Abstract
Background: Docetaxel is the first-line treatment for castration-resistant prostate cancer (CRPC). The limited survival benefit associated with the quick emergence of resistance and systemic toxicity diminishes its efficacy in high-dose monotherapy. YK-4-279 is a small molecule inhibitor of ETV1 that plays an important role in the progression of prostate cancer. The aim of this study was to evaluate the hypothesis that the combination of docetaxel and YK-4-279 will have a synergistic effect on inhibiting growth and accelerating apoptosis in human prostate cancer cells.
Methods: Cell growth assessed using CCK-8 and trypan blue exclusion assays. Cell apoptosis was determined by morphological assessment in cells stained with propidium iodide. Standard scratch migration and Matrigel-coated transwell invasion assays were used to assess cell migration and invasion, respectively. Western blotting was used to investigate the levels of ETV1, AR, PSA, p-STAT3, survivin, Bcl-2, and p-Akt in prostate cancer cells.
Results: The combination of low-dose docetaxel and YK-4-279 synergistically inhibited growth and induced apoptosis in human prostate cancer cells. The combination also more efficiently suppressed the migration and invasion of LNCaP and PC-3 cells. The combination of low-dose docetaxel and YK-4-279 caused a stronger decrease in the levels of ETV1, AR, PSA, p-STAT3, survivin, Bcl-2, and p-Akt in LNCaP cells and of p-Akt, Bcl-2, and p-STAT3 in PC-3 cells compared with either drug alone.
Conclusions: These data suggest that the combination of docetaxel and YK-4-279 may be an effective approach for inhibiting the growth and metastasis of prostate cancer. This could permit a decrease in the docetaxel dose necessary for patients with CRPC and thereby lower its systemic toxicity.
Keywords: docetaxel, YK-4-279, prostate cancer, combination, synergistic action.
Introduction
Prostate cancer is the most frequently occurring cancer and the second leading cause of cancer-related deaths among men in the United States 1. Patients diagnosed with localized stage disease are sensitive to various treatments and are often curable; however, ~40% of all cases will eventually progress to a metastatic stage 2.
Since the 1940s, targeting androgen signaling using androgen deprivation therapy (ADT) has been the mainstay of clinical interventions for metastatic hormone-sensitive prostate cancer 3. However, its initial effectiveness is only transient (2-3 years) 4, and most men relapse with castration-resistant prostate cancer (CRPC) and die soon thereafter 5. For patients with CRPC, the common treatment is docetaxel-based chemotherapy to prolong survival and maintain a good quality of life 6. However, treatment using high doses of docetaxel ultimately cause toxicity and resistance, which means that there are only limited options for patients progressing on or after docetaxel 7. Recently, some novel agents including abiraterone, cabazitaxel, and enzalutamide were approved for patients with CRPC following docetaxel failure or resistance, but their efficiency is limited 8. Therefore, there is an urgent need to improve docetaxel-based regimens to reduce toxicity and increase efficacy.
Prostate cancer that progresses to lethal CRPC has been associated with ETS gene fusions, PTEN loss, and androgen receptor (AR) amplification 9. In particularly, ETS transcription factor (mainly ETV1 or ERG) fusions occur frequently in prostate cancer 10-11, and ~50% of human prostate cancers containing ETS gene fusions 12. One study suggested that ETV1 expression promotes autonomous testosterone production to reactivate AR signaling in aggressive disease 13. It was suggested that ETV1 plays an important role in the progression of CRPC and can indirectly mediate AR signaling.
YK-4-279 monotherapy can inhibit the growth and metastasis of ETV1 fusion-positive prostate cancer xenografts 14-15. This suggests that YK-4-279 could be used as a small molecule inhibitor of ETV1. Docetaxel, a semi-synthetic second-generation taxane, can slow down the progression of prostate cancer, and it retains antitumor activity in CRPC patients 16. It can inhibit proliferation and induce apoptosis by binding to β-tubulin and causing cell-cycle arrest 17.
The addition of docetaxel to ADT has survival benefits compared with ADT alone in patients with metastatic hormone-sensitive prostate cancer 18-19. This finding suggests that there is an interaction between AR signaling and docetaxel sensitivity. Thus, we hypothesized that the combination of YK-4-279 and docetaxel will synergistically inhibit growth and accelerate apoptosis in human prostate cancer cells.
Materials and methods
Cell culture and reagents
Two human prostate cancer cell lines (LNCaP and PC-3) were obtained from the American Type Culture Collection (Rockville, MD, USA). YK-4-279 was purchased from MCE (MedChem Express, HY-1450, USA) and docetaxel was from Aladdin (D107319). The cells were cultured as described previously 20. Docetaxel and YK-4-279 were dissolved in DMSO (Sigma, USA); the final concentration of DMSO was 0.1% in all experiments.
Cell viability
For CCK-8 assays 21, cells were seeded at a density of 2 × 10 4 cells/ml of medium in a 96-well plate (0.1 ml/well) and incubated for 24 h. Then, the cells were treated with different concentrations of docetaxel (1 nM) and YK-4-279 (0.1 µM, 0.5 µM, or 1.0 µM) for 72 h. After treatment, the media were replaced with fresh media and 10 µl CCK-8 (Dojindo, Kumamoto, Japan) was added to each well. After a 1-h incubation, the absorbance at 450 nm was measured on a microplate reader.
For the trypan blue exclusion assays 22, cells were seeded at a density of 2 × 104 cells/ml of medium in 35-mm tissue culture dishes (2 ml/dish) and incubated for 24 h. The cells were then treated with different concentrations of docetaxel (0.1 nM, 0.5 nM, or 1 nM) and YK-4-279 (0.1 µM, 0.5 µM, or 1.0 µM ) for 72 h. Then, single cell suspensions were prepared and the number of viable cells was counted using a hemocytometer under a light microscope by mixing 80 µl of cell suspension and 20 µl of 0.4% trypan blue solution for 5 min. Blue cells were counted as dead and the cells that did not absorb dye were counted as live.
