Abstract
Transforming growth factor beta (TGF-β) is a multifunctional protein that induces gene expression of cartilage-specific molecules, but its exact role in the process of chondrogenesis is unclear. Because recent studies suggest that TGF-β can facilitate chondrogenic precursor cells differentiating into chondrocytes, we sought to determine whether TGF-β prevents denervation-induced reduction of endochondral bone formation in an experimental model. Mice were treated daily with recombinant human TGF-β1 (rhTGF-β1) for 3 weeks. We found that rhTGF-β1 not only prevented denervation-induced reduction of gene expression of type II collagen, type X collagen, aggrecan, Indian hedgehog, and parathyroid hormone-related peptide, but also synergized endochondral differentiation. These results demonstrate that short-term systemic administration of TGF-β substantially prevents denervation-induced reduction of endochondral bone formation via stimulating endochondral differentiation. Potential therapeutic applications will be pursued in further studies that address the molecular biological mechanism of TGF-β on endochodral bone formation after denervation in animal models.
Keywords: TGF-β1, denervation, endochondral differentiation, ColII, ColX, aggrecan.
Introduction
Denervation is associated with reduced skeletal bone formation in patients and experimental models 1, 2, resulting in low bone density, diminished linear growth velocity (possibly long-term) and an increased risk of fractures, osteoporosis and reduced peak bone mass. Recent studies have shown that members of the transforming growth factor-β (TGF-β)/activin/bone morphogenetic protein (BMP) cytokine family play an important role in stimulation of bone formation 3-5. Most members of the TGF-β superfamily are expressed in cartilage tissue 6-8 and may affect skeletal histogenesis of cartilage formation and growth. TGF-β promotes chondrogenesis in cultures of early undifferentiated mesenchymal cells in vitro 9, 10 and stimulates chondrogenic differentiation in vivo 11-13. TGF-β also stimulates collagen synthesis 14, 15 and chemotaxis of osteoblast-like cells 16, 17 and promotes soft tissue wound healing after denervation in vivo 18. TGF-β is expressed at all stages during fracture repair, and exogenous TGF-β has dramatic effects on gene expression and differentiation of cartilage and bone cells during bone repair 19. These findings illustrate that TGF-β can regulate mesenchymal cell and chondrocyte function, but the mechanism is unclear.
Studies have demonstrated that TGF-β, BMP-2, and growth/differentiation factor-5 (GDF-5, also known as cartilage-derived morphogenetic protein-1, CDMP-1) 20-22 rapidly induce type II collagen expression in chondrocytes, suggesting a critical signaling role for the TGF-β superfamily in chondrocyte-specific gene expression. A member of the hedgehog family, Indian hedgehog (Ihh), expresses skeletal elements in prehypertrophic chondrocytes in the growth plate 23-25 and stimulates chondrocyte differentiation 26. Ectopic expression of Ihh up-regulates parathyroid hormone-related protein (PTHrP) gene expression 25, while TGF-β stimulates expression of PTHrP in articular chondrocytes 27. We therefore investigated the role of TGF-β in regulating denervation-induced reduction of endochondral bone formation in mice and the pathway that regulates endochondral differentiation.
Materials and methods
Animals
Denervation was induced as previously described by sciatic nerve crush 5. Briefly, 5-week old male C57BL/6 mice (Harlan Company, Indianapolis, IN) weighing 16-18 g were acclimated for 1 week at an ambient temperature of 25ºC with 12 h light and dark cycles and were fed rodent chow and water ad libitum. The 6-week-old mice were divided into sham-operated (n = 7) and sciatic nerve crush (n = 7) groups.
All mice were treated by subcutaneous injection with either vehicle or rhTGF-β1 100 μg/kg daily 2 hrs before operation. Mice were given intraperitoneal (IP) injections of 2.5 mg/kg calcein on day 2 and 25 mg/kg tetracycline-HCl (Achromycin) on day 8. Mice were weighed and then killed quickly by cervical dislocation after deep anesthesia induction with methoxyflurane. Rib cartilage, articular cartilage and intervertebral disc were isolated from each animal and pooled respectively for total RNA or culture.
