Abstract
Background
To determine the association between serum 25(OH)D and dry eye syndrome (DES) incidence. This study was also designed to determine whether serum 25(OH)D levels were associated with ocular parameter of DES patients.
Material/Methods
This is a case-control study with 70 DES cases and 70 healthy controls. Clinical data included body mass index (BMI, kg/m2), smoking history, diabetes, and blood pressure. Serum 25(OH)D was chosen as the main parameter and reflected the level of vitamin D. The DES parameters included ocular surface disease index (OSDI) scales, tear film breakup time (TBUT) and Schirmer test I. The differences in each parameter between case and control groups were detected and the association of serum 25(OH)D and DES parameter were detected.
Results
It was shown that 25(OH)D levels were lower in patients with DES than in healthy controls. When the 25(OH)D levels was stratified, vitamin D deficiency was more common in the DES cases. In advanced studies, it was found that there were statistically significant associations between serum 25(OH) D levels and the Schimer test, TBUT, and OSDI scales.
Conclusions
A significant association between serum 25(OH)D level and DES incidence was detected in this study. Considering the relatively small sample size of this study, larger studies are needed in the future.
MeSH Keywords: 24,25-Dihydroxyvitamin D 3; Case-Control Studies; Dry Eye Syndromes
Background
Ocular surface is protected by tears, and tear abnormalities or ocular surface disorders could result in dry eye syndrome (DES) [1]. DES is a common and complex condition with reduced ocular comfort and impaired visual performance. Nowadays, DES is regarded as one of the most common ocular disorders throughout the world [2,3]. The treatment of DES is based on local application of eye drops, including artificial tears and anti-inflammatory agents [4]. Although most DES patients treated with eye drops will feel more comfortable, it is hard to cure DES. In addition, for some individuals, there are no satisfactory therapeutics that can provide relief from DES ocular discomfort symptoms. Thus, better understandings of the risk factors and pathogenesis of DES would help in primary prevention of DES and effective therapy development. The generally recognized risk factors for DES included elder age, reproductive factors, tobacco smoking, contact lenses use, ocular surgery, systemic diseases, and dry environment contact [5,6]. Better understandings of risk factors and related lifestyle changes of DES patients can be helpful in the DES related discomforts management [7].
Vitamin D is a fat soluble vitamin that is involved in a number of physiological and pathological processes [8,9]. Vitamin D formulations have been widely used in different diseases and have been reported to be effective in the treatment or prevention of osteoporosis, cancers, and cardiovascular disorders [10–12]. In advanced in-vivo and in-vitro studies, vitamin D has been reported to enhance immunity, relieve inflammatory reaction and regulate cell cycles [13–15]. The application of vitamin D in treatment of ocular surface disorders has been reported. In a previous study, it was suggested that there is a possible role for vitamin D in modulating corneal wound healing, thus have important implications for therapeutic use of vitamin D at the ocular surface [16]. In another study based on mouse, rabbit, and human samples, it was determined that 25-hydroxyvitamin D[(3)(25(OH)D(3)] and/or its active metabolite, 1α,25-dihydroxyvitamin D[(3)(1,25(OH)(2)D(3)] can enhance corneal epithelial barrier function [17].
Several epidemiological studies were conducted to evaluate the association between vitamin D or vitamin D deficiency and incidence rate of DES [18–21], however, no conclusions were reached. A cross-sectional study that included 17,542 adults from Korea found that lower serum 25(OH)D levels were associated with incidence rates of DES. However, in another cross-sectional study containing male patients, it was reported that vitamin D levels were not significantly associated with the presence or severity of DES [18]. The purpose of our case-control study was to determine the effect of serum 25(OH)D on DES incidence. The study was also designed to determine whether serum 25(OH)D levels were associated with ocular parameters of DES patients.
Material and Methods
Ethical approval of this current study was obtained from the Human Research Ethics Committee of Changshu No 2 People’s Hospital.
