Abstract
Background
Prenatal exposure to organophosphate pesticides (OPs) has been associated with impaired child development. Pesticide exposure determinants need to be studied in order to identify sources and pathways of pesticide exposure. The aim of this paper is to describe prenatal exposure to OPs and evaluate the associated factors in pregnant women.
Methods
The study population consisted of pregnant women (n = 573) who participated in the INMA birth cohort study in Valencia (Spain, 2003–2006). OP metabolites were analyzed in maternal urine at the 32nd week of gestation using a liquid chromatography-high resolution mass spectrometry method. The analysis included non-specific (diethyl phosphate [DEP], diethyl thiophosphate [DETP], dimethyl thiophosphate [DMTP], dimethyl dithiophosphate [DMDTP]) and specific metabolites (2-diethylamino-6-methyl-4-pyrimidinol [DEAMPY], 2-isopropyl-4-methyl-6-hydroxypyrimidine [IMPY], para-nitrophenol [PNP], and 3,5,6-trichloro-2-pyridinol [TCPY]). Information about the sociodemographic, environmental, and dietary characteristics was obtained by questionnaire. The association between log-transformed OPs and covariates was analyzed using multivariable interval censored regression.
Results
The detection frequencies were low, DMTP and TCPY being the most frequently detected metabolites (53.8% and 39.1%, respectively). All the OP metabolites were positively associated with maternal intake of fruits and vegetables. Other maternal characteristics related to the OPs were body mass index (BMI) before pregnancy and smoking habit during pregnancy. Women with lower BMI and those who did not smoke presented higher OP concentrations. Moreover, mothers who had a yard or garden with plants at home or who lived in an urban area were also more exposed to OPs.
Conclusions
The OP detection frequencies and the concentrations observed in our study population were low, compared with most of the previously published studies. Given the high vulnerability of the fetus to neurotoxicant exposure, further research on the determinants of the body burden of OPs during pregnancy would be necessary. The knowledge gained from such studies would enhance the effectiveness of public health control and future recommendations in order to reduce the risk to both the health of pregnant women and the health and development of their children.
Electronic supplementary material
The online version of this article (doi:10.1186/s12940-017-0255-z) contains supplementary material, which is available to authorized users.
Keywords: Pesticides, Diet, Vegetables, Fruit, Agriculture, Development
Background
Pesticides are widely used in the agricultural areas of Spain. The sales of these products over the last decade have exceeded 600 million euros annually, thus accounting for 10% of the total sales in Europe [1]. The Valencia Region is the second largest agricultural area in Spain and one of the largest pesticide users in this country. This region was responsible for more than 12% of the total national pesticide consumption in 2009 [2]. The organophosphate pesticides (OPs) are the most widely used active substance in insecticides, followed by pyrethroids and carbamates [3]. As a result of their massive use, especially chlorpyrifos, OPs spread through the environment and contaminate water, soil, and atmosphere, resulting in a potential risk to humans and the environment [4].
Pesticides are also used in domestic settings, pyrethroids being the most common active ingredient used in residential insecticides [5]. However, some OPs, such as chlorpyrifos, were commonly found in the composition of domestic insecticides in Spain [6]. As of 2008, the use of chlorpyrifos as a domestic pesticide was phased out in the EU (Directive 98/8/CE).
Prenatal exposure to OPs was first described in three birth cohort studies conducted in USA. Chlorpyrifos was detected in cord blood samples from newborns participating in the Columbia Center for Children’s Environmental Health in New York, showing a high correlation with maternal blood, which may indicate that this pesticide crosses the placenta barrier [7]. Urinary levels of some OP metabolites have also been measured among pregnant women from the Children’s Environmental Health Study in New York [8] and from the Health Assessment of Mothers and Children of Salinas (CHAMACOS study), an agricultural area in California [9]. These latter studies found detectable levels of different OP metabolites in nearly all urine samples.
There is increasing public concern about the effects associated to the exposure to pesticides during early development [10]. Fetuses and children are especially more vulnerable to exposure to environmental pollutants in comparison to adults, since their organs and systems are still developing and their detoxification mechanisms are not yet fully mature [11]. There is increasing evidence of a relationship between prenatal exposure to OPs and child neurodevelopment [12]. In addition, OPs have also been linked in epidemiology studies to shorter time of gestation [13, 14], low birth weight [15, 16], increased child blood pressure [17], respiratory outcomes [18], obesity and diabetes [19, 20]. To date, very few longitudinal studies have investigated factors associated with pesticide exposure in pregnant women [8, 21–24].
The aim of this study is to describe prenatal exposure to OPs and evaluate the associated factors in pregnant women participating in a birth cohort study located in a Spanish area with intense agricultural activity. Studies on pesticide exposure determinants are needed to identify sources and pathways of pesticide exposure and to contribute to policies aimed at reducing exposure.
Methods
Study population
This study is framed within the INfancia y Medio Ambiente (INMA) Project (Environment and Childhood), the aim of which is to investigate the effects of environmental exposure, diet, and genetics on fetal and child development in a cohort of pregnant women and their offspring in different regions of Spain (http://www.proyectoinma.org/). The study protocol has been reported elsewhere [25]. Briefly, pregnant women were recruited at the beginning of their pregnancy (10–13 weeks of gestation) in the region of Valencia (2003–2005, n = 855). These women were followed up until the third trimester of pregnancy (n = 794). The final study population consisted of 573 pregnant women with complete data on OP exposure. The main reason for the decrease in the study population was the unavailability of urine samples for OP measurement (n = 122) and the limited resources for the analysis of OPs in all available samples (n = 99).
Analysis of OP metabolites in urine
Urine samples were collected at the third trimester of pregnancy (mean ± sd: 32.2 ± 1.4 weeks of gestation). Samples were separated into aliquots of 10 mL and then frozen at −20 °C until analysis in the Public Health Laboratory of Valencia. One aliquot was used for the analytical determination of metabolites in urine by using an ultra-high pressure liquid chromatography coupled with high resolution mass spectrometry method (UPLC-HRMS) [26]. Briefly, metabolites from hydrolyzed urine were extracted by liquid-liquid extraction with 10 ml of acetonitrile followed by a QuEChERS extraction [27]. The acetonitrile layer obtained was evaporated to dryness, dissolved in 200 μL of methanol: water (10/90, v/v) containing 0.1% of acetic acid, ultra-centrifuged (11,000 rpm, 3 min and 10 °C) and transferred into an injection vial for analysis on an Accela liquid chromatography UHPLC system from ThermoFisher Scientific (Bremen, Germany). Separation was performed by using a Hypersil Gold column (100 × 116 2.1 mm, 1.9 μ) with a flow rate of 400 μL min−1 and an injection volume of 10 μL. Mobile phase components were 0.1% acetic acid aqueous solution (A) and methanol containing 0.1% acetic acid (B). Targeted mass analysis was performed on an Orbitrap mass spectrometer Exactive™ analyzer (Thermo Scientific, Bremen, Germany) operating in both positive and negative ESI modes. The ion source parameters were 3.5 kV (positive mode) and 2.5 kV (negative mode); sheath gas flow-rate: 55; auxiliary gas flow-rate: 10; skimmer voltage: 23 V; heater temperature: 300 °C; capillary temperature: 150 °C; capillary voltage: 45 V, and tube lens voltage: 120 V. The system operated at a resolving power of 50,000 (250 ms). Each metabolite was identified and confirmed following the criteria of relative retention time (RRT), mass tolerance value of the molecular ion <5 ppm, isotopic pattern, and fragment ions.
The analysis included four non-specific metabolites of exposure to OPs (Table 1): diethyl phosphate (DEP), diethyl thiophosphate (DETP), dimethyl thiophosphate (DMTP), dimethyl dithiophosphate (DMDTP), and the four specific metabolites: 2-diethylamino-6-methyl-4-pyrimidinol (DEAMPY), 2-isopropyl-4-methyl-6-hydroxypyrimidine (IMPY), para-nitrophenol (PNP), and 3,5,6-trichloro-2-pyridinol (TCPY). Sum (molar) variables were also calculated: sumDEP: DEP + DETP, sumDMP: DMTP + DMDTP, sumDAP: sumDEP + sumDMP, and sumOPs: DEAMPY + IMPY + PNP + TCPY.