Measuring apoptosis
Apoptosis was determined by morphologically assessing cells stained with propidium iodide (PI) 22. Cells were seeded at a density of 2 × 104 cells/ml in 35-mm tissue culture dishes (2 ml/dish) and incubated for 24 h. They were then treated with docetaxel (0.5 nM) and/or YK-4-279 (0.5 µM) for 72 h. After treatment, cytospin slides were prepared using a smear centrifuge and fixed with acetone/methanol (1:1) for 10 min at room temperature. The cells were stained with 1 µg/ml PI in PBS for 10 min. Then we identified apoptotic cells using a fluorescence microscope. Cells with classical morphological features including nuclear condensation, cell shrinkage, and the formation of apoptotic bodies were counted as apoptotic. At least 400 cells in approximately 10 randomly selected fields were counted in each sample.
Scratch migration assays
For scratch migration assays 23, cells were seeded at a density of 5 × 105 cells/ml in 35-mm tissue culture dishes (2 ml/dish) and incubated for 24 h. The cell surface was then scratched using a sterile 200-µl pipette tip (Axygen, Union City, CA, USA) after washing with PBS. Then, the scratched cells were rinsed gently with PBS three times and complete medium was added (0.2% FBS). The cells were then treated with different concentrations of docetaxel and/or YK-4-279 for an additional 24 h. Images were captured using an inverted microscope. The distance that cells migrated compared with baseline measurements was measured using Image J software.
Invasion assays
For Matrigel-coated transwell invasion assays 24, 600 µl complete medium (20% FBS) was added to the lower chamber. The cells were pre-treated with YK-4-279 or docetaxel for 48h. Then, the cells were trypsinized, resuspended in complete medium (1% FBS), and seeded at a density of 5 × 105 cells/ml in the top chamber (200 µl/chamber; Corning) containing a Matrigel-coated membrane. The cells were then treated with different concentrations of docetaxel and/or YK-4-279 for an additional 24 h. Next, the medium and the cells remaining in the top chambers were removed. After fixing with methanol and staining with 0.1% crystal violet, the number of cells that had invaded to the lower membrane was counted and images were captured under an inverted microscope (Olympus).
Western blotting
After treatment, the protein lysates were prepared as described previously 25. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore). After blocking nonspecific binding sites with blocking buffer, the membranes were incubated overnight at 4˚C with the following primary antibodies: #4060 for phospho-Akt, #9145 for phospho-Stat3, #5365 for PSA/KLK3, #2808 for survivin, #2870 for Bcl-2, #4370 for phospho-p44/42 MAPK (ERK-1/2), #12153 for IL-6, #3202 for AR (all from Cell Signaling Technology, Beverly, MA), and ab81086 for ETV1 (Abcam, Cambridge, MA, USA). β-actin (Cell Signaling Technology, Beverly, MA) was used as a loading control. Following removal of the primary antibody, the membranes were washed three times with TBST (TBS containing 0.05% Tween 20) at room temperature and then incubated with fluorochrome-conjugated secondary antibody for 2 h. The membranes were then washed with TBST three times and signals were detected using ECL in the dark room.
Statistical analysis
The potential synergistic effects of docetaxel and YK-4-279 were assessed using the isobole method with the equation Ac/Ae + Bc/Be = combination index (CI) 26. Ac and Bc represent the concentration of drug A and drug B used in the combination, respectively, and Ae and Be represent the concentration of drug A and B that produced the same magnitude of effect when administered alone. If CI is <1, then the drugs are considered to act synergistically; if CI is >1 or =1, then the drugs act in an antagonistic or additive manner, respectively. Comparisons of cell viability, apoptosis, migration, and invasion were analyzed using ANOVA with Tukey-Kramer multiple comparison tests.
Results
Effects of docetaxel and YK-4-279 alone or in combination on prostate cancer cell growth and apoptosis
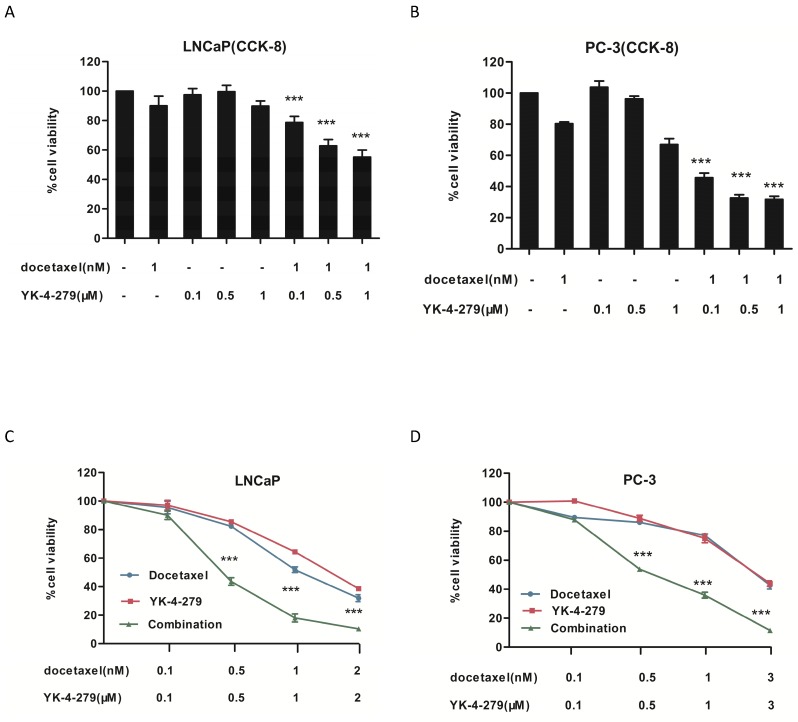
The effects of docetaxel and YK-4-279 alone or in combination on the growth of human prostate cancer cells were determined using the CCK-8 and trypan blue exclusion assays. Human LNCaP (androgen-dependent), PC-3 (androgen-independent) prostate cancer cells were treated with different concentrations of docetaxel and YK-4-279 for 72 h. As shown in Fig. 1A and B, treatment with YK-4-279 (0.1 µM, 0.5 µM, and 1.0 µM) alone and in combination with docetaxel (1 nM) inhibited the growth of both LNCaP and PC-3 cells in a dose-dependent manner. Treatment with the combination of docetaxel and YK-4-279 had a stronger inhibitory effect on cell growth than either drug alone. When the concentration ratio of YK-4-279 and docetaxel was 1:1000, co-treatment with YK-4-279 and docetaxel exhibited a stronger decrease in cell viability compared with the other two combination groups. As shown in Fig. 1C and D, docetaxel and YK-4-279 single and combined treatments affected the viability of LNCaP and PC-3 cells in a dose-dependent manner. The half maximal inhibitory concentrations (IC50) of YK-4-279 were 1.48 µM and 2.03 µM in LNCaP and PC-3 cells, respectively, and the IC50 of docetaxel alone was 1.16 nM and 2.07 nM in LNCaP and PC-3 cells, respectively. Specifically, the IC50 of docetaxel was decreased to 0.41 nM and 0.66 nM in LNCaP and PC-3 cells respectively, whereas those of YK-4-279 decreased to 0.41 µM and 0.66 µM. The combination indexes (CIs) for the IC50 were 0.64 and 0.64 in LNCaP and PC-3 cells, respectively. These results suggest that the combination of YK-4-279 and docetaxel synergistically inhibited the growth of both LNCaP and PC-3 cells.