Unless indicated otherwise, all chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). All mice were cared for in accordance with the National Research Council's Guide for the Care and Use of Laboratory Animals. The experiments were conducted at University of Chicago, laboratory animal facility, in barrier housing within AAALAC-accredited animal quarters under protocols reviewed and approved by the Institutional Animal Care and Use Committee.
Preparation of rhTGF-β1
RhTGF-β1 was purchased from Sigma and bioactivity of samples was confirmed using the mink lung inhibition assay 28. Samples were in 0.1% mouse serum albumin (MSA), 0.04 M sodium citrate buffer at pH 6.2.
Mouse rib cartilage primary culture
Chondrocytes were isolated from mouse rib cartilages as described previously 29. Briefly, cartilaginous rib cages were pre-incubated in 3 mg/ml collagenase (Sigma) in DMEM (Gibco/Invitrogen, Carlsbad, CA) for 45 min at 37ºC and rinsed with PBS. They were further incubated in 3 mg/ml collagenase in DMEM at 37ºC for 5 hrs, with undigested parts discarded. Chondrocytes were collected and rinsed with medium twice before seeding at 1.0 x 105 cells/cm2 in tissue culture plates. The cells were grown in DMEM supplemented with antibiotics (100 U/ml penicillin G sodium, 0.25 μg/ml amphotericin B, and 100 μg/ml streptomycin sulfate) and 10% FBS (Gibco/Invitrogen).
Quantitative real-time PCR
Total RNA was prepared from mouse cartilages by the single-step method as described previously 30,31. RNA was suspended in RNase/DNase-free water (Gibco/Invitrogen) and quantified with an Agilent 2100 Bioanalyzer using the RNA 6000 Nano Assay (Agilent Technologies, Palo Alto, CA). First-strand cDNA synthesis was performed using the SuperScript first-stand synthesis system for real-time PCR (Life Technologies, Rockville, MD) with oligo (dT) as the primer according to manufacturer's protocol. As an additional quality control, GAPDH mRNA primers were added to each RNA sample prior to cDNA synthesis. Oligonucleotide primers and Taqman probes were obtained from Applied Biosystems. Real-time PCR was performed in a Smart Cycler (Cepheid, Sunnyvale, CA). Measurements were taken at the end of the 72ºC extension step in each cycle, and the second-derivative method was used to calculate the threshold cycle. Melt curve analysis showed a single sharp peak for all samples. Real-time PCR was also performed with primers specific for GAPDH. Data were normalized to GAPDH mRNA.
Western blot analysis
Aliquots of the cartilage extract (100 μg protein) were loaded on a 4-20% tris-glycerine gel (Novex, San Diego, CA) and transblotted onto nitrocellulose-ECL membranes (Amersham International, Amersham, UK). Membranes were blocked with 10% non-fat dry milk in Tris-buffered saline, pH 7.6, containing 0.05% Tween-20 (TTBS) for 1 h. Type II collagen (ColII), type X collagen (ColX) and aggrecan protein levels were determined using a rabbit polyclonal anti-human ColII, ColX and aggrecan antibody as primary antibody and peroxidase-conjugated goat anti-rabbit IgG as a secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). β-actin levels were determined to control for equal loading of the lanes by using a rabbit monoclonal anti-β-actin antibody as primary antibody and peroxidase-conjugated goat anti-rabbit IgG as a secondary antibody (Sigma). The blots were then washed in TTBS for 5 min x 3, in Tris-buffered saline, pH 7.6, for 5 min, incubated in enhanced chemiluminescence reagents (Amersham Life Sciences, Buckingham, UK), exposed on radiographic film (Eastman Kodak, Rochester, NY) and quantified by densitometry. The levels were expressed as arbitrary units based on GR/β-actin ratios.
Determination of GAG synthesis rates and GAG accumulation
The chondrocytes were labeled with 2 μCi/ml of [35S]-sulfate for 6 hrs after treatment with rhTGF-β1 (1 ng/ml) for 1 hr and/or dipalmitoylated Ihh (dpIhh) 32 and PTHrP (1-34) (Sigma). Glycosaminoglycan (GAG) synthesis was determined by measuring incorporation of [35S]-sulfate into material precipitated with cetylpyridinium chloride after protease digestion 33. DNA contents were determined by the fluorometric procedure as described previously 34. To detect sulfated proteoglycan accumulation, cultures were fixed with 3% acetic acid (adjusted to pH 1.0; using HCl) for 5 minutes and stained with 0.1% Alcian blue in 3% acetic acid (pH 1.0) 35.
Statistics
Results are given as means ± SEM. Student's t-test or analysis of variance (ANOVA) followed by Schaffer's test was used for statistical analysis as appropriate.
Results
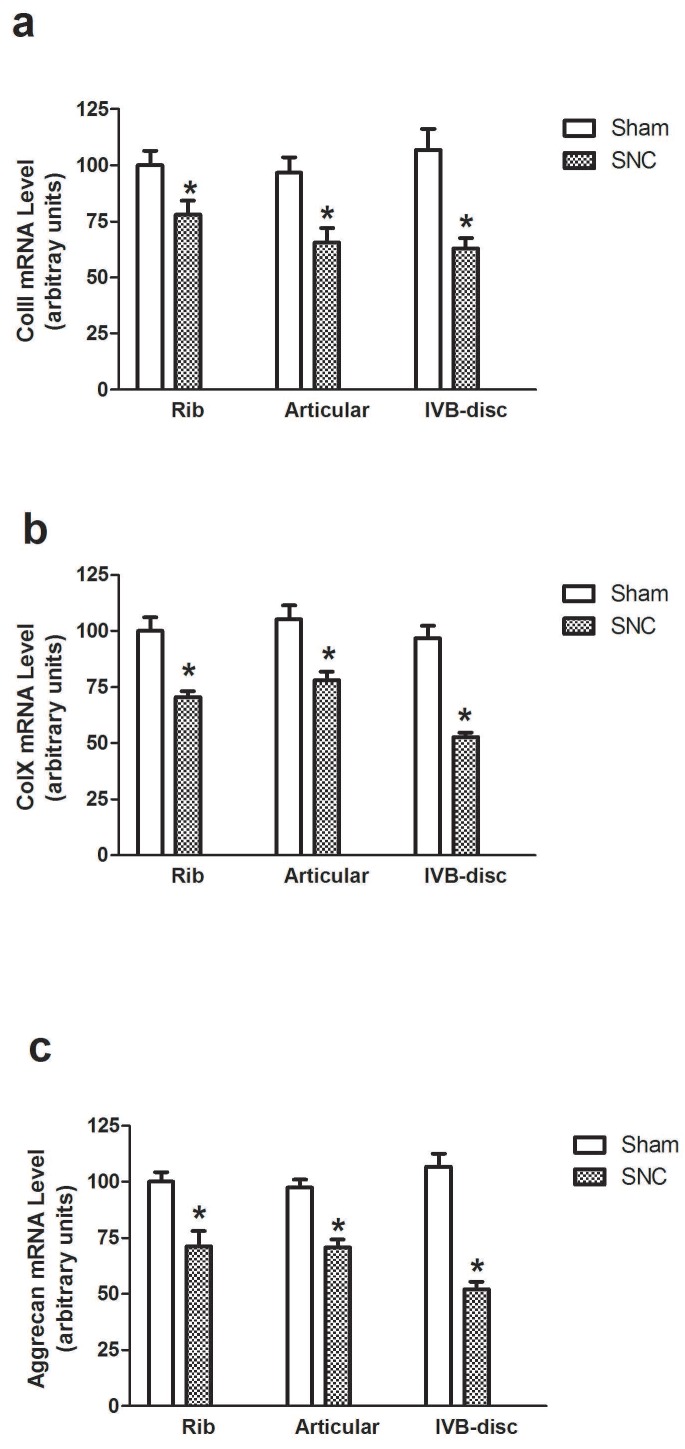
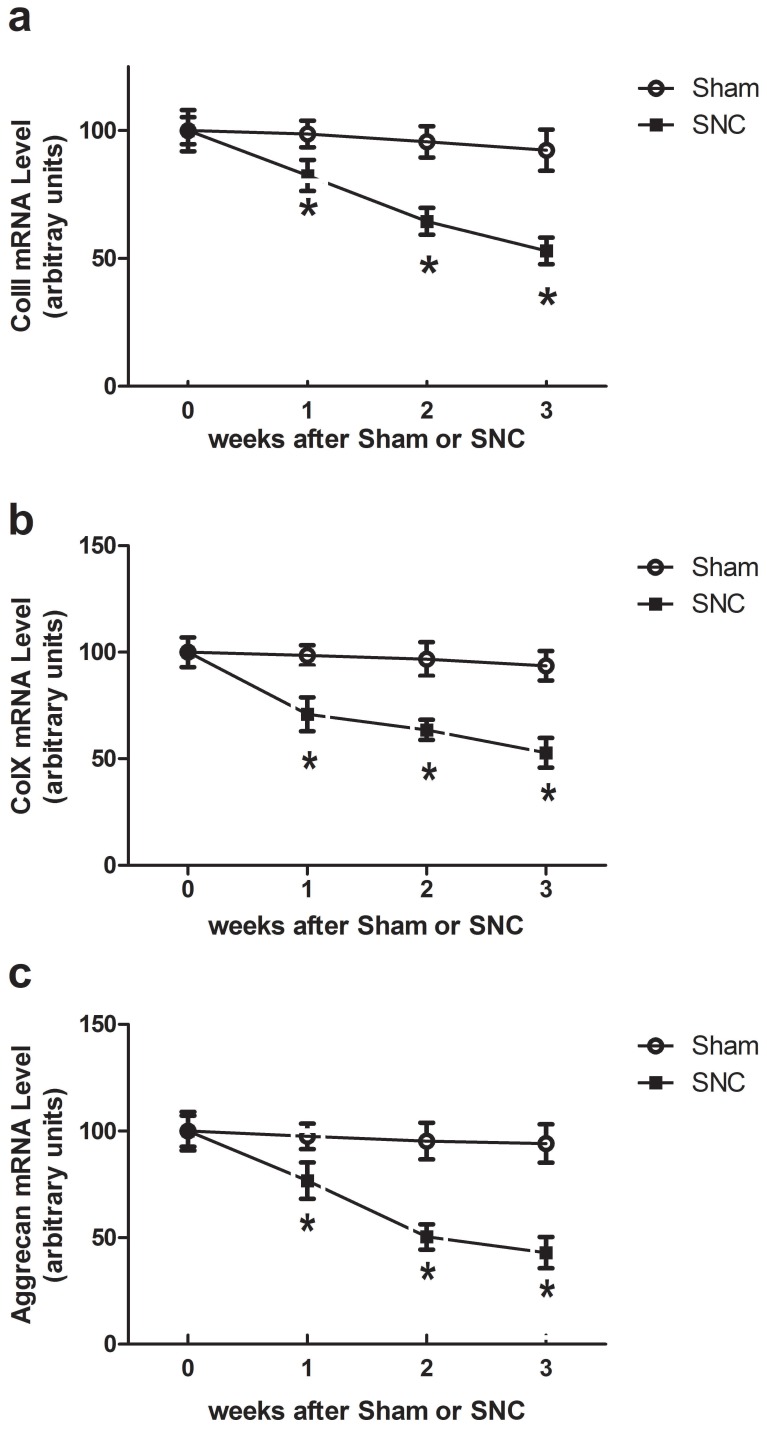
Using real-time PCR analysis, we detected the expression of genes encoding ColII, ColX and aggrecan, the phenotypic markers of endochondral differentiation cells, in rib cartilage, articular cartilage and intervertebral disc of mice at weeks 1, 2 and 3 after sham-operated and sciatic nerve crush. Sciatic nerve crush reduced ColII, ColX and aggrecan mRNA levels in all regions of rib cartilage, articular cartilage and intervertebral disc of mice while the greatest reduction was seen in the intervertebral disc (Fig. 1). The time point results showed that the Sciatic nerve crush reduced ColII, ColX and aggrecan mRNA levels in the intervertebral disc of mice beginning at week 1 and continuing to week 3 (Fig. 2).
Figure 1.
Effect of denervation on ColII (a), ColX (b) and aggrecan (c) gene expression. Data expressed as mean ± SEM (n = 7) with * p < 0.05, sciatic nerve crush (SNC) vs sham-operation (sham) by Student's t-test.Fig.2
Figure 2.
Time course effects of denervation on mRNA levels in intervertebral disc of mice. Data expressed as mean ± SEM (n = 7) with * p < 0.05, SNC vs sham by Student's t-test. The time point 0 hr represents normal mice prior to operation.
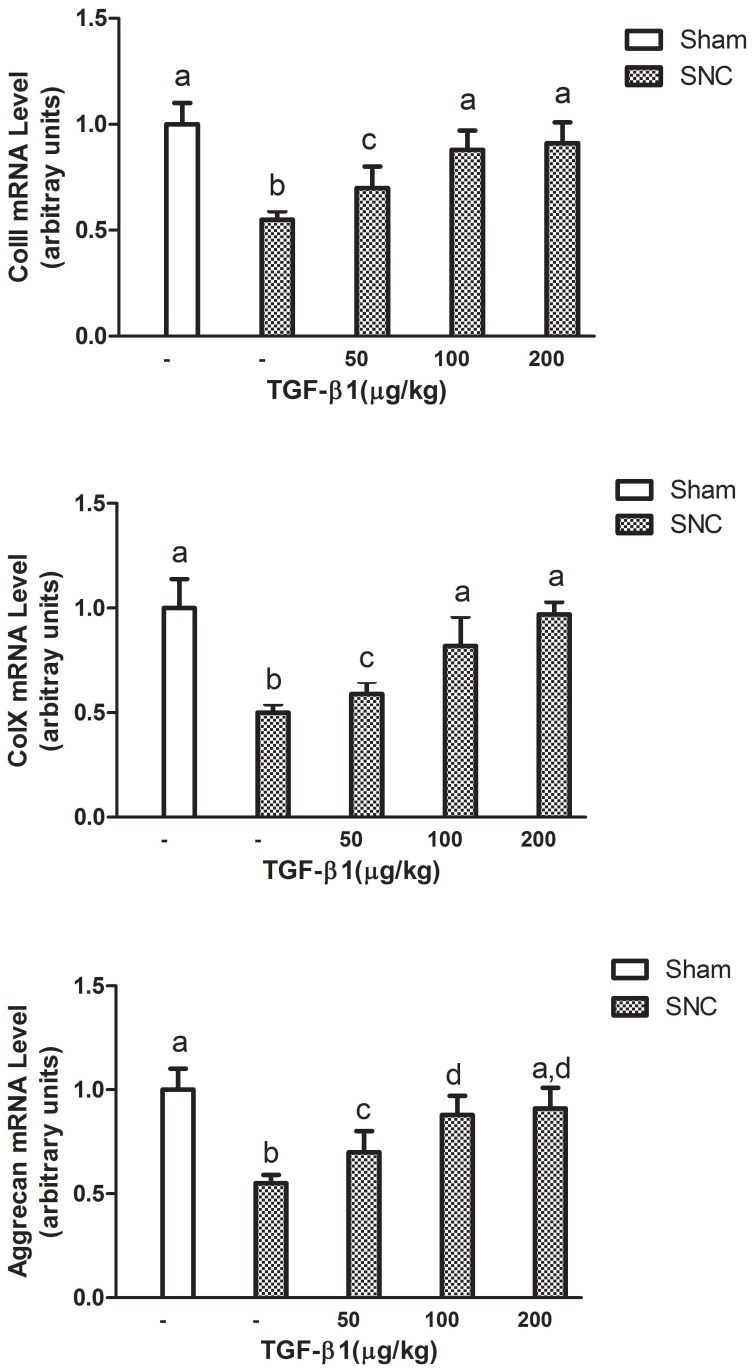
We next measured ColII, ColX and aggrecan mRNA levels in the intervertebral disc of mice with rhTGF-β1 treatment at week 3 after sciatic nerve crush. The real-time PCR analysis showed that all ColII, ColX and aggrecan mRNA levels in the intervertebral disc of sciatic nerve crushed-mice were significantly increased starting at 50 μg/kg of rhTGF-β1. The effects were observed in a consistent dose-dependent manner as 100 μg/kg of rhTGF-β1 further enhanced the mRNA levels of ColII, ColX and aggrecan to the normal ranges (Fig. 3).
Figure 3.
Dose dependent effects of rhTGF-β1 on mRNA levels in intervertebral disc of mice. Data expressed as mean ± SEM (n = 7), * p < 0.05 with different letter among all groups by ANOVA.
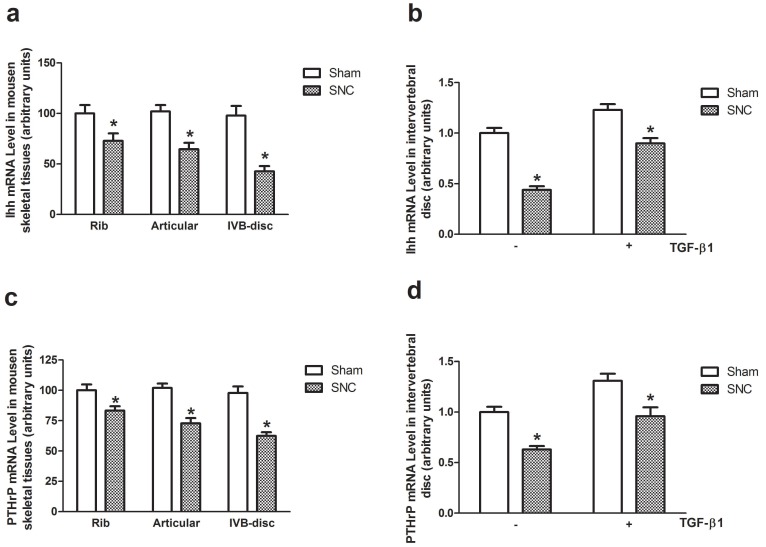
The Ihh mRNA and PTHrP mRNA levels were detected by real-time PCR in rib cartilage, articular cartilage and intervertebral disc of mice at week 3 after sham-operated and sciatic nerve crush. The greatest reduction of both Ihh mRNA and PTHrP mRNA levels were observed in intervertebral disc (Fig. 4a and 4c). With rhTGF-β1 treatment, the Ihh mRNA levels were significantly increased in both sham-operated and sciatic nerve crushed-mice (Fig. 4b). The similar effects of rhTGF-β1 treatment on PTHrP mRNA levels were observed in intervertebral disc of sham-operated and sciatic nerve crushed mice as well (Fig. 4d).
Figure 4.
The Ihh and PTHrP gene expression. The effects of denervation on Ihh (a) and PTHrP (c) mRNA levels. Data expressed as mean ± SEM (n = 7) with * p < 0.05, SNC vs sham by Student's t-test. The effects of rhTGF-β1 on Ihh (b) and PTHrP (d) mRNA levels. Data expressed as mean ± SEM (n = 7), * p < 0.05 with different letter among all groups by ANOVA.