Table 1.
OP metabolites analyzed and possible precursor compounds
| Possible precursor compound | Metabolite | Acronym | LOD (μg/L) |
|---|---|---|---|
| Chlorethoxyphos, chlorpyrifos, coumaphos, diazinon, disulfoton, ethion, parathion, phorate, phosalone, sulfotep, terbufos | Diethyl phosphate | DEPa | 10 |
| Diethyl thiophosphate | DETPa | 3.2 | |
| Azinphos-methyl, dichlorvos, dicrotophos, dimethoate, fenitrothion, fenthion, malathion, methyl parathion, trichlorfon, chlorpyrifos-methyl, methidathion, mevinphos, oxydemeton-methyl, phosmet, primiphos-methyl, temephos, tetrachlorvinphos, isazofos-methyl, naled | Dimethyl thiophosphate | DMTPa | 1.6 |
| Dimethyl dithiophosphate | DMDTPa | 1.6 | |
| Chlorpyrifos, chlorpyrifos-methyl | 3,5,6-trichloro-2-pyridinol | TCPYb | 0.8 |
| Parathion, methyl parathion | p-nitrophenol | PNPb | 0.8 |
| Pirimiphos-methyl | 2-diethylamino-6-methyl-4-pyrimidinol | DEAMPYb | 1.6 |
| Diazinon | 2-isopropyl-4-methyl-6-hydroxypyrimidine | IMPYb | 1.6 |
LOD Limit of determination
aNon-specific compound
bSpecific compound
The analytical suitability of the method was checked each working day through the analysis of the quality controls previously described by Roca et al. [26]. In accordance with this quality procedure, in each analytical batch, various quality control samples (QC) were prepared by spiking blank urine samples at LoQ, QCinter, and QChigh levels to calculate the extraction efficiency and ensure a good quantification of real samples. The QCs were subjected to the same extraction and analysis procedures as real samples and calibration curve points.
OP concentrations were expressed in μg/L and in μg/g of creatinine in order to correct for urinary dilution. Creatinine was determined by the Jaffé method (kinetic with target measurement, compensated method) with Roche reagents in a Hitachi 911 auto-analyzer.
Covariates
The women filled in two questionnaires during pregnancy, one at the first trimester (mean ± sd: 12.1 ± 1.5 weeks of gestation) and the other at the third trimester (mean ± sd: 31.8 ± 1.7 weeks of gestation). The questionnaires were administered by trained interviewers and focused on sociodemographic, environmental, and lifestyle information during pregnancy. The maternal covariates obtained were maternal age (years), country of birth (Spain, other), educational level (up to primary, secondary, university), body mass index before pregnancy (BMI, kg/m2), parity (0, 1, >1), employment status during pregnancy (employed, not employed), smoking habit during pregnancy (smoker, non-smoker), season of sampling (winter, spring, summer, fall), area of residence (urban, metropolitan, semi-urban, rural), use of indoor pesticides (no, yes), yard with plants at home (no, yes), outdoor pesticides application (no, yes), residence near fields or greenhouses (no, residence near fields, residence near fields sprayed with pesticides). We also obtained information about the paternal or maternal occupation and identified those related to the use of pesticides (agriculture, gardening).
We defined social class based on the maternal or paternal occupation during pregnancy with the highest social class, according to a widely used Spanish adaptation of the coding system of the International Standard Classification of Occupations approved in 1988 (ISCO88) [28]. Class I + II included managerial jobs, senior technical staff, and commercial managers; class III included skilled non-manual workers; and class IV + V, manual workers.
The Valencia Region was divided into four sub-areas according to the population density and land uses: urban, metropolitan, semi-urban, and rural.
Dietary information was collected by using a validated semi-quantitative food frequency questionnaire (FFQ) of 101 food items [29]. The FFQ was administered during the third trimester of pregnancy (the same time point as the general questionnaire) and covered average intakes from the previous 3 months. Information about the intake of different fruits and vegetables was obtained and grouped as follows (expressed as daily servings): citrus fruits (orange and orange juice), stone fruits (prune, peach, nectarine, and apricot), kiwis, apples and pears, bananas, watermelon, fruit vegetables (courgette, aubergine, cucumber, tomato, and pepper), green vegetables, (lettuce, spinach, chard, and cabbage), other vegetables (green beans, onion, carrot, and pumpkin). Total fruit and vegetable intakes were also calculated.
Statistical analysis
Maximum likelihood estimation of censored linear regression models was used to estimate the geometric mean (GM) and 95% confidence intervals (95%CI) for OP metabolites [30, 31]. The compounds were log-transformed so as to approach normality. This scheme also applies to molar summed variables. The OP metabolite concentrations were expressed as μg/g of creatinine, as μg/L, and as molar (nm/g of creatinine and nm/L). All individual compounds were included in the definition of sum variables, although, when studied separately, we only considered those with a detection frequency > 30%.
The association between OP metabolite concentrations and the sociodemographic, environmental, and dietary characteristics was evaluated by multivariable interval censored regression models in order to deal with values below the LOD. These models were built using a backward elimination procedure: first, all the covariates related to the OP metabolite concentrations at the univariate level (p < 0.1) were included in the initial models. Then, those variables not associated with the OP metabolite concentrations in the multivariable model using the Likelihood Ratio Test (p > 0.1) were sequentially excluded. For comparability purposes, final models were adjusted for all the covariates that were retained for at least one of the compounds. For this analysis the unadjusted OP concentrations were used including creatinine as a covariate.
In a further analysis, total fruit and vegetable intake variables were replaced by the subgroup dietary variables (citrus fruits, stone fruits, kiwis, apples and pears, bananas, watermelon, fruit vegetables, green vegetables, other vegetables) in order to evaluate their influence on OP metabolite concentrations.
Results
Sociodemographic, environmental, and dietary characteristics of the study population are shown in Table 2. Nearly all the participants were Spaniards, 25% of them had finished university studies, around 80% worked during pregnancy, and nearly half of them belonged to the lowest social class. More than 60% of the women used domestic pesticides at home during pregnancy. Only 16 of the fathers and none of the mothers had an occupation related with the use of pesticides (agriculture or gardening).
Table 2.