Figure 1.
Effects of docetaxel and YK-4-279 alone or in combination on LNCaP and PC-3 cell growth. A and B. LNCaP or PC-3 cells was were seeded at a density of 2 × 10 4 cells/ml of medium in a 96-well plate (0.1ml/well) and incubated for 24h.The cells were then treated with docetaxel or YK-4-279 for 72 hours and cell growth was evaluated by CCK-8 assay. C and D. LNCaP or PC-3 cells was cultured at a density of 2 × 10 4 cells/ml in 35 mm tissue culture dishes (2ml/dish) for 24 h. The cells were then treated with docetaxel or YK-4-279 for 72 h. The number of viable cells was determined by the tyrpan blue exclusion assay and expressed as percentages of solvent-treated control. Each value represents mean±S.E. from three separate experiments. Significant numbers of viable cells between a combination group and a single-agent-treated group were analyzed by ANOVA with Tukey-Kramer multiple comparison test (*p<0.05, **p<0.01, ***p<0.001).
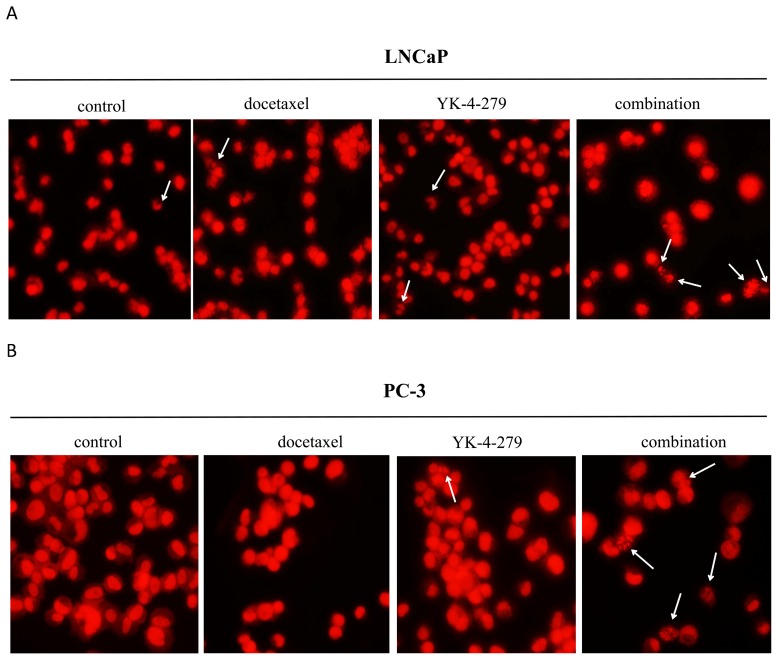
Next, the effects of docetaxel and YK-4-279 alone or in combination on prostate cancer cell apoptosis were determined by morphologically assessing cells stained with PI. Apoptotic cells were identified according to classic morphologic features such as nuclear condensation, cell shrinkage, and the formation of apoptotic bodies 23. Morphologically distinct apoptotic cells from representative samples are shown in Fig. 2. As shown in Table 1, treating cells with docetaxel (0.5 nM) and YK-4-279 (0.5 µM) alone resulted in a small number of apoptotic cells. In contrast, the combination of docetaxel and YK-4-279 caused a strong increase in the number of apoptotic cells, suggesting that the combination of docetaxel and YK-4-279 had a more potent effect on stimulating apoptosis than either agent alone.
Figure 2.
Effect of docetaxel and YK-4-279 alone or in combination on LNCaP and PC-3 cell apoptosis. The nuclear morphology changes were analyzed by fluorescence microscopy in ×200 magnification using the propidium iodide nuclear fluorescent dye staining. These analyses were performed 72h after treatment. Arrows represent the apoptotic cells. A: LNCaP cells, B: PC-3 cells.
Table 1.
Effect of docetaxel and YK-4-279 alone or in combination on LNCaP and PC-3 cell apoptosis
| Treatment | Apoptotic cells (%) | Dead cells (%) | ||
|---|---|---|---|---|
| LNCaP | PC-3 | LNCaP | PC-3 | |
| Control | 2.32±0.31 | 3.38±0.89 | 3.67±1.15 | 4.01±0.55 |
| Docetaxel | 4.95±0.57 | 4.38±0.86 | 5.33±0.58 | 4.88±0.78 |
| YK-4-279 | 5.52±0.84 | 4.72±0.81 | 6.67±0.58 | 4.94±0.38 |
| Combination | 17.03±3.12*** | 17.04±0.64*** | 18.67±0.15*** | 19.96±2.11*** |
LNCaP or PC-3 cells was cultured at a density of 2 × 10 4 cells/ml for 24 h. The cells were then treated with docetaxel (0.5nM) and YK-4-279(0.5µM) alone or in combination for 72h. Apoptosis was determined by morphological assessment. Each value represents mean±S.E from three separate experiments. Significant numbers of apoptotic cells between a combination group and a single-agent-treated group were analyzed by ANOVA with Tukey-Kramer multiple comparison test (*p<0.05, **p<0.01, ***p<0.001).
