and PPBS can be both diagnosed by the radiologist because of renal obstruction and related urinary tract damage. Most reports recommend a protocol of primary ultrasound for initial diagnosis of hydronephrosis and a voiding cysto-urethrogram for the evaluation of the functional obstruction at the bladder neck [1–3,5]. Excretory efficacy of the renal system is evaluated either by intravenous urography and/or DMSA scans in both PBS and PPBS [2,5].
As evident from our study, primary detection and diagnosis of PPBS may fall entirely in the domain of the Radiologist, especially more so in patients with subtle or unilateral abdominal wall defects. The Radiologist plays a role not only during ultrasound evaluation of the renal status but also in localizing undescended testis, as shown in our patient. The testis is known to be abdominal in PBS and inguinal in PPBS, respectively [2]. The later was true in our patient. Testicular localisation not only facilitates surgical planning for orchidopexy but is also vital because some testes, even those surgically treated, may sooner or later become malignant [9]. Therefore, a regular ultrasound surveillance of the testes in PBS/PPBS, whether scrotal or abdominal, should be advised, both pre and post-orchidopexy.
Imaging approach in our patient was similar and we performed sonography, intravenous urography and voiding cysto-urethrograpy. Renal anomaly in our patient was also found to be severe, as there was only one functioning kidney and the other one was dysplastic, non-functioning and appeared as “absent” both on ultrasound and on intravenous urography. The dysplastic left kidney, paradoxically had a (upper segment) hydroureter, which was seen on ultrasound (Figure 2B) and on MRI (Figure 5A, 5B). This feature of the dysplastic kidney with a dilated ureter is well-known and have been described in previous reports. The urinary bladder in our patient was found to be chronically obstructed and the posterior urethra appeared mildly dilated.
We used MR imaging evaluation of the renal system, which was found to be invaluable in documenting the dysplastic non-functional left kidney. Since excellent evaluation of the entire renal system, including ureters and urinary bladder, can be non-invasively documented by MRI, we propose that it should be included in the standard imaging protocol of PPBS and PBS. Lately, one report by Garcia-Roig et al. has highlighted the role of MR urography in detecting renal dysplasia and dysfunction in PPBS [10]. Although we agree with these authors in proposing MR as a routine work-up modality, we would not prefer to use contrast or diuresis in these infants, as they may already have impaired renal function and electrolyte balance. Furthermore, aggressive MR interrogation would extend examination time and increase the added risk of prolonged sedation. In our patient,a limited examination by coronal T2-W and T1-W sequences provided adequate and relevant clinical information.
Some investigators believe that PPBS is identical to the megacystis megaureter syndrome [4].The latter disease, however, lacks abdominal wall and testicular descent abnormalities and is, in our opinion, a distinctly different entity. Another disease that should be included in the differential diagnosis of PPBS and PBS is the extremely rare megacystis–microcolon-intestinal hypoperistalsis syndrome (MMIHS) [11]. This disease is an extreme form of PBS with associated functional ileus and obstruction of the gastrointestinal tract and microcolon, which is frequently fatal [11]. Electron microscopy shows vacuolar degeneration in the smooth muscles of the small bowel and urinary bladder in MMIHS [11].
The management approach in PBS and PPBS is similar and includes orchidopexy with abdominoplasty. The approach to the urinary system has to be individualised and may require urinary diversion or decompressions or nephrectomy [4]. Complete surgical management, therefore, addresses all three aspects of the triad. There is as yet a lack of consensus on the optimal management approach in these children. Some authors prefer surgical intervention, while others disagree and believe the outcome is poor due to high morbidity and mortality in “total reconstruction”, Japanese researchers have achieved good results with renal transplantation [4]. The final outcome in both PBS and PPBS depends on the severity of renal tract anomalies and other associated anomalies [2,3].