Patients
In this study, a case-control study design was used. A total of 70 DES patients and 70 matched controls were included in this study. All the DES patients were diagnosed from May 2015 to January 2016 at the out-patient clinic of the Department of Ophthalmology, Changshu No 2 People’s Hospital, Changshu, People’s Republic of China. The control group was patients without DES in the same time period. The inclusion criteria were: (1) matched to the diagnosis criteria of DES, (2) aged over 18 years, and (3) signed the informed consents before inclusion in the study. The exclusion criteria contained: (1) unable to co-operate in the study handling, (2) Sjogren syndrome, rheumatoid arthritis, lupus erythematosus, or any other immune diseases; (3) mental disorder or serious systemic disease, such cancer, hematological system disease, or hyperthyroidism; (4) pregnancy or breast-feeding in women; (5) participation in other ophthalmic clinical trials; (6) other ocular disease, such as ocular surgery history within the recent six months, use of any ophthalmic eye drops or contact lenses within recent one month, eyelid or eyelash abnormalities, nasolacrimal apparatus abnormalities glaucoma, uveitis, retinal hemorrhage, or optic neuritis.
DES diagnosis
Patients with DES were diagnosed using experts’ consensus about clinical diagnosis and treatment of dry eye (2013). The diagnostic criteria includes: (1) subjective symptoms of dryness, foreign body sensation, burning sensation, fatigue, discomfort, visual acuity fluctuation, and tear film breakup time (TBUT) test ≤5 seconds or Schirmer I test (without surface anesthesia) ≤5 mm/5 minutes; (2) the subjective symptoms of ocular discomfort and TBUT test 5–10 seconds or Schirmer I test (without surface anesthesia) ≤5–10 mm/5 minutes combined with corneal fluorescein staining positive.
Clinical data collection
Clinical data, including body mass index (BMI, kg/m2), smoking history, diabetes status, and blood pressure were obtained through questioning of the participants or reviewing their medical records. The ocular surface disease index (OSDI) quantitation was conducted by trained investigators. The OSDI scale questionnaire included 12 questions, containing three aspects of eye symptoms, visual function, and environmental stimulation. Detailed information was collected from each participant regarding ocular dryness, foreign body, visual fatigue, and other subjective discomfort symptoms. The response grades were divided into no symptoms (0 point), occasional presence (0.5 points), intermittent (1 point), and persistent discomfort (2 point). Detailed explanations of each point was used to reach a relative accurate conclusion. The DES-related examinations were evaluated after a full ophthalmologic examination was performed. All the ocular surface examinations were performed by one study investigator (MYF). The examinations were conducted in the following order: TBUT, keratoepitheliopathy examination, and Schirmer I test. All the clinical data was collected and recorded in electronic tables.
Vitamin D detection
The longer half-life of 25(OH)D, allows it to remains stable in serum and as such it can be used to reflect the level of vitamin D. In this study, 25(OH)D was chosen as a main study parameter. Peripheral blood samples from each participant were collected and serum samples were frozen at −70°C until 25(OH)D detection was conducted. Serum 25(OH)D levels were measured by a reverse phase liquid chromatography method (Agilent Technologies, CA, USA). The accuracy quality control of low, medium, and high quality was to be 85~115% with the accuracy value within 15%.
Statistical analysis
The statistical analysis was performed using Package for SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA). The data of each index is shown as the mean ± standard deviation (SD). Comparison of discontinuous variable parameters was done using the chi-square test or Fisher’s exact test. The differences in the continuous variables were detected using non-paired t-test. Pearson correlation analysis was used in the detection of the associations between different continuous variables. A value of p<0.05 was considered to be statistically significant.
Results
Clinical characteristics
Basic clinical characteristics of patients with DES and healthy controls are presented in Table 1. In this study, 70 DES cases (43 males and 27 females) and 70 matched controls (36 males and 34 females) were included. The mean BMI in the DES group and the control group was 23.2±4.4 kg/m2 and 23.6±5.1 kg/m2, respectively. There were no differences in smoking status, diabetes prevalence, and blood pressure between the DES group and the control group. When the Schirmer I test was considered, the DES group (9.4±3.9 mm/5 minutes) demonstrated a significantly lower value compared with the control group (13.9±5.3 mm/5 minutes, p<0.001). In addition, a significantly reduced TBUT was detected in the DES cases (DES group, 6.1±2.4 second; control group, 13.4±3.8 seconds, p<0.001). Compared with the control group, the mean OSDI value was significantly higher in the DES group (p<0.001).
Table 1.