Effects of docetaxel and YK-4-279 alone or in combination on LNCaP and PC-3 cell motility
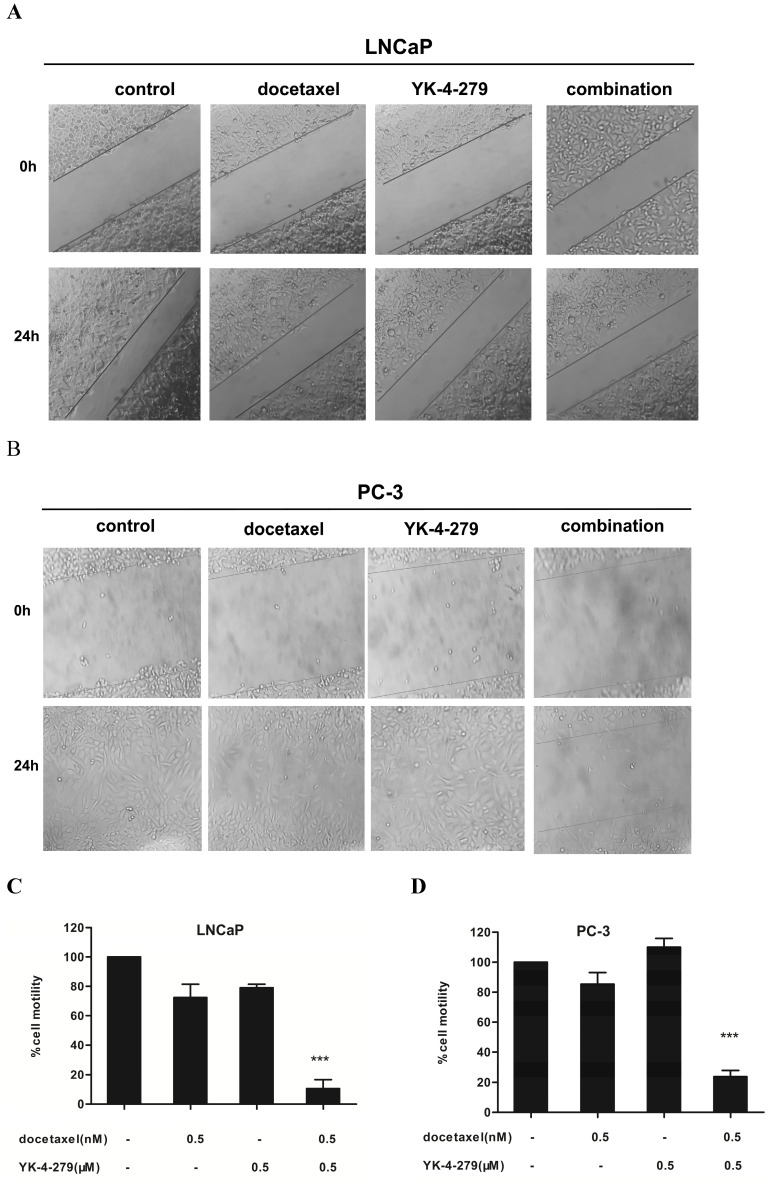
The effects of docetaxel and YK-4-279 alone or in combination on the migration of LNCaP and PC-3 cells was determined by a standard scratch migration assay. Prior to performing this experiment, the effects of time on the growth of LNCaP and PC-3 cells treated with docetaxel and YK-4-279 alone or in combination was determined using trypan blue exclusion assays (not shown). The number of viable cells in the combination treatment group was comparable to the individual treatments within 24 h; therefore, the co-treatment had no effects on the proliferation of LNCaP and PC-3 cells within 24 hours. These findings suggest that if a scratch assay was performed within 24 h, the effects of the co-treatment would not be due to cytotoxicity but instead due to the inhibition of cell migration. Treating LNCaP and PC-3 cells with docetaxel and/or YK-4-279 had significantly different effects on the number of migrated cells (Fig. 3A and B). More cells in the control and single agent-treated groups migrated cells than in the combination treatment group. The combination of docetaxel (0.5 nM) and YK-4-279 (0.5 µM) caused an 88% and 77% decrease in the number of migrating LNCaP and PC-3 cells, respectively, compared with control (Fig. 3C and D). The combination of docetaxel (0.5 nM) and YK-4-279 (0.5 µM) had a more potent effect on inhibiting the migration of LNCaP cells compared with PC-3 cells.
Figure 3.
Effects of docetaxel and YK-4-279 alone or in combination on LNCaP and PC-3 cell motility. LNCaP and PC-3 cells were seeded at a density of 5 × 105 cells/ml in 35 mm tissue culture dishes (2ml/dish) for 24 h. After the scratch finished, the cells were then treated with different concentrations of docetaxel and YK-4-279 alone or in combination for an additional 24h.Cell motility was quantified by measuring the distance between the migrating cell boundaries. Motility was expressed relative to vehicle treated conditions. Significant cell motility between a combination group and a single-agent-treated group were analyzed by ANOVA with Tukey-Kramer multiple comparison test (*p<0.05, **p<0.01, ***p<0.001).