Sociodemographic, environmental, and dietary characteristics of the study population, INMA-Valencia, Spain, 2003–2006
| Study population (n = 573) | Population not included (n = 221) | P-value1 | ||||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Maternal age (years) | <25 | 60 | 10.5 | 31 | 14 | 0.216 |
| 25–29 | 196 | 34.2 | 85 | 38.5 | ||
| 30–34 | 230 | 40.1 | 75 | 33.9 | ||
| ≥35 | 87 | 15.2 | 30 | 13.6 | ||
| Country of birth | Spain | 509 | 88.8 | 189 | 85.5 | 0.200 |
| Other | 64 | 11.2 | 32 | 14.5 | ||
| Educational level | Up to primary | 182 | 31.8 | 88 | 39.8 | 0.069 |
| Secondary | 248 | 43.3 | 90 | 40.7 | ||
| University | 143 | 25.0 | 43 | 19.5 | ||
| Parity | 0 | 324 | 56.5 | 114 | 51.6 | 0.002 |
| 1 | 212 | 37.0 | 75 | 33.9 | ||
| >1 | 37 | 6.5 | 32 | 14.5 | ||
| Employment status during pregnancy | Non-employed | 101 | 17.6 | 38 | 17.2 | 0.886 |
| Employed | 472 | 82.4 | 183 | 82.8 | ||
| Social classc | I + II (higher) | 137 | 23.9 | 37 | 16.7 | 0.021 |
| III | 161 | 28.1 | 55 | 24.9 | ||
| IV + V (lower) | 275 | 48.0 | 129 | 58.4 | ||
| Smoking habit during pregnancy | Non-smoker | 447 | 78.0 | 159 | 74.3 | 0.271 |
| Smoker | 126 | 22.0 | 55 | 25.7 | ||
| Season of sampling | Winter | 73 | 12.7 | 42 | 29.8 | <0.001 |
| Spring | 106 | 18.5 | 41 | 29.1 | ||
| Summer | 199 | 34.7 | 21 | 14.9 | ||
| Fall | 195 | 34.0 | 37 | 26.2 | ||
| Area of residence | Urban | 56 | 9.8 | 15 | 6.8 | 0.629 |
| Metropolitan | 276 | 48.2 | 111 | 50.7 | ||
| Semi-urban | 206 | 36.0 | 79 | 36.1 | ||
| Rural | 35 | 6.1 | 14 | 6.4 | ||
| Use of indoor pesticides | No | 212 | 37.1 | 72 | 33.6 | 0.375 |
| Yes | 360 | 62.9 | 142 | 66.4 | ||
| Yard with plants at home | No | 393 | 68.7 | 159 | 74.3 | 0.127 |
| Yes | 179 | 31.3 | 55 | 25.7 | ||
| Outdoor pesticides application | No | 487 | 85.1 | 187 | 87.4 | 0.423 |
| Yes | 85 | 14.9 | 27 | 12.6 | ||
| Residence near fields or greenhouses | No | 315 | 55.1 | 131 | 61.2 | 0.097 |
| Residence near fields | 164 | 28.7 | 45 | 21 | ||
| Residence near fields sprayed with pesticides | 93 | 16.3 | 38 | 17.8 | ||
| BMI before pregnancya,b | 23.8 | 4.5 | 23.7 | 5.0 | 0.263 | |
| Intake of vegetables (daily servings)a,b | 2.2 | 1.3 | 2.2 | 1.3 | 0.991 | |
| Intake of fruits (daily servings)a,b | 2.5 | 1.7 | 2.6 | 1.8 | 0.369 | |
1 P-value from Chi-square test
amean and standard deviation
b p-values from Mann-Whitney test
cClass I + II included managerial jobs, senior technical staff, and commercial managers; class III included skilled non-manual workers; and class IV + V, manual workers
Differences between the study population (n = 573) and the excluded women (those who arrived at the third trimester visit but for whom OP metabolite measurements are unavailable, n = 221) were assessed. We observed statistically significant differences according to the parity, social class, and season of sampling. Among the excluded women there was a higher proportion of parity > 1, lower social class, and urine samples taken during winter.
The frequencies of detection of OP metabolites were low, in fact only DMTP and TCPY were detected in more than 30% of the samples. The GM of the concentrations ranged from 0.02 μg/g of creatinine for IMPY and DEAMPY to 2.76 μg/g of creatinine for DEP. Regarding the sum variables, the GM of the concentrations ranged from 3.07 μg/g of creatinine for sumDEP to 16.29 μg/g of creatinine for sumDAP (Table 3).
Table 3.
Urinary OP concentrations (creatinine adjusted and unadjusted and molar) in pregnant women from INMA-Valencia cohort (Spain, 2003–2006, n = 573)
| Creatinine adjusted (μg/g) | Unadjusted (μg/L) | Molar concentrations creatinine adjusted (nm/g) | Molar concentrations (nm/L) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N > LOD | % > LOD | Min | Max | GM | 95% CI | Min | Max | GM | 95% CI | GM | 95% CI | GM | 95% CI | |||||
| DEP | 50 | 8.7 | <LOD | 66.4 | 1.04 | 0.54 | 2.00 | <LOD | 71.2 | 1.73 | 1.05 | 2.85 | 6.66 | 3.55 | 12.47 | 11.23 | 6.81 | 18.52 |
| DETP | 43 | 7.5 | <LOD | 56.5 | 0.09 | 0.03 | 0.27 | <LOD | 32.0 | 0.22 | 0.1 | 0.49 | 0.60 | 0.21 | 1.68 | 1.29 | 0.57 | 2.91 |
| DMTP | 308 | 53.8 | <LOD | 1023.0 | 2.76 | 2.23 | 3.42 | <LOD | 940.4 | 2.39 | 1.93 | 2.97 | 19.45 | 15.69 | 24.11 | 16.83 | 13.56 | 20.89 |
| DMDTP | 88 | 15.4 | <LOD | 189.5 | 0.06 | 0.03 | 0.14 | <LOD | 467.2 | 0.07 | 0.03 | 0.14 | 0.50 | 0.24 | 1.07 | 0.42 | 0.19 | 0.91 |
| DEAMPY | 32 | 5.6 | <LOD | 394.3 | 0.02 | 0.01 | 0.09 | <LOD | 293.0 | 0.03 | 0.01 | 0.12 | 0.14 | 0.04 | 0.56 | 0.19 | 0.05 | 0.67 |
| IMPY | 67 | 11.7 | <LOD | 787.5 | 0.02 | 0.01 | 0.07 | <LOD | 744.2 | 0.03 | 0.01 | 0.07 | 0.19 | 0.07 | 0.54 | 0.17 | 0.06 | 0.48 |
| PNP | 56 | 9.8 | <LOD | 28.7 | 0.04 | 0.02 | 0.09 | <LOD | 24.0 | 0.04 | 0.02 | 0.09 | 0.33 | 0.15 | 0.73 | 0.29 | 0.12 | 0.66 |
| TCPY | 224 | 39.1 | <LOD | 142.3 | 0.57 | 0.45 | 0.72 | <LOD | 117.3 | 0.49 | 0.39 | 0.62 | 2.88 | 2.29 | 3.62 | 2.47 | 1.95 | 3.13 |
| sumDEP | 76 | 13.3 | 6.6 | 98.2 | 3.07 | 2.21 | 4.27 | 6.6 | 78.0 | 4.72 | 3.76 | 5.93 | 19.57 | 14.11 | 27.13 | 29.78 | 23.71 | 37.42 |
| sumDMP | 314 | 54.8 | 1.6 | 1023.8 | 4.84 | 4.05 | 5.77 | 1.6 | 1015.8 | 4.2 | 3.52 | 5.02 | 32.79 | 27.44 | 39.19 | 28.48 | 23.81 | 34.07 |
| sumDAP | 337 | 58.8 | 8.2 | 1030.4 | 16.29 | 14.67 | 18.09 | 8.2 | 1022.4 | 14.64 | 13.25 | 16.18 | 107.25 | 96.45 | 119.27 | 96.15 | 86.87 | 106.42 |
| sumOP | 283 | 49.4 | 2.4 | 789.1 | 3.83 | 3.36 | 4.36 | 2.4 | 745.8 | 3.44 | 3.03 | 3.91 | 22.41 | 19.69 | 25.51 | 20.18 | 17.78 | 22.91 |
The detection frequency for the summed variables was defined as the proportion of individuals with at least one detected compound
DEP Diethyl phosphate; DETP Diethyl thiophosphate; DMTP Dimethyl thiophosphate; DMDTP Dimethyl dithiophosphate; DEAMPY 2-diethylamino-6-methyl-4-pyrimidinol; IMPY 2-isopropyl-4-methyl-6-hydroxypyrimidine; PNP para-nitrophenol; TCPY 3,5,6-trichloro-2-pyridinol; sumDEP DEP + DETP; sumDMP DMTP + DMDTP; sumDAP sumDEP + sumDMP; sumOP DEAMPY + IMPY + PNP + TCPy
The limit of determination was 10 μg/L for DEP, 2.3 μg/L for DETP, 1.6 μg/L for DMTP, 1.6 μg/L for DMDTP, 0.8 μg/L for TCPY, 0.8 μg/L for PNP, 1.6 μg/L for DEAMPY, and 1.6 μg/L for IMPY.
LOD limit of determination
Min minimum value
Max maximum value
GM geometric mean
The characteristics of the study population related to the OP metabolite concentrations can be found in Table 4. Smoking habit was significantly statistically associated with DMTP concentrations, women who smoked during pregnancy being the ones who presented lower levels. The pattern with the other compounds was similar but not significant. We observed that women who smoked during pregnancy consumed less fruit (mean = 2.24; standard deviation [sd] = 1.29 daily servings) than non-smoker women (mean = 2.58; sd = 1.62 daily servings, p-value Mann Whitney test =0.001). Smokers also consumed fewer vegetables than non-smokers (mean = 2.22; sd = 1.42 vs. mean = 2.24; sd = 1.29 daily servings), but the differences were not statistically significant (Additional file 1: Table S1).