Demographics and clinical characteristics in DES patients and normal controls.
| DES group (n=70) | Control group (n=70) | P value | |
|---|---|---|---|
| Age (years) | 49.2±12.2 | 51.5±15.6 | 0.332 |
| Gender | |||
| Male | 43 | 36 | 0.153 |
| Female | 27 | 34 | |
| BMI (kg/m2) | 23.2±4.4 | 23.6±5.1 | 0.620 |
| Smoking | 19 (27.1%) | 21 (30.0%) | 0.524 |
| Diabetes | 22 (32.4%) | 18 (25.7%) | 0.287 |
| SBP (mmHg) | 128.6±22.4 | 131.2±18.2 | 0.452 |
| DBP (mmHg) | 78.2±12.7 | 80.8±11.8 | 0.212 |
| Schirmer test (mm/5 min) | 9.4±3.9 | 13.9±5.3 | <0.001 |
| Fluorescein TBUT (s) | 6.1±2.4 | 13.4±3.8 | <0.001 |
| OSDI (points) | 43.8±18.8 | 13.6±4.3 | <0.001 |
SBP – systolic blood pressure; DBP – diastole blood pressure; OSDI – ocular surface disease index.
25(OH)D levels in patients with DES
The serum 25(OH)D levels in patients with DES and the control patients are shown in Table 2. Serum 25(OH)D levels were significantly lower in the DES group than in the healthy control group (DES group, 19.3±5.8; control group, 31.6±7.3, p<0.001). Furthermore, serum 25(OH)D levels in both male and female patients were lower compared with the healthy controls (p<0.05). When the participants were divided into different vitamin D status (25(OH)D, <10 ng/mL, 10~20 ng/mL, 20~30 ng/mL, and ≥30 ng/mL), deficiency of vitamin D was more common in the DES group (p<0.001).
Table 2.
Serum Vitamin 25(OH)D levels of patients with DES and the control group.
| DES group (n=70) | Control group (n=70) | P value | |
|---|---|---|---|
| Serum 25(OH)D | 19.3±5.8 | 31.6±7.3 | <0.001 |
| Gender | |||
| Male | 20.6±5.3 | 33.5±9.9 | <0.001 |
| Female | 17.2±6.2 | 29.8±7.1 | <0.001 |
| Serum 25(OH)D | |||
| <10 ng/mL | 4 | 1 | <0.001 |
| 10~20 ng/mL | 32 | 6 | |
| 20~30 ng/mL | 32 | 25 | |
| ≥30 ng/mL | 2 | 48 | |
Association between serum 25(OH) D levels and DES parameters
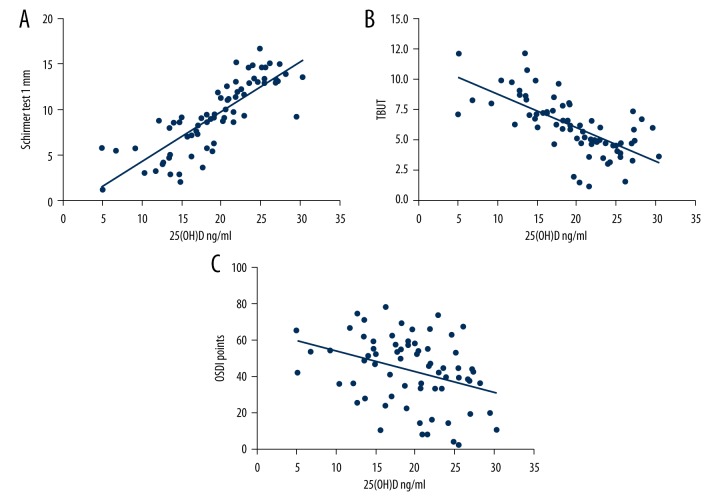
Considering that serum 25(OH)D level was significantly lower in the DES group, we conducted additional analyses on the association between serum 25(OH) D levels and DES parameters. Through Pearson correlation analysis, it was found that serum 25(OH)D level was associated with increased Schimer test I (r=0.8248, p<0.001). In addition, there was an inverse correlation between serum 25(OH)D and ODSI scores (r=−0.3348, p=0.005) and TBUT (r=−0.6806, p<0.001). All the detailed data are shown in Figure 1.
Figure 1.