Effects of docetaxel and YK-4-279 alone or in combination on LNCaP and PC-3 cell invasion
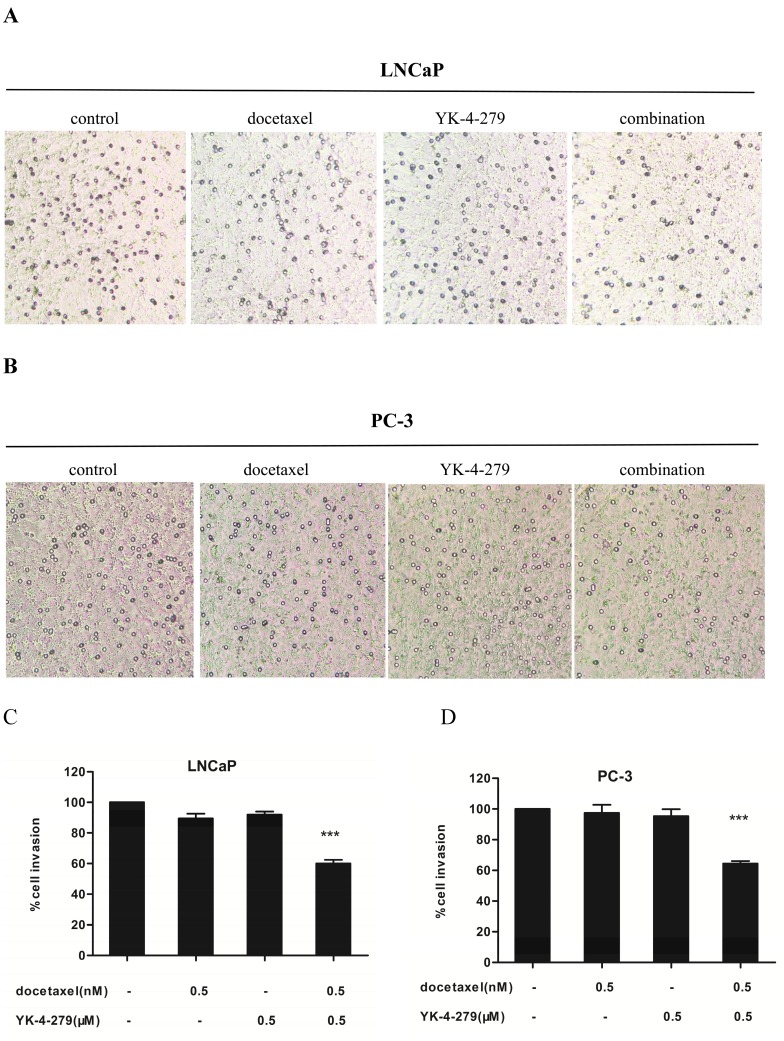
The effects of docetaxel and YK-4-279 alone or in combination on the invasiveness of LNCaP and PC-3 cells was determined using Matrigel-coated transwell invasion assays. There was a significant difference in the invasiveness of LNCaP and PC-3 cells treated with a single agent and the combination treatment (Fig. 4A and B). Treatment with docetaxel (0.5 nM) and YK-4-279 (0.5 µM) alone had little or no effect on the number of cells invading through the Matrigel-coated inserts. However, combination treatment led to a 40% and 36% decrease in the number of invading LNCaP and PC-3 cells, respectively, compared with control (Fig. 4C and D). The combination of docetaxel (0.5 nM) and YK-4-279 (0.5 µM) had more potent effect on inhibiting the invasion of LNCaP cells compared with PC-3 cells.
Figure 4.
Effects of docetaxel and YK-4-279 alone or in combination on LNCaP and PC-3 cell invasion. LNCaP and PC-3 cells were pre-treated for 48 hours with YK-4-279 and docetaxel alone or in combination. The cells were trypsinized and resuspended in complete medium (1%FBS) and seeded at a density of 5 × 105 cells/ml in the top chamber(200µl/chamber), while 600ul complete medium (20%FBS) was added to the lower chamber. The cells were treated with docetaxel and YK-4-279 alone or in combination for an additional 24h.After fixation and staining, the cells that had invaded to the lower membrane of the inserts were counted and images were captured. Invasion was expressed relative to vehicle treated conditions. Significant invasion (% of control) between a combination group and a single-agent-treated group were analyzed by ANOVA with Tukey-Kramer multiple comparison test (*p<0.05, **p<0.01, ***p<0.001).
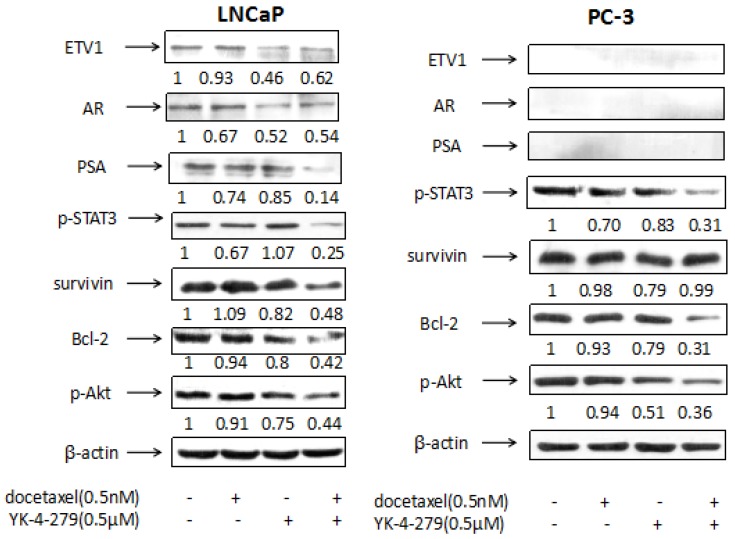
Effects of docetaxel and YK-4-279 alone or in combination on the levels of ETV1, AR, PSA, p-STAT3, survivin, Bcl-2 and p-Akt in LNCaP and PC-3 cells
The levels of ETV1, AR, PSA, p-STAT3, survivin, Bcl-2, and p-Akt in LNCaP and PC-3 cells were determined using western blotting. The PI3K/Akt/m TOR signaling pathway has many functions, including the regulation of cellular growth, proliferation, migration, and angiogenesis 27. It also plays an important role in facilitating prostate cancer progression to CRPC and is highly activated in prostate cancer 28. Low-dose docetaxel alone did not alter the expression of p-Akt in either LNCaP or PC-3 cells whereas the combination treatment strongly decreased expression in both cell lines, suggesting that YK-4-279 functions as a docetaxel chemosensitizer. As shown in Fig. 5, in LNCaP cells, the level of p-Akt relative to control (1.00) was 0.91 in cells treated with docetaxel, 0.75 in cells treated with YK-4-279, and 0.44 in cells treated with the combination of docetaxel and YK-4-279. In PC-3 cells, the level of p-Akt was 1.00 in control, 0.94 in cells treated with docetaxel, 0.51 in cells treated with YK-4-279, and 0.36 in cells treated with the combination.
Figure 5.
Effects of docetaxel and YK-4-279 alone or in combination on the levels of ETV1, AR, PSA, p-STAT3, survivin, Bcl-2 and p-Akt in LNCaP and PC-3 cells. LNCaP and PC-3 cells were cultured at a density of 1 × 105 cells/ml in 100 mm tissue culture dishes (10ml/dish) for 24 h. The cells were then treated with docetaxel (0.5nM) and YK-4-279(0.5µM) alone or in combination for 24h (for analysis of p-Akt, survivin, PSA, p-STAT3 and AR) and 48 h (for analysis of Bcl-2). The levels of AR,Bcl-2, p-STAT3, PSA,p-Akt and survivin were determined by the Western blot analysis. The band density was measured and normalized for actin.