Conclusions
Regardless of the surgical management, the role of the radiologist remains vital not only in the diagnostic work-up but also in follow-up of both PBS and PPBS patients. The imaging approach for a comprehensive evaluation of the renal system and also for the exclusion of other associated anomalies is highlighted by our report. Our experience shows that although PPBS is a rare variety of PBS, increased awareness of all the aspects of these diseases is essential for the radiologist, especially in large maternal and paediatric centres. We report a new occurrence of PPBS, a rare entity, which has not been reported earlier from India. Lastly, we propose a short T2W coronal MR examination as a standard imaging protocol in PBS and PPBS.
Learning points
Pseudo Prune Belly Syndrome (PPBS) is a rare entity similar to Prune Belly syndrome (PBS). It is also referred to as an incomplete expression of PBS.
The clinical presentation of PPBS is subtle, compared to PBS, and may remain unrecognised, unless encountered by the radiologist who may often be the primary physician to detect and diagnose the problem, as is true in some other clinical entities as well. Therefore, the necessity for an increased awareness of this anomaly by our specialists needs to be emphasised.
Urinary system anomalies are not milder in PPBS, as compared to PBS, and may be as severe and one may even encounter a dysplastic kidney, as in our infant.
The role of a brief MR-T2 W coronal evaluation of the renal system is proposed as a standard protocol in PBS and PPBS patients.
The radiologist should focus not only on a comprehensive evaluation of the renal system but also on localisation and surveillance of the undescended testis, whether pexed or not.
Associated anomalies affect prognosis and surgical outcome of PBS and PPBS, and their exclusion/documentation is the responsibility of the radiologist.
Footnotes
Conflict of interest
None.
References
- 1.Ghritlaharey RK, Gupta G, Kushwaha AS, Chanchlani R. Prune belly syndrome associated with incomplete VACTERL. J Indian Assoc Pediatr Surg. 2007;12(1):39–41. [Google Scholar]
- 2.Woodhouse CR, Ransley PG, Innes-Williams D. Prune belly syndrome – report of 47 cases. Arch Dis Child. 1982;57:856–59. doi: 10.1136/adc.57.11.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younous S, Zarrouki Y, Boutbaoucht M, et al. Prune belly syndrome associated with full spectrum of VACTERL in a New Born. J Clin Neonatol. 2012;1(1):49–51. doi: 10.4103/2249-4847.92234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zugor V, Schott GE, Labanaris AP. The prune belly syndrome: Urological aspects and long term outcomes of a rare disease. Pediatr Rep. 2012;4(2):e20. doi: 10.4081/pr.2012.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellah RD, States LJ, Duckett JW. Pseudoprune-Belly syndrome: Imaging findings and clinical outcome. Am J Roentgenol. 1996;167(6):1389–93. doi: 10.2214/ajr.167.6.8956564. [DOI] [PubMed] [Google Scholar]
- 6.Granberg CF, Harrison SM, Dajusta D, et al. Genetic basis of prune belly syndrome: screening for HNF1β gene. J Urol. 2012;187(1):272–78. doi: 10.1016/j.juro.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grimsby GM, Harrison SM, Granaberg CF, et al. Impact and frequency of extra genito-urinary manifestations of prune belly syndrome. J Pediatr Urol. 2015;11(5):280.e1–6. doi: 10.1016/j.jpurol.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byon M, Kim GJ. Prune-belly syndrome detected by ultrasound in the first trimester and the usefulness of vesicocentesis as a modality of treatment. Obstet Gynecol Sci. 2013;56(4):265–68. doi: 10.5468/ogs.2013.56.4.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humphrey PA, Shuch B. Seminoma in cryptorchid testis in prune belly syndrome. J Urol. 2015;194(3):799–800. doi: 10.1016/j.juro.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Roig ML, Grattan-Smith JD, Arlen AM, et al. Detailed evaluation of the upper urinary tract in patients with prune belly syndrome using magnetic resonance urography. J Pediatr Urol. 2016;12(2):122.e1–7. doi: 10.1016/j.jpurol.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Levin TL, Soghier L, Blitman NM, et al. Megacystis-microcolon-intestinal hypoperistalsis and prune belly: Overlapping syndromes. Pediatr Radiol. 2004;34(12):995–98. doi: 10.1007/s00247-004-1260-2. [DOI] [PubMed] [Google Scholar]