Correlation of serum 25(OH)D with (A) Schirmer test results (r=0.8248, p<0.001); (B) TBUT (r=−0.6806, p<0.001); and (C) OSDI scales (r=−0.3348, p=0.005). The correlation analyses were calculated in 70 DES cases.
Discussion
In this study, it was shown that 25(OH)D levels were lower in patients with DES than in healthy controls. When the 25(OH)D levels was stratified, vitamin D deficiency was more common in the DES cases. In further analysis, it was found that there were statistical significant associations between serum 25(OH) D levels and the Schimer test, TBUT value and OSDI scale.
DES is a multifactorial disorder and various risk factors were associated with the incidence of DES. Nowadays, there is more interest in the association between vitamin D and DES cases. The major conclusions of this case-control study were consistent with previous cross-sectional study conclusions, in that low serum 25(OH)D levels were associated with DES [20]. In previous cross-sectional data analysis, it was found that low serum 25(OH)D levels were associated with DES in Korean adults. These results provide clues that vitamin D supplementation might be used in DES treatment. In another case-control study with 34 patients with serum vitamin D deficiency and 21 control patients with normal vitamin D levels, it was found that vitamin D deficiency decreases the TBUT and Schirmer test values [21]. In our study, significant lower vitamin D level was detected in DES patients and it was found that significant associations were detected in both male and female groups. In addition, the advanced correlation analysis results showed more interesting and meaningful association between serum 25(OH) D and severity and ocular discomfort in DES cases. Rather than considering vitamin D deficiency as a risk factor for DES as in a previous study, more attention was focused on the association between vitamin D and ocular physical examination and indisposed symptoms in this current study. Positive correlation between serum vitamin D level and DES severity suggests there is the potential for application of vitamin D for treatment or prevention of DES. Ass shown in the first study on the effect of vitamin D on the DES in Chinese population, this study provides additional knowledge on the effect of vitamin D on the DES development. However, even though the association of vitamin D and DES was detected in different studies, the study of the detailed mechanism was lacking. There were several possible explains for the effect of vitamin D on the DES development.
It has been recognized that systemic autoimmune diseases or immune abnormalities could result in the increased risk of DES [22]. Considering the remarkable effect of vitamin D on the immune system, it could be conjectured that the immunoregulatory effect of vitamin D might influence the development of DES. For instance, in a case-control study with 107 Sjogren syndrome (SS) patients and 74 healthy controls, plasma vitamin D levels in SS patient group were significantly lower than in the control group [23]. As dry eye was one of the most frequent symptoms of SS, and vitamin D deficiency was frequent found in patients with SS, vitamin D might be associated with the SS-related DES incidence. Besides, there were several previous studies that reported on the associations between DES and systemic autoimmune disorders, including rheumatism and lupus erythematosus. Abnormal autoimmune status might result in a higher incidence of DES and the protective immunomodulatory effect of vitamin D in the regulation of immune cells might help in the prevention of DES incidence.
There was a previous study that reported that vitamin D deficiency decreased the TBUT and Schirmer test values and may be associated with dry-eye symptoms in non-Sjogren syndrome [21], thus suggesting a potential mechanism beyond the autoimmune effect of vitamin D may exist. Chronic inflammation induced by autoimmune disorders or environmental stimulating factors in ocular surface cells has been considered to be involved in DES. Vitamin D has demonstrated anti-inflammatory effects in different pathological processes [24]. For instance, vitamin D could decrease the inflammatory response of cancer and inhibited the proliferation of cancer cells in-vitro [25]. Considering that inflammation was one of the key pathogenesis and phenotype of DES, higher level vitamin D would decreased the inflammatory response in the ocular surface and then improve the severity of DES.
The production of vitamin D in the metabolic system is from two main sources. In general, vitamin D is mainly produced by cutaneous synthesis upon skin exposure to ultraviolet light and partial vitamin D is gained from food sources. 1,25(OH)D and 25(OH)D were two key intermediate products in vitamin D metabolism. Because of the short half-life of 1,25(OH)D (5–7 days) and the long half-life of 25(OH)D (20~30 days), the serum 5(OH)D level is commonly used as the indicator of vitamin D status and it was used in the present study. Vitamin D receptor (VDR) is a member of the nuclear hormone receptor superfamily and plays an important role in vitamin D function. In a case-control study of 64 DES cases and 51 controls, the single nucleotide polymorphisms (SNPs) in the VDR genes also varied between DES cases and controls [26]. Thus, the influence of SNPs in the vitamin D related gene might explain the association between serum vitamin D and DES incidence.