Bcl-2 family members are important in the regulation and control of the intrinsic apoptosis pathway. Although the exact mechanism of action of docetaxel is not well-understood, it is believed to inhibit Bcl-2 and Bcl-x activity by decreasing their gene expression to promote apoptosis in prostate cancer cells 29. In the current study, the expression of Bcl-2 was significantly decreased in cells treated with the combination of docetaxel and YK-4-279 compared with the control in both LNCaP and PC-3 cells. This suggests that decreasing Bcl-2 levels may play a role in the apoptosis induced by the combination of docetaxel and YK-4-279. As shown in Fig. 5, in LNCaP cells the levels of Bcl-2 relative to control (1.00) was 0.94 in cells treated with docetaxel, 0.80 in cells treated with YK-4-279, and 0.42 in cells treated with the combination of docetaxel and YK-4-279. In PC-3 cells, the level of Bcl-2 relative to control (1.00) was 0.93 in cells treated with docetaxel, 0.79 in cells treated with YK-4-279, and 0.31 in cells treated with the combination.
Survivin is a member of the inhibitor of apoptosis protein family and has been actively pursued as a target for cancer treatment because its overexpression is correlated to recurrence, metastasis, and therapeutic resistance 30. Moreover, accumulating evidence suggests that survivin plays a pivotal role in the progression of prostate cancer 31. In the current study, the combination of docetaxel and YK-4-279 significantly suppressed survivin expression in LNCaP cells but not in PC-3 cells, suggesting that the mechanism of action of the combination treatment differs in LNCaP and PC-3 cells. As shown in Fig. 5, in LNCaP cells the levels of survivin relative to control (1.00) was 1.09 in cells treated with docetaxel, 0.82 in cells treated with YK-4-279, and 0.48 in cells treated with the combination of docetaxel and YK-4-279. In PC-3 cells, the level of survivin relative to control (1.00) was 0.98 in cells treated with docetaxel, 0.79 in cells treated with YK-4-279, and 0.99 in cells treated with the combination.
Signal transducer and activator of transcription 3 (STAT3) is an important oncogenic protein that regulates genes involved in cell proliferation, differentiation, and invasion 32. The constitutive activation of STAT3 has been implicated in promoting the oncogenesis and progression of prostate cancer, and it occurs frequently in primary prostate adenocarcinomas 33-34. Some studies demonstrated that the migration and invasion of prostate cancer cells can be inhibited by suppressing the activation of STAT3 35. In the current study, the combination of docetaxel and YK-4-279 potently decreased the levels of p-STAT3 in both LNCaP and PC-3 cells. As shown in Fig. 5, in LNCaP cells the levels of p-STAT3 relative to control (1.00) was 0.67 in cells treated with docetaxel, 1.07 in cells treated with YK-4-279, and 0.25 in cells treated with the combination of docetaxel and YK-4-279. In PC-3 cells, the levels of p-STAT3 relative to control (1.00) were 0.70 in cells treated with docetaxel, 0.83 in cells treated with YK-4-279, and 0.31 in cells treated with the combination.
Treating LNCaP cells with docetaxel (0.5 nM) or YK-4-279 (0.5 µM) alone moderately decreased the levels of PSA. However, the combination of docetaxel (0.0005 µM) and YK-4-279 (0.5 µM) had a stronger effect on decreasing PSA levels. Specifically, PSA levels were 1.00 in control, 0.74 in cells treated with docetaxel, 0.85 in cells treated with YK-4-279, and 0.14 in cells treated with the combination of docetaxel and YK-4-279. In contrast, PSA levels were not detected in PC-3 cells with the treatments of docetaxel and YK-4-279 alone or in combination.
Treating LNCaP cells with docetaxel (0.5 nM) or YK-4-279 (0.5 µM) alone decreased the levels of AR and ETV1, and the combination treatment had no additional effect. AR levels were 1.00 in control, 0.67 in cells treated with docetaxel, 0.52 in cells treated with YK-4-279, and 0.54 in cells treated with the combination of docetaxel and YK-4-279. ETV1 levels were 1.00 in control, 0.93 in cells treated with docetaxel, 0.46 in cells treated with YK-4-279, and 0.62 in cells treated with the combination of docetaxel and YK-4-279. AR and ETV1 levels were not detected in PC-3 cells with the treatments of docetaxel and YK-4-279 alone or in combination.
Discussion
Previous studies suggested that docetaxel, the first line treatment for CRPC, has significant toxic effects and often results in resistance when used as a high-dose monotherapy for prostate cancer 36. Based on the concept that a multitude of cellular targets may conquer drug resistance and decrease adverse effects, many studies using docetaxel-related co-treatments with one or two other drugs have been carried out; however, the effective therapies remain limited 37. Thus, the current study aimed to identify a drug with low or no cytotoxicity that could synergistically inhibit proliferation and induce apoptosis in human prostate cancer cells when used in combination with docetaxel.
ETV1, a member of the ETS transcription factor family, can direct androgen metabolism and confer aggressive prostate cancer in targeted mice and patients 10. ETS gene fusions play an important role of driving prostate cancer development and progression to lethal CRPC 9. YK-4-279, a small molecule inhibitor of ETV1, can inhibit ETV1 biological activity in fusion-positive LNCaP prostate cancer cells 14-15. In addition, YK-4-279 inhibits EWS-FLI1 activity, induces apoptosis in Ewing's sarcoma cell lines, and slows down tumor growth in mouse xenograft models 38. YK-4-279 functions in Ewing's sarcoma cells by blocking the interaction between EWS-FLI1 and RHA. However, the mechanism by which YK-4-279 inhibit ERG- and ETV1-derived malignant phenotypes in prostate cancer cells both in vitro and in vivo is unclear 14. In the present study, we tested the hypothesis that YK-4-279 can synergize with docetaxel to lead to greater cell death than treatment with docetaxel alone.