Table 4.
Sociodemographic, environmental, and dietary characteristics associated with the OP concentrations. INMA-Valencia cohort (Spain, 2003–2006)a
| DMTP | TCPY | sumDEP | sumDMP | sumDAP | sumOP | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| beta | 95% CI | beta | 95% CI | beta | 95% CI | beta | 95% CI | beta | 95% CI | beta | 95% CI | ||||||||
| Smoking habit | No | ||||||||||||||||||
| Yes | −1.22** | −1.93 | −0.51 | −0.13 | −0.78 | 0.53 | 0.19 | −0.19 | 0.56 | −0.23 | −0.76 | 0.31 | −0.53 | −0.87 | −0.19 | −0.02 | −0.42 | 0.39 | |
| Level of education | Primary | ||||||||||||||||||
| Secondary | −0.40 | −1.05 | 0.26 | 0.36 | −0.27 | 0.99 | 0.00 | −0.45 | 0.45 | −0.23 | −0.76 | 0.31 | −0.07 | −0.39 | 0.24 | 0.38* | −0.01 | 0.77 | |
| University | 0.27 | −0.48 | 1.02 | 0.36 | −0.38 | 1.09 | 0.10 | −0.29 | 0.49 | 0.31 | −0.30 | 0.93 | 0.13 | −0.24 | 0.49 | 0.25 | −0.21 | 0.71 | |
| Season of sampling | Winter | ||||||||||||||||||
| Spring | −0.88* | −1.90 | 0.14 | 2.31** | 1.15 | 3.46 | −0.52* | −1.13 | 0.09 | −0.55 | −1.39 | 0.29 | −0.25 | −0.74 | 0.24 | 0.89** | 0.26 | 1.53 | |
| Summer | 0.58 | −0.33 | 1.48 | 3.26** | 2.17 | 4.35 | −0.31 | −0.83 | 0.22 | 0.65* | −0.10 | 1.39 | 0.27 | −0.16 | 0.71 | 1.15** | 0.56 | 1.73 | |
| Fall | 0.74 | −0.16 | 1.64 | 2.74** | 1.64 | 3.83 | 0.27 | −0.21 | 0.76 | 0.64* | −0.10 | 1.39 | 0.32 | −0.11 | 0.76 | 0.74** | 0.15 | 1.33 | |
| Zone of residence | Urban | ||||||||||||||||||
| Metropolitan | −0.75 | −1.69 | 0.18 | 0.15 | −0.76 | 1.05 | −0.56** | −1.06 | −0.05 | −0.60 | −1.37 | 0.16 | −0.37 | −0.82 | 0.07 | 0.02 | −0.54 | 0.58 | |
| Semi-urban | −0.81 | −1.80 | 0.18 | −0.46 | −1.43 | 0.51 | −0.15 | −0.66 | 0.36 | −0.63 | −1.44 | 0.19 | −0.22 | −0.70 | 0.25 | −0.30 | −0.90 | 0.30 | |
| Rural | −0.58 | −2.01 | 0.84 | −0.82 | −2.25 | 0.62 | −0.33 | −1.11 | 0.44 | −0.38 | −1.55 | 0.79 | −0.15 | −0.83 | 0.53 | −0.24 | −1.11 | 0.63 | |
| Yard with plants at home | No | ||||||||||||||||||
| Yes | 1.00** | 0.23 | 1.76 | 0.64* | −0.10 | 1.38 | −0.11 | −0.57 | 0.35 | 0.76** | 0.13 | 1.39 | 0.24 | −0.13 | 0.61 | 0.16 | −0.30 | 0.62 | |
| Application of outdoor pesticides | No | ||||||||||||||||||
| Yes | −0.65 | −1.62 | 0.31 | 0.02 | −0.91 | 0.94 | 0.55** | 0.01 | 1.10 | −0.55 | −1.34 | 0.25 | −0.12 | −0.59 | 0.34 | 0.11 | −0.47 | 0.68 | |
| Residence near fields or greenhouses | No | ||||||||||||||||||
| Residence near fields | −0.18 | −0.82 | 0.46 | −0.22 | −0.84 | 0.40 | −0.11 | −0.48 | 0.27 | −0.22 | −0.75 | 0.30 | −0.15 | −0.46 | 0.16 | 0.05 | −0.33 | 0.44 | |
| Residence near fields sprayed with pesticides | 0.26 | −0.53 | 1.05 | 0.07 | −0.71 | 0.84 | 0.07 | −0.37 | 0.50 | 0.12 | −0.53 | 0.77 | 0.07 | −0.31 | 0.45 | 0.46* | −0.01 | 0.93 | |
| Fruit intake (daily servings) | 0.26** | 0.04 | 0.47 | 0.05 | −0.15 | 0.26 | 0.20** | 0.09 | 0.31 | 0.21** | 0.03 | 0.38 | 0.14** | 0.03 | 0.24 | 0.13* | 0.00 | 0.26 | |
| Vegetables intake (daily servings) | 0.26** | 0.09 | 0.43 | 0.16* | 0.00 | 0.32 | 0.08 | −0.01 | 0.17 | 0.23** | 0.10 | 0.37 | 0.12** | 0.04 | 0.20 | 0.08 | −0.02 | 0.19 | |
| BMI before pregnancy | −0.36** | −0.67 | −0.05 | −0.39 | −0.70 | −0.08 | 0.02 | −0.15 | 0.19 | −0.32** | −0.58 | −0.07 | −0.16** | −0.31 | −0.01 | −0.16* | −0.35 | 0.02 | |
BMI Body mass index; CI Confidence intervals; DMTP Dimethyl thiophosphate; TCPy 3,5,6-trichloro-2-pyridinol; SumDEP Sum of diethylphosphates (DEP + DETP); SumDMP Sum of dimethylphosphates (DMTP + DMDTP); SumDAP Sum of dialkyl phosphates (SumDEP + SumDMP); SumOP Sum of specific compounds (DEAMPY + IMPY + PNP + TCPY)
aAll models were adjusted by smoking habit, educational level, season of sampling, zone of residence, yard with plants at home, application of outdoor pesticides, residence near fields or greenhouses, fruit intake, vegetable intake, BMI before pregnancy, and creatinine
*p < 0.1
**p < 0.05
Women with university studies had the lowest concentrations of sumDAP. Season of urine sampling was associated with nearly all the compounds, samples taken during summer or fall being those which had the highest concentrations. Women who lived in an urban zone had higher OP metabolites, as did women who lived near fields or greenhouses sprayed with pesticides. Women who had a yard or garden with plants at home had the highest concentrations of DMTP, TCPy, and sumDMP, and women who applied outdoor pesticides had the highest concentrations of sumDEP. The intake of fruit and vegetables during the third trimester of pregnancy was positively associated with the OP metabolite concentrations, and the BMI before pregnancy was inversely associated with nearly all the compounds. We analyzed the differences in the intake of fruits and vegetables according to the maternal BMI and observed that women with BMI > 25 Kg/m2 consumed more vegetables (2.35 weekly servings for women with BMI = 25–30 Kg/m2 and 2.30 weekly servings for women with BMI ≥ 30 Kg/m2) than women with BMI < 25 Kg/m2 (2.19 weekly servings), but differences were not statistically significant (p-value Kruskal Wallis test = 0.416) (Additional file 1: Table S1).
Women whose partners had an occupation related with the use of pesticides had higher levels of TCPY (mean [sd] = 7.2 [15.3] μg/g of creatinine) than those whose partners did not work with pesticides (mean [sd] = 2.8 [8.1] μg/g of creatinine, p-value Mann Whitney test = 0.046).