A major strength of our study was that the method used to assess serum vitamin D levels was sensitive and therefore might produce more robust conclusions. However, several limitations in this study should be reported. First, the sample size of this study was relatively small and it has been demonstrated that larger sample sizes are requirements for advanced study analysis. Considering that this was a hospital-based study, it was hard to include a large sample size in the participant selection process. Additionally, population-based studies are required for the detection of the association between vitamin D and DES status. Second, even though 5(OH)D is the most commonly used index for vitamin D detection, there are other indices reflecting the intracellular effect of vitamin D that were not reported in this study. The association of these related indices might provide additional knowledge to aid in our understanding of the mechanism of vitamin D on DES. Future prospective studies were needed to assess other serum markers of vitamin D in addition to 25(OH)D in the DES cases. In advanced studies, the effect of dietary vitamin D intake, vitamin D supplementation, and ultraviolet radiation exposure on the development of DES should be examined. A third limitation of our study was that that using a case-control study design, selection bias could influence the conclusions.
Conclusions
A significant association between serum 25(OH)D level and DES incidence was detected in this study. In addition, serum 25(OH)D was associated with the DES parameters and ocular discomfort status. It could be conjectured that vitamin D might be a potentially favorable adjunctive option for patients with DES. Considering the relatively small sample size of this study, larger studies with longer follow-up duration are needed.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Source of support: This study was funded by the by the Foundation for Young Scholars of Suzhou, China (Grant No. kjxw2015044)
References
- 1.Yokoi N, Sonomura Y, Kato H, et al. Three percent diquafosol ophthalmic solution as an additional therapy to existing artificial tears with steroids for dry-eye patients with Sjogren’s syndrome. Eye. 2015;29:1204–12. doi: 10.1038/eye.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall LL, Roach JM. Treatment of dry eye disease. Consult Pharm. 2016;31(2):96–106. doi: 10.4140/TCP.n.2016.96. [DOI] [PubMed] [Google Scholar]
- 3.Zhu W, Wu Y, Li G, et al. Efficacy of polyunsaturated fatty acids for dry eye syndrome: A meta-analysis of randomized controlled trials. Nut Rev. 2014;72:662–71. doi: 10.1111/nure.12145. [DOI] [PubMed] [Google Scholar]
- 4.Lee J. Systematic review of thermal massage therapy for the treatment of dry eye syndrome. Value Health. 2015;18(7):A344. [Google Scholar]
- 5.Lee JH, Lee W, Yoon JH, et al. Relationship between symptoms of dry eye syndrome and occupational characteristics: The Korean National Health and Nutrition Examination Survey 2010–2012. BMC Ophthalmol. 2015;15:147. doi: 10.1186/s12886-015-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ren G, Li H, Qiao W, et al. Possible association of killer cell immunoglobulin-like receptor genotypes and haplotypes with dry eye disease in a Han Chinese population. Mol Vis. 2015;21:948–54. [PMC free article] [PubMed] [Google Scholar]
- 7.Na KS, Han K, Park YG, et al. Depression, stress, quality of life, and dry eye disease in Korean women: A population-based study. Cornea. 2015;34:733–38. doi: 10.1097/ICO.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 8.Dou R, Ng K, Giovannucci EL, et al. Vitamin D and colorectal cancer: Molecular, epidemiological and clinical evidence. Br J Nutr. 2016;115:1643–60. doi: 10.1017/S0007114516000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosen Y, Daich J, Soliman I, et al. Vitamin D and autoimmunity. Scand J Rheumatol. 2016;45(6):439–47. doi: 10.3109/03009742.2016.1151072. [DOI] [PubMed] [Google Scholar]