The current study demonstrated for the first time that the combination of docetaxel and YK-4-279 synergistically inhibits the growth of LNCaP and PC-3 prostate cancer cells. The combination of low-dose docetaxel (0.5 nM) and YK-4-279 (0.5 µM) had a more potent inhibitory effect on the growth of LNCaP and PC-3 cells than either agent used individually at a higher dose (docetaxel, 1 nM; YK-4-279, 1 µM; Fig. 1C and D). Moreover, the combination of low-dose docetaxel and YK-4-279 had a stronger effect on inducing apoptosis and decreasing motility and invasion in both LNCaP and PC-3 cells. Although PC-3 cells do not contain an ETV1 rearrangement and are androgen-independent, the combination of docetaxel and YK-4-279 still had a stronger effect on inhibiting growth, inducing apoptosis, and decreasing motility and invasion via a different mechanism in LNCaP cells than single drug alone. Overall, this study provides preclinical proof of concept that the combination of docetaxel with YK-4-279 results in a synergistic anti-tumor response in non-CRPC and CRPC models.
The AR is a transcription factor, and AR activation promotes the growth and progression of prostate cancer 39. Chromosomal translocations are frequently found in prostate cancer. For example, including ETV1 rearrangements causes the overexpression of ETV1, which cooperates with AR signaling 14. In addition, ETV1 upregulates the expression of AR target genes as well as genes involved in steroid biosynthesis and metabolism, resulting in activation of the AR transcriptional program 10. PSA is an androgen-regulated gene, and increased PSA levels indicate active AR signaling 40-41. In the current study, in fusion-positive LNCaP cells YK-4-279 decreased the levels of ETV1 and AR. When used in combination with docetaxel it caused a stronger decrease in PSA levels than either drug alone. In addition, the combination of docetaxel and YK-4-279 significantly suppressed the expression of survivin, BCl-2, p-Akt, and p-STAT3 in fusion-positive LNCaP cells, suggesting that co-treatment could affect more than one signaling pathway to induce apoptosis and inhibit the growth, migration, and invasion of prostate cancer cells. In fusion-negative PC-3 cells, although co-treatment did not affect AR signaling and the levels of survivin, the levels of BCl-2, p-Akt, p-STAT3 were significantly decreased, which explains why the combination of docetaxel and YK-4-279 could induce apoptosis and inhibit the growth, migration, and invasion of PC-3 cells.
In conclusion, the results of the current study demonstrated that the combination of low-dose docetaxel and YK-4-279 strongly inhibited growth and induced apoptosis in human prostate cancer cells. Moreover, the combination more efficiently suppressed the migration and invasion ability of PC-3 cells. These activities were accompanied by inhibition of the expression of ETV1, AR, PSA, p-STAT3, survivin, Bcl-2, and p-Akt in LNCaP cells and of p-Akt, Bcl-2, and p-STAT3 in PC-3 cells. Thus, the combination of docetaxel and YK-4-279 may be an effective approach for inhibiting the growth and metastasis of prostate cancer.
Acknowledgments
The present study was supported by funds from the Guangdong Province Leadership Grant 2011, Chinese National Science Foundation Grants ( 81272452 ), Rutgers Cancer Institute of New Jersey (CCSG P30-CA072720 RSD), and Hundred Talent Project of Guangdong University of Technology (220418008).We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
References
- 1.Roychowdhury S, Chinnaiyan AM. et al. Advancing precision medicine for prostate cancer through genomics. Journal of Clinical Oncology. 2013;31:1866–1873. doi: 10.1200/JCO.2012.45.3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim TD, Jin F, Shin S. et al. Histone demethylase JMJD2A drives prostate tumorigenesis through transcription factor ETV1. The Journal of clinical investigation. 2016;126:706–720. doi: 10.1172/JCI78132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Long BJ, Grigoryev DN, Nnane IP. et al. Antiandrogenic effects of novel androgen synthesis inhibitors on hormone-dependent prostate cancer. Cancer research. 2000;60:6630–6640. [PubMed] [Google Scholar]
- 4.Cai C, Chen S, Ng P. et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer research. 2011;71:6503–6513. doi: 10.1158/0008-5472.CAN-11-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rickman DS, Chen YB, Banerjee S. et al. ERG cooperates with androgen receptor in regulating trefoil factor 3 in prostate cancer disease progression. Neoplasia. 2010;12:1031–1040. doi: 10.1593/neo.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano M, Shoji S, Higure T. et al. Low-dose docetaxel, estramustine and prednisolone combination chemotherapy for castration-resistant prostate cancer. Molecular and Clinical Oncology. 2016;4:942–946. doi: 10.3892/mco.2016.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sridhar SS, Freedland SJ, Gleave ME. et al. Castration-resistant prostate cancer: from new pathophysiology to new treatment. European urology. 2014;65:289–299. doi: 10.1016/j.eururo.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Antonarakis ES, Lu C, Wang H. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. New England Journal of Medicine. 2014;371:1028–1038. doi: 10.1056/NEJMoa1315815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grasso CS, Wu YM, Robinson DR. et al. The mutational landscape of lethal, castration-resistant prostate cancer. Nature. 2012;487:239–243. doi: 10.1038/nature11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attard G, Swennenhuis JF, Olmos D. et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Research. 2009;69:2912–2918. doi: 10.1158/0008-5472.CAN-08-3667. [DOI] [PubMed] [Google Scholar]
- 11.Mehra R, Tomlins SA, Yu J. et al. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer research. 2008;68:3584–90. doi: 10.1158/0008-5472.CAN-07-6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu L, Zhao JC, Kim J. et al. ERG is a critical regulator of Wnt/LEF1 signaling in prostate cancer. Cancer research. 2013;73:6068–6079. doi: 10.1158/0008-5472.CAN-13-0882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baena E, Shao Z, Linn DE. et al. ETV1 directs androgen metabolism and confers aggressive prostate cancer in targeted mice and patients. Genes & development. 2013;27:683–698. doi: 10.1101/gad.211011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rahim S, Beauchamp EM, Kong Y. et al. YK-4-279 inhibits ERG and ETV1 mediated prostate cancer cell invasion. PloS one. 2011;6:e19343. doi: 10.1371/journal.pone.0019343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahim S, Minas T, Hong SH. et al. A small molecule inhibitor of ETV1, YK-4-279, prevents prostate cancer growth and metastasis in a mouse xenograft model. PloS one. 2014;9:e114260. doi: 10.1371/journal.pone.0114260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackler NJ, Pienta KJ. Drug insight: use of docetaxel in prostate and urothelial cancers. Nature clinical practice Urology. 2005;2:92–100. doi: 10.1038/ncpuro0099. [DOI] [PubMed] [Google Scholar]