The relationship between the intake of specific fruits and vegetables with the OP metabolite concentrations has been also evaluated (Fig. 1). The intake of green vegetables during pregnancy was significantly and positively associated with all the compounds, except for TCPy. Other groups of fruits and vegetables also associated with a considerable number of compounds (>3) were stone fruits, kiwis, apples and pears, and fruit vegetables. TCPy was the OP metabolite least associated with the intake of fruits and vegetables, and sumDAP was the OPs metabolite group associated with the intake of the highest number of different fruits and vegetables.
Fig. 1.
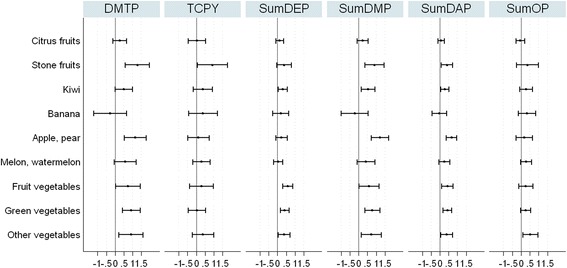
Association between the intake of different types of fruits and vegetables during pregnancy and the OP concentrations. All models were adjusted by smoking habit, educational level, season of sampling, zone of residence, yard with plants at home, application of outdoor pesticides, residence near fields or greenhouses, fruit intake, vegetable intake, body mass index before pregnancy, and creatinine.
Discussion
In this Spanish birth cohort study we assessed maternal exposure to OPs and evaluated the associated factors. The detection frequencies were low, DMTP and TCPY being the most frequently detected metabolites. The concentrations were positively associated with maternal intake of fruits and vegetables during pregnancy, especially the intake of green and fruit vegetables, stone fruits, kiwis, and apples and pears. Other maternal characteristics related to the OP metabolite concentrations were BMI before pregnancy and smoking habit during pregnancy. Women with lower BMI and those who did not smoke presented higher OP metabolite concentrations. Moreover, mothers who had a yard or garden with plants at home or lived in the urban area were also more exposed to OPs.
Other epidemiological studies have assessed the maternal exposure to OPs during pregnancy (Table 5). The first birth cohort studies where OP metabolites were analyzed in maternal urine were conducted in USA, one in New York City [8], and the other in an agricultural community in Salinas Valley, California [32, 33]. In both studies most of the metabolite concentrations were higher than in our study; DEP was the only one with concentrations a little higher in our study than in the USA cohorts. However, the detection frequency of this metabolite in our cohort was lower, probably due to a higher limit of detection. In our cohort the percentage of women using indoor pesticides (63%) was higher than in the New York cohort (46%), but urinary OP metabolite concentrations did not associate with this indoor use obtained by questionnaire. The possible reason could be related with differences in the time window of exposure reflected by both urinary metabolites and questionnaire. Whilst urinary biomarkers reflect acute or short-term exposure better, questionnaires may assess exposure during a period of time greater than the half-life of these substances. Another possible reason is that pyrethroids, but not OPs, were the main active substance found in the domestic insecticides [5], although some OPs, such as chlorpyrifos, diazinon, and fenitrothion were still allowed as domestic insecticides when urine samples were taken.
Table 5.
OP concentrations and associated factors among pregnant women observed in previous studies
| Study | Location | n | Metabolites measured | Levels | LOD | Factors studied | Factors associated with OPs |
|---|---|---|---|---|---|---|---|
| Berkowitz et al., 2003 [8] | New York, USA | 386 | TCPy | 11.3 μg/g a | 12 μg/L | Indoor pesticides use, maternal age, ethnicity, marital status, maternal education, season | Lower/middle school (−) |
| PCP | 7.3 μg/ga | 23.0 μg/L | - | ||||
| Castorina et al., 2003, 2010 [32, 33] | California, USA | 481 | DMP | 1.7 μg/La | 0.7 μg/L | - | - |
| DMTP | 6.3 μg/La | 0.5 μg/L | |||||
| DMDTP | 0.5 μg/La | 0.3 μg/L | |||||
| DEP | 1.1 μg/La | 0.3 μg/L | |||||
| DETP | 0.9 μg/La | 0.2 μg/L | |||||
| DEDTP | 0 μg/La, d | 0.1 μg/L | |||||
| PNP | 0.3 μg/La | 0.1 μg/L | |||||
| TCPy | 3.2 μg/La | 0.3 μg/L | |||||
| Ye et al., 2009 [34] | Oslo, Norway | 110 (pooled for analysis) | DMP | 12.97 μg/g (10.39 μg/L)b | 0.1 μg/L | - | - |
| DMTP | 13.21 μg/g (9.91 μg/L)b | 0.1 μg/L | |||||
| DMDTP | 0.98 μg/g (0.75 μg/L)b | 0.1 μg/L | |||||
| DEP | 2.54 μg/g (1.94 μg/L)b | 0.1 μg/L | |||||
| DETP | 1.51 μg/g (1.19 μg/L)b | 0.1 μg/L | |||||
| DEDTP | 0.02 μg/g (0.18 μg/L)b | 0.01 μg/L | |||||
| Total DAP | 31.23 μg/g (24.20 μg/L)b | ||||||
| TCPy | 2.95 μg/g (2.33 μg/L)b | 0.15 μg/L | |||||
| Rotterdam (Netherlands) | 100 | DMP | 19.78 μg/g (14.87 μg/L)b | 0.1 μg/L | |||
| DMTP | 19.42 μg/g (15.14 μg/L)b | 0.1 μg/L | |||||
| DMDTP | 0.85 μg/g (0.65 μg/L)b | 0.1 μg/L | |||||
| DEP | 5.02 μg/g (3.83 μg/L)b | 0.1 μg/L | |||||
| DETP | 2.98 μg/g (2.49 μg/L)b | 0.1 μg/L | |||||
| DEDTP | 0.12 μg/g (0.10 μg/L)b | 0.01 μg/L | |||||
| Total DAP | 48.18 μg/g (37.08 μg/L)b | ||||||
| TCPy | 4.38 μg/g (3.61 μg/L)b | 0.15 μg/L | |||||
| USA (NHANES) | 119 | DMP | 2.33 μg/g (3.22 μg/L)b | 0.5 μg/L | |||
| DMTP | 6.47 μg/g (7.09 μg/L)b | 0.4 μg/L | |||||
| DMDTP | 1.22 μg/g (1.50 μg/L)b | 0.2 μg/L | |||||
| DEP | 2.36 μg/g (2.38 μg/L)b | 0.1 μg/L | |||||
| DETP | 1.22 μg/g (1.59 μg/L)b | 0.1 μg/L | |||||
| DEDTP | 0.18 μg/g (0.17 μg/L)b | 0.1 μg/L | |||||
| Total DAP | 13.78 μg/g (16.96 μg/L)b | ||||||
| TCPy | 2.59 μg/g (2.77 μg/L)b | 0.4 μg/L | |||||
| Wang et al., 2012 [14] | Shanghai, China | 187 | DMP | 25.75 μg/g (17.19 μg/L)b | Nr | - | - |
| DMTP | 11.99 μg/g (8.01 μg/L)b | ||||||
| DEP | 9.03 μg/g (6.03 μg/L)b | ||||||
| DETP | 9.45 μg/g (6.31 μg/L)b | ||||||
| DEDTP | P25: 0.94 μg/g | ||||||
| Yolton et al., 2013 [21] | Cincinnati, USA | 100 | SumDEP | 9.4 (8–11.1) nmol/g creatininec | 0.6 μg/L for DMP, 0.2 μg/L for DMTP, 0.5 μg/L for DMDTP, 0.6 μg/L for DEP, 0.4 μg/L for DETP and 0.4 μg/L for DEDTP | sex, race, maternal education, marital status, maternal employment, fresh fruit and vegetable intake, organic use | black (−), not married living alone (−), higher education (+) and veg/fruit intake (+) |
| SumDMP | 46.4 (40.2–53.7) nmol/g creatininec | Black (−), higher education (+), not married (−), employed (+), veg/fruit intake (+), organic use (+) | |||||
| SumDAP | 73.5 (64.8–83.4) nmol/g creatininec | Black (−), higher education (+), not married (−), employed (+), veg/fruit intake (+), organic use (+) | |||||
| Fortenberry et al., 2014 [35] | Mexico DF, Mexico | 187 | TCPy | 1.76 (1.55. 2.02) μg/L c | 0.10 μg/L | - | - |
| Colapinto et al., 2015 [22] | Canada | 1850 | DMP | 3.19 μg/Lb | 1.0 μg/L | Maternal tea consumption | - |
| DMTP | 3.29 μg/Lb | 0.6 μg/L | |||||
| DMDTP | 0.48 μg/Lb | 0.3 μg/L | |||||
| DEP | 2.46 μg/Lb | 1.0 μg/L | |||||
| DETP | 0.67 μg/Lb | 0.3 μg/L | |||||
| DEDTP | <0.15 μg/L | 0.3 μg/L | |||||
| Forde et al., 2015 [36] | Caribbean Islands | 150 | DEP | 1.65 (1.39–1.97) μg/Lc | 1.0 μg/L | - | - |
| DETP | DF < 60% | 0.3 μg/L | |||||
| DMP | 1.60 (1.33–1.94) μg/L c | 1.0 μg/L | |||||
| Lewis et al., 2015 [23] | Puerto Rico | 54 | TCPy | 0.4 μg/Lb | 0.1 μg/L | Sociodemographic, consumption of fruits, vegetables, and legumes in the 48 h prior to urine collection, and home pest-related issues | Decreasing age (−), grape juice intake (+), raisins (+) |
| IMPY | <LOD | 0.1 μg/L | Decreasing age (−), married (−), insects inside home (−) | ||||
| PNP | 0.5 μg/Lb | 0.1 μg/L | Unemployed (+), collards intake (−), spinach intake (−) | ||||
| DEP | 0.9 μg/Lb | 0.5 μg/L | Decreasing age (−), collards intake (+), peanuts intake (+), pesticides applied by participant (+) | ||||
| DETP | 0.5 μg/Lb | 0.3 μg/L | Age (−), grape juice intake (+), raisins (+) | ||||
| DEDTP | <LOD | 0.1 μg/L | Cherries intake (+), pesticides stored at home (−) | ||||
| DMP | 1.4 μg/Lb | 0.5 μg/L | Married (+), grapes intake (−) | ||||
| DMTP | 0.8 μg/Lb | 0.1 μg/L | Married (+) | ||||
| DMDTP | 0.2 μg/Lb | 0.1 μg/L | Collards intake (+) | ||||
| Sokoloff et al., 2016 [24] | Canada | 1884 | DMP | 23 (22–24) nmol/L | 1.00 μg/L | Maternal and sampling characteristics, pesticide use and dwelling characteristics, vegetables and fruit consumption, grain products consumption | |
| DMTP | 20 (19–22) nmol/L | 0.60 μg/L | |||||
| DMDTP | 2 (2–2) nmol/L | 0.30 μg/L | |||||
| DEP | 14 (14–15) nmol/L | 1.00 μg/L | |||||
| DETP | 3 (3–3) nmol/L | 0.60 μg/L | |||||
| DEDTP | DF < 50% | 0.30 μg/L | |||||
| SumDMP | 52 (49–55) nmol/L | 0.30 μg/L | Education (+), parity (−), BMI (−), smokers (−), afternoon sample collection (+), in fasting status (−), sample collected in winter (+), consumption of sweet peppers (+), tomatoes (+), beans and dry beans (+), citrus fruits (+), apple juice (+), soy and rice beverages (+), cold cereal (+), white and whole grain bread (+) | ||||
| SumDEP | 19 (18–19) nmol/L | 0.30 μg/L | Education (+), household income (+), parity (−), BMI (−), smoker (−), sample collected in winter (+), consumption of citrus fruit (+), apple juice (+), pasta (−), | ||||
| SumDAP | 78 (74–82) nmol/L | 0.30 μg/L |
aMedian
bGM
cGM (95%CI)
dNo instrument response
(+) positive association with OPs
(−) negative association with OPs
Nr: not reported
DF: detection frequency
LOD: limit of dermination
Ye et al. [34] compared urinary OP metabolites among pregnant women from Norway, Netherlands, and USA. The OP metabolite concentrations were found to be higher in women from Rotterdam (Netherlands) than women from Oslo (Norway) and USA (NHANES) [34]. Regarding the comparison with our study, the metabolite concentrations observed in our study population were lower than those in the women from Norway, Netherlands, and USA. TCPy, DMTP, DEP, and DETP concentrations in our cohort were much lower than those reported for pregnant women from Shanghai (China) [14], Cincinnati (USA) [21], Mexico DF [35], and Canada [22, 24]. Similar concentrations of DEP and TCPy to those of our study were found in the Caribbean Islands [36] and Puerto Rico [23] studies, respectively, although the detection frequencies in our study were lower.
Maternal educational level was associated with TCPy concentrations in women from New York [8], women with lower/middle studies being the ones who had lower concentrations in comparison to women with a higher educational level. A similar pattern was observed in women from Cincinnati [21] and from Canada (Mirec Study) [24]: those with a higher education presented higher urinary concentrations of sumDEP and sumDMP. Other factors associated with exposure to OPs in the Cincinnati study were race, marital status, employment, and vegetable and fruit intake. Lower sumDEP, sumDMP, and sumDAP concentrations were observed among black, unmarried, and unemployed women. Similarly to our study, the intakes of vegetables and fruits were positively associated with OP metabolite concentrations. OP exposure in pregnant women from Puerto Rico was studied in relationship with their sociodemographic characteristics, consumption of specific vegetables and fruits 48 h prior to urine collection, and home pest-related issues [23]. Overall, OP metabolite concentrations were inversely associated with maternal age and positively associated with some vegetable and fruit items, such as raisins, collards, peanuts, grape juice, and cherries.
We observed that maternal BMI before pregnancy was inversely and consistently associated with nearly all the metabolites. This association has also been described in the Mirec Study [24], where BMI before pregnancy was inversely associated with both sumDMP and sumDEP. The authors commented that a possible explanation could be the awareness on the importance of good nutrition among women with BMI < 25 Kg/m2. In our study, we did not find statistically significant differences in the intake of vegetables and fruits according to the pre-pregnancy BMI. These results suggest that there could be another factor, maybe metabolic, that might be promoting the association between BMI and OP metabolites.
Another maternal characteristic related to the OP exposure in our study population was the place of residence. Women who lived in the urban area were more exposed to OPs. This finding was expected since Valencia is a city with a long agricultural tradition and the urban area is widely surrounded by fields with an intensive use of agriculture. In addition, women who lived near fields sprayed with pesticides presented higher sumOP concentrations. In fact, this variable has been considered a useful alternative to biomonitoring and a good proxy of OP concentrations [37].
This is one of the largest birth cohorts to evaluate prenatal exposure to OPs and to study the associated factors, and, as far as we know, the first one conducted in Spain. The longitudinal design of our study will allow evaluation of postnatal exposure and the possible associated effects on child development.
A limitation in this study could be that a single spot measurement may not adequately characterize exposure over the whole pregnancy, given that OPs have a fast clearance from the body. In fact, results from the CHAMACOS cohort showed impairment in children’s cognitive performance at 7 years old [38] associated to an average of two DAP metabolite measures during pregnancy, but not when the one-point-in-time measures were evaluated. We collected urine samples from all the INMA participants over two different periods during pregnancy, the first and third trimesters, but due to limited resources to measure OP metabolites in all the urine samples we only have one measurement. The average of two OP metabolite measurements would have probably been a more adequate proxy of prenatal exposure. Additionally, there was a long time lag between collection of samples and analysis. The low concentrations observed in this population could be related with this limitation; in fact, Hoppin et al. [39] observed that urinary TCPY concentrations decreased with the time elapsed since collection at room temperature; however, our samples were frozen at −20 °C immediately after collection and this drop in OP metabolite concentrations is expected not be so important. Our FFQ covered average intakes during the 3 months before the sampling, when the half-life of these compounds is shorter. In any case, we observed several statistical associations with the intake of some fruits and vegetables.
Another important consideration is the limitation of measuring OP metabolites instead of parent compounds. The metabolism of parent compounds results in the production of active oxon forms, responsible for the developmental neurotoxicity [40]. These oxon forms are metabolically converted to less toxic compounds (DAPs) via hydrolysis. However, the hydrolysis of parent compounds present in food and the environment also generates DAP metabolites that are not toxic when consumed [41–43]. Therefore urinary non-specific metabolites may represent exposure to parent pesticides and to preformed derivatives from food and the environment, and the measurement of DAP metabolites could overestimate the real exposure to parent compounds. In fact, Yolton et al. [21] observed that higher urinary concentrations of DE metabolites were associated with improved attention in infants. This positive association seems to be related to the DAP metabolites present in vegetables and fruits that could be acting as a proxy of the nutrients and antioxidants present in diet. Despite this limitation, DAP metabolites have been extensively used in epidemiological studies because these metabolites are common to the majority of OP pesticides, and laboratory methods are not available for pesticide-specific metabolites. Consequently, the non-specific DAPs provide valuable information about cumulative exposure to the OP class [44].
Finally, we were able to provide an estimate of the geometric mean for all metabolites using censored regression. However, this estimation could be imprecise due to the distributional assumptions of these models and the high proportion of undetected values.
Conclusions
In conclusion, the OP metabolite detection frequencies and the concentrations observed in our study population were low, compared with previously published studies. For TCPy, the metabolite of chlorpyrifos, the concentrations were low despite the fact that it was still allowed as a domestic pesticide in Spain when the urine sampling was performed. Some maternal characteristics were associated with prenatal OP exposure. More in-depth knowledge of the determinants of the body burden of OPs during pregnancy would enhance the effectiveness of public health control and future recommendations for decreasing the levels of these compounds, thus reducing the risk for the health of pregnant women and the health and development of their children.
Acknowledgements
The authors would particularly like to thank all the participants for their generous collaboration.
Funding
This study was supported by grants from the Instituto de Salud Carlos III [FIS-FEDER 13/1944, 13/2032, 14/0891, 14/1687, 16/1288 and Miguel Servet-FEDER CP15/0025]; Conselleria de Sanitat, Generalitat Valenciana (FISABIO UGP 15–230) and EU (FP7-ENV-2011 DENAMIC cod 282,957).
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to ethical concerns but are available from the corresponding author on reasonable request. All INMA questionnaires and protocols are available at http://www.proyectoinma.org/.
Authors’ contributions
Authors contributed to the article as follows: SL conceived the study, supervised the data collection and data analysis, and prepared the manuscript. MM conducted data analysis of the association of interest. MR and LLG participated in data collection. All authors provided critical revision of the manuscript and helped with data interpretation and manuscript preparation. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Informed consent was obtained from all participants and the study was approved by the Ethic Committees of the La Fe hospital (Valencia).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- BMI
Body mass index before pregnancy
- DEAMPY
2-diethylamino-6-methyl-4-pyrimidinol
- DEP
Diethyl phosphate
- DETP
Diethyl thiophosphate
- DMDTP
Dimethyl dithiophosphate
- DMTP
Dimethyl thiophosphate
- FFQ
Food frequency questionnaire
- GM
Geometric mean
- IMPY
2-isopropyl-4-methyl-6-hydroxypyrimidine
- INMA
INfancia y Medio Ambiente
- LOD
Limit of determination
- OPs
Organophosphate pesticides
- PBA
Phenoxybenzoic acid
- PCP
Pentachlorophenol
- PNP
Para-nitrophenol
- sumDAP
sumDEP + sumDMP
- sumDEP
DEP + DETP
- sumDMP
DMTP + DMDTP
- sumOP
DEAMPY + IMPY + PNP + TCPY
- TCPy
3,5,6-trichloro-2-pyridinol
- UPLC-HRMS
High resolution mass spectrometry
Additional file
Intake of fruit and vegetables according to the maternal sociodemographic and life style characteristics. (DOC 49 kb)
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s12940-017-0255-z) contains supplementary material, which is available to authorized users.
Contributor Information
Sabrina Llop, Phone: (+34) 961925941, Email: llop_sab@gva.es.
Mario Murcia, Email: Murcia_mur@gva.es.
Carmen Iñiguez, Email: inyiguez_car@gva.es.
Marta Roca, Email: marta_roca@iislafe.es.
Llúcia González, Email: gonzalez_llu@gva.es.
Vicent Yusà, Email: yusa_vic@gva.es.
Marisa Rebagliato, Email: rebaglia@med.uji.es.
Ferran Ballester, Email: ballester_fer@gva.es.
References
- 1.European Crop Protection. Industry Statistics 2001–2010. 2010. http://www.ecpa.eu/industry-statistics. Accessed 12 May 2016.
- 2.Asociación de empresarios para la producción de las plantas (AEPLA). Report 2009. 2009. http://www.eumedia.es/portales/files/documentos/AEPLA2009.pdf. Accessed 15 Apr 2016.
- 3.Spanish Ministry of Agriculture F and E. Annual statistics about the pesticide use in the agriculture. 2013. http://www.magrama.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/estadisticas-medios-produccion/fitosanitarios.aspx. Accessed 20 May 2016.
- 4.Ccanccapa A, Masia A, Andreu V, Pico Y. Spatio-temporal patterns of pesticide residues in the Turia and Jucar rivers (Spain) Sci Total Environ. 2016;540:200–210. doi: 10.1016/j.scitotenv.2015.06.063. [DOI] [PubMed] [Google Scholar]
- 5.Moreno MJ, Melia LA, Oltra Moscardo MT, Jimenez PR. Current situation in Spain of aerosol insect sprays registered for household use by the environmental health authorities. Rev EspSalud Publica. 2003;77:383–391. [PubMed] [Google Scholar]
- 6.Sociedad Española de Sanidad Ambiental Study of the use of organophosphate insecticide Chlorpyrifos in urban spaces. Rev Salud Ambient. 2009;9(Supl 1):1–20. [Google Scholar]
- 7.Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111:749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkowitz GS, Obel J, Deych E, Lapinski R, Godbold J, Liu Z, et al. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect. 2003;111:79–84. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bradman A, Eskenazi B, Barr DB, Bravo R, Castorina R, Chevrier J, et al. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts JR, Karr CJ, Council On Environmental Health Pesticide exposure in children. Pediatrics. 2012;130:e1765–e1788. doi: 10.1542/peds.2012-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selevan SG, Kimmel CA, Mendola P. IDentifying critical windows of exposure for children’s health. EnvironHealth Perspect. 2000;108 Suppl 3:451–455. [DOI] [PMC free article] [PubMed]
- 12.Gonzalez-Alzaga B, Lacasana M, Guilar-Garduno C, Rodriguez-Barranco M, Ballester F, Rebagliato M, et al. A systematic review of neurodevelopmental effects of prenatal and postnatal organophosphate pesticide exposure. Toxicol Lett. 2014;230:104–121. doi: 10.1016/j.toxlet.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 13.Eskenazi B, Harley K, Bradman A, Weltzien E, Jewell NP, Barr DB, et al. Association of in utero organophosphate pesticide exposure and fetal growth and length of gestation in an agricultural population. Environ Health Perspect. 2004;112:1116–1124. doi: 10.1289/ehp.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang P, Tian Y, Wang XJ, Gao Y, Shi R, Wang GQ, et al. Organophosphate pesticide exposure and perinatal outcomes in shanghai, China. Environ Int. 2012;42:100–104. doi: 10.1016/j.envint.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 15.Rauch SA, Braun JM, Barr DB, Calafat AM, Khoury J, Montesano AM, et al. Associations of prenatal exposure to organophosphate pesticide metabolites with gestational age and birth weight. Environ Health Perspect. 2012;120:1055–1060. doi: 10.1289/ehp.1104615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, et al. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ Health Perspect. 2004;112:1125–1132. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harari R, Julvez J, Murata K, Barr D, Bellinger DC, Debes F, et al. Neurobehavioral deficits and increased blood pressure in school-age children prenatally exposed to pesticides. Environ Health Perspect. 2010;118:890–896. doi: 10.1289/ehp.0901582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raanan R, Balmes JR, Harley KG, Gunier RB, Magzamen S, Bradman A, et al. Decreased lung function in 7-year-old children with early-life organophosphate exposure. Thorax. 2016;71:148–153. doi: 10.1136/thoraxjnl-2014-206622. [DOI] [PubMed] [Google Scholar]
- 19.Bost-Legrand A, Warembourg C, Massart C, Chevrier C, Bonvallot N, Monfort C, et al. Prenatal exposure to persistent organic pollutants and organophosphate pesticides, and markers of glucose metabolism at birth. Environ Res. 2016;146:207–217. doi: 10.1016/j.envres.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Slotkin TA. Does early-life exposure to organophosphate insecticides lead to prediabetes and obesity? ReprodToxicol. 2011;31:297–301. doi: 10.1016/j.reprotox.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yolton K, Xu Y, Sucharew H, Succop P, Altaye M, Popelar A, et al. Impact of low-level gestational exposure to organophosphate pesticides on neurobehavior in early infancy: a prospective study. Environ Health. 2013;12:79. doi: 10.1186/1476-069X-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Colapinto CK, Arbuckle TE, Dubois L, Fraser W. Tea consumption in pregnancy as a predictor of pesticide exposure and adverse birth outcomes: the MIREC study. Environ Res. 2015;142:77–83. doi: 10.1016/j.envres.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 23.Lewis RC, Cantonwine DE, Del Toro LV, Calafat AM, Valentin-Blasini L, Davis MD, et al. Distribution and determinants of urinary biomarkers of exposure to organophosphate insecticides in Puerto Rican pregnant women. Sci Total Environ. 2015;512–513:337–344. doi: 10.1016/j.scitotenv.2015.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sokoloff K, Fraser W, Arbuckle TE, Fisher M, Gaudreau E, LeBlanc A, et al. Determinants of urinary concentrations of dialkyl phosphates among pregnant women in Canada - results from the MIREC study. Environ Int. 2016;94:133–140. doi: 10.1016/j.envint.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Guxens M, Ballester F, Espada M, Fernandez MF, Grimalt JO, Ibarluzea J, et al. Cohort profile: the INMA--INfancia y Medio Ambiente--(environment and childhood) project. Int J Epidemiol. 2012;41:930–940. doi: 10.1093/ije/dyr054. [DOI] [PubMed] [Google Scholar]
- 26.Roca M, Leon N, Pastor A, Yusa V. Comprehensive analytical strategy for biomonitoring of pesticides in urine by liquid chromatography-orbitrap high resolution masss pectrometry. J Chromatogr A. 2014;1374:66–76. doi: 10.1016/j.chroma.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 27.Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J AOAC Int. 2003;86:412–431. [PubMed] [Google Scholar]
- 28.Domingo-Salvany A, Regidor E, Alonso J, Varez-Dardet C. Proposal for a social class measure. Working Group of the Spanish Society of epidemiology and the Spanish Society of Family and Community Medicine. Aten Primaria. 2000;25:350–363. doi: 10.1016/S0212-6567(00)78518-0. [DOI] [PubMed] [Google Scholar]
- 29.Vioque J, Navarrete-Munoz EM, Gimenez-Monzo D, Garcia-de-la-Hera M, Granado F, Young IS, et al. Reproducibility and validity of a food frequency questionnaire among pregnant women in a Mediterranean area. Nutr J. 2013;12:26. doi: 10.1186/1475-2891-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Newman MC, Dixon PM, Looney BB, Pinder JE. Estimating mean and variance for environmental samples with below detection limit observations. Water Resources Butlletin. 1989;25:905–916. doi: 10.1111/j.1752-1688.1989.tb05406.x. [DOI] [Google Scholar]
- 31.Lubin JH, Colt JS, Camann D, Davis S, Cerhan JR, Severson RK, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112:1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castorina R, Bradman A, Fenster L, Barr DB, Bravo R, Vedar MG, et al. Comparison of current-use pesticide and other toxicant urinary metabolite levels among pregnant women in the CHAMACOS cohort and NHANES. Environ Health Perspect. 2010;118:856–863. doi: 10.1289/ehp.0901568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castorina R, Bradman A, McKone TE, Barr DB, Harnly ME, Eskenazi B. Cumulative organophosphate pesticide exposure and risk assessment among pregnant women living in an agricultural community: a case study from the CHAMACOS cohort. Environ Health Perspect. 2003;111:1640–1648. doi: 10.1289/ehp.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye X, Pierik FH, Angerer J, Meltzer HM, Jaddoe VW, Tiemeier H, et al. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol a in pooled urine specimens from pregnant women participating in the Norwegian mother and child cohort study (MoBa) Int J Hyg Environ Health. 2009;212:481–491. doi: 10.1016/j.ijheh.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fortenberry GZ, Meeker JD, Sanchez BN, Barr DB, Panuwet P, Bellinger D, et al. Urinary 3,5,6-trichloro-2-pyridinol (TCPY) in pregnant women from Mexico City: distribution, temporal variability, and relationship with child attention and hyperactivity. Int J Hyg Environ Health. 2014;217:405–412. doi: 10.1016/j.ijheh.2013.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forde MS, Robertson L, Laouan Sidi EA, Cote S, Gaudreau E, Drescher O, et al. Evaluation of exposure to organophosphate, carbamate, phenoxy acid, and chlorophenol pesticides in pregnant women from 10 Caribbean countries. Environ Sci Process Impacts. 2015;17:1661–1671. doi: 10.1039/C5EM00247H. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Alzaga B, Hernandez AF, Rodriguez-Barranco M, Gomez I, Guilar-Garduno C, Lopez-Flores I, et al. Pre- and postnatal exposures to pesticides and neurodevelopmental effects in children living in agricultural communities from south-eastern Spain. Environ Int. 2015;85:229–237. doi: 10.1016/j.envint.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal exposure to organophosphate pesticides and IQ in 7-year-old children. Environ Health Perspect. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoppin JA, Ulmer R, Calafat AM, Barr DB, Baker SV, Meltzer HM, et al. Impact of urine preservation methods and duration of storage on measured levels of environmental contaminants. J Expo Sci Environ Epidemiol. 2006;16:39–48. doi: 10.1038/sj.jea.7500435. [DOI] [PubMed] [Google Scholar]
- 40.Flaskos J. The developmental neurotoxicity of organophosphorus insecticides: a direct role for the oxon metabolites. Toxicol Lett. 2012;209:86–93. doi: 10.1016/j.toxlet.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 41.Chen L, Zhao T, Pan C, Ross JH, Krieger RI. Preformed biomarkers including dialkylphosphates (DAPs) in produce may confound biomonitoring in pesticide exposure and risk assessment. J Agric Food Chem. 2012;60:9342–9351. doi: 10.1021/jf303116p. [DOI] [PubMed] [Google Scholar]
- 42.Quirós-Alcalá L, Bradman A, Smith K, Weerasekera G, Odetokun M, Barr DB, et al. Organophosphorous pesticide breakdown products in house dust and children’s urine. J Expo Sci Environ Epidemiol. 2012;22:559–568. doi: 10.1038/jes.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu C, Bravo R, Caltabiano LM, Irish RM, Weerasekera G, Barr DB. The presence of dialkylphosphates in fresh fruit juices: implication for organophosphorus pesticide exposure and risk assessments. J Toxicol Environ Health Part A. 2005;68:209–227. doi: 10.1080/15287390590890554. [DOI] [PubMed] [Google Scholar]
- 44.Bravo R, Caltabiano LM, Weerasekera G, Whitehead RD, Fernandez C, Needham LL, et al. Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification. J Expo Anal Environ Epidemiol. 2004;14:249–259. doi: 10.1038/sj.jea.7500322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to ethical concerns but are available from the corresponding author on reasonable request. All INMA questionnaires and protocols are available at http://www.proyectoinma.org/.