- 10.Yun BH, Chon SJ, Choi YS, et al. The effect of prolonged breast-feeding on the development of postmenopausal osteoporosis in population with insufficient calcium intake and vitamin D level. Osteoporos Int. 2016;27:2745–53. doi: 10.1007/s00198-016-3585-8. [DOI] [PubMed] [Google Scholar]
- 11.Bjorkhem-Bergman L, Bergman P. Vitamin D and patients with palliative cancer. BMJ Support Palliat Care. 2016;6:287–91. doi: 10.1136/bmjspcare-2015-000921. [DOI] [PubMed] [Google Scholar]
- 12.Mitra S, Nayak PK, Agrawal S, et al. Vitamin D status and cardio-metabolic risk in Indian postmenopausal women. J Clin Diagn Res. 2016;10(3):QC17–20. doi: 10.7860/JCDR/2016/17839.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kongsbak M, Levring TB, Geisler C, von Essen MR. The vitamin d receptor and T cell function. Front Immunol. 2013;4:148. doi: 10.3389/fimmu.2013.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Curiel I, Marin-Luevano P, Trujillo V, et al. Calcitriol prevents inflammatory gene expression in macrovascular endothelial cells. Br J Biomed Sci. 2016;73:74–78. doi: 10.1080/09674845.2016.1162376. [DOI] [PubMed] [Google Scholar]
- 15.de Braganca AC, Volpini RA, Canale D, et al. Vitamin D deficiency aggravates ischemic acute kidney injury in rats. Physiol Rep. 2015;3(3) doi: 10.14814/phy2.12331. pii: e12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reins RY, Hanlon SD, Magadi S, McDermott AM. Effects of topically applied vitamin D during corneal wound healing. PLoS One. 2016;11:e0152889. doi: 10.1371/journal.pone.0152889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin Z, Pintea V, Lin Y, et al. Vitamin D enhances corneal epithelial barrier function. Invest Ophthalmol Vis Sci. 2011;52(10):7359–64. doi: 10.1167/iovs.11-7605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shetty R, Sethu S, Deshmukh R, et al. Corneal dendritic cell density is associated with subbasal nerve plexus features, ocular surface disease index, and serum vitamin D in evaporative dry eye disease. BioMed Res Int. 2016;2016:4369750. doi: 10.1155/2016/4369750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jee D, Kang S, Yuan C, et al. Epidemiologic Survey Committee of the Korean Ophthalmologic Society. Serum 25-hydroxyvitamin D levels and dry eye syndrome: differential effects of vitamin D on ocular diseases. PLoS One. 2016;11:e0149294. doi: 10.1371/journal.pone.0149294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoon SY, Bae SH, Shin YJ, et al. Low Serum 25-hydroxyvitamin D levels are associated with dry eye syndrome. PloS One. 2016;11:e0147847. doi: 10.1371/journal.pone.0147847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtul BE, Ozer PA, Aydinli MS. The association of vitamin D deficiency with tear break-up time and Schirmer testing in non-Sjogren dry eye. Eye. 2015;29:1081–84. doi: 10.1038/eye.2015.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Generali E, Cantarini L, Selmi C. Ocular involvement in systemic autoimmune diseases. Clin Rev Allergy Immunol. 2015;49:263–70. doi: 10.1007/s12016-015-8518-3. [DOI] [PubMed] [Google Scholar]
- 23.Erten S, Sahin A, Altunoglu A, et al. Comparison of plasma vitamin D levels in patients with Sjogren’s syndrome and healthy subjects. Int J Rheum Dis. 2015;18:70–75. doi: 10.1111/1756-185X.12298. [DOI] [PubMed] [Google Scholar]
- 24.Azizieh F, Alyahya KO, Raghupathy R. Association between levels of vitamin D and inflammatory markers in healthy women. J Inflamm Res. 2016;9:51–57. doi: 10.2147/JIR.S103298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chirumbolo S. Vitamin D3 in cancer prevention and therapy: The nutritional issue. Horm Mol Biol Clin Investig. 2015;23(3):71–78. doi: 10.1515/hmbci-2015-0011. [DOI] [PubMed] [Google Scholar]
- 26.Hallak JA, Tibrewal S, Mohindra N, et al. Single nucleotide polymorphisms in the BDNF, VDR, and DNASE 1 genes in dry eye disease patients: A case-control study. Invest Ophthalmol Vis Sci. 2015;56(10):5990–96. doi: 10.1167/iovs.15-17036. [DOI] [PMC free article] [PubMed] [Google Scholar]