- 17.Petrioli R, Francini E, Roviello G. Is there still a place for docetaxel rechallenge in prostate cancer. World journal of clinical oncology. 2015;6:99–103. doi: 10.5306/wjco.v6.i5.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeney CJ, Chen YH, Carducci M. et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. New England Journal of Medicine. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gravis G, Fizazi K, Joly F. et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. The lancet oncology. 2013;14:149–158. doi: 10.1016/S1470-2045(12)70560-0. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Liu Y, Wu J. et al. Mechanistic Study of Inhibitory Effects of Atorvastatin and Docetaxel in Combination on Prostate Cancer. Cancer Genomics Proteomics. 2016;13:151–160. [PMC free article] [PubMed] [Google Scholar]
- 21.Manohar SM, Padgaonkar AA, Jalota-Badhwar A. et al. Cyclin-dependent kinase inhibitor, P276-00, inhibits HIF-1α and induces G2/M arrest under hypoxia in prostate cancer cells. Prostate Cancer & Prostatic Disease. 2012;15:15–27. doi: 10.1038/pcan.2011.51. [DOI] [PubMed] [Google Scholar]
- 22.Huang H, Cao K, Malik S. et al. Combination of 12-O-tetradecanoylphorbol-13-acetate with diethyldithiocarbamate markedly inhibits pancreatic cancer cell growth in 3D culture and in immunodeficient mice. International Journal of Molecular Medicine. 2015;35:1617–1624. doi: 10.3892/ijmm.2015.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagaprashantha LD, Vatsyayan R, Singhal J. et al. Anti-cancer effects of novel flavonoid vicenin-2 as a single agent and in synergistic combination with docetaxel in prostate cancer. Biochemical pharmacology. 2011;82:1100–1109. doi: 10.1016/j.bcp.2011.07.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Tao H, Fang Z. et al. Anti-Tumor Activity of TRPM8 Inhibitor BCTC in Prostate Cancer DU145 Cells. Oncology Letters. 2016;11:225–230. doi: 10.3892/ol.2015.3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng X, Cui XX, Avila GE. et al. Atorvastatin and celecoxib inhibit prostate PC-3 tumors in immunodeficient mice. Clinical cancer research. 2007;13:5480–5487. doi: 10.1158/1078-0432.CCR-07-0242. [DOI] [PubMed] [Google Scholar]
- 26.Huang H, Xuan C, Li D. et al. Combination of α-Tomatine and Curcumin Inhibits Growth and Induces Apoptosis in Human Prostate Cancer Cells. Plos One. 2015;10:e0144293. doi: 10.1371/journal.pone.0144293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bitting RL, Armstrong AJ. Targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocrine-related cancer. 2013;20:R83–R99. doi: 10.1530/ERC-12-0394. [DOI] [PubMed] [Google Scholar]
- 28.Quan Y, Wang N, Chen Q. et al. SIRT3 inhibits prostate cancer by destabilizing oncoprotein c-MYC through regulation of the PI3K/Akt pathway. Oncotarget. 2015;6:26494–26507. doi: 10.18632/oncotarget.4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haldar S, Chintapalli J, Croce CM. Taxol induces bcl-2 phosphorylation and death of prostate cancer cells. Cancer Research. 1996;56:1253–1255. [PubMed] [Google Scholar]
- 30.Liu HY, Yu X, Liu H. et al. Co-targeting EGFR and survivin with a bivalent aptamer-dual siRNA chimera effectively suppresses prostate cancer. Scientific Reports. 2016;6:30346. doi: 10.1038/srep30346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakahara T, Kita A, Yamanaka K. et al. YM155, a novel small-molecule survivin suppressant, induces regression of established human hormone-refractory prostate tumor xenografts. Cancer Research. 2007;67:8014–8021. doi: 10.1158/0008-5472.CAN-07-1343. [DOI] [PubMed] [Google Scholar]
- 32.Liang M, Zhan F, Zhao J. et al. CPA-7 influences immune profile and elicits anti-prostate cancer effects by inhibiting activated STAT3. Bmc Cancer. 2016;16:504. doi: 10.1186/s12885-016-2488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mora LB, Buettner R, Seigne J. et al. Constitutive activation of Stat3 in human prostate tumors and cell lines: direct inhibition of Stat3 signaling induces apoptosis of prostate cancer cells. Cancer Research. 2002;62:6659–6666. [PubMed] [Google Scholar]
- 34.Canesin G, Evans-Axelsson S, Hellsten R. et al. The STAT3 Inhibitor Galiellalactone Effectively Reduces Tumor Growth and Metastatic Spread in an Orthotopic Xenograft Mouse Model of Prostate Cancer. European Urology. 2015;69:400–404. doi: 10.1016/j.eururo.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Sun M, Liu C, Nadiminty N. et al. Inhibition of Stat3 activation by sanguinarine suppresses prostate cancer cell growth and invasion. Prostate. 2012;72:82–9. doi: 10.1002/pros.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karanika S, Karantanos T, Kurosaka S. et al. GLIPR1-ΔTM synergizes with docetaxel in cell death and suppresses resistance to docetaxel in prostate cancer cells. Molecular cancer. 2015;14:122. doi: 10.1186/s12943-015-0395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Attia RT, Tolba MF, Trivedi R. et al. The chemomodulatory effects of glufosfamide on docetaxel cytotoxicity in prostate cancer cells. PeerJ. 2016;4:e2168. doi: 10.7717/peerj.2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Minas TZ, Han J, Javaheri T. et al. YK-4-279 effectively antagonizes EWS-FLI1 induced leukemia in a transgenic mouse model. Oncotarget. 2015;6:37678–94. doi: 10.18632/oncotarget.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darshan MS, Loftus MS, Thadani-Mulero M. et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer research. 2011;71:6019–6029. doi: 10.1158/0008-5472.CAN-11-1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Botrel TE, Clark O, Lima Pompeo AC. et al. Efficacy and Safety of Combined Androgen Deprivation Therapy (ADT) and Docetaxel Compared with ADT Alone for Metastatic Hormone-Naive Prostate Cancer: A Systematic Review and Meta-Analysis. PloS one. 2016;11:e0157660. doi: 10.1371/journal.pone.0157660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen YU, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Current opinion in pharmacology. 2008;8:440–448. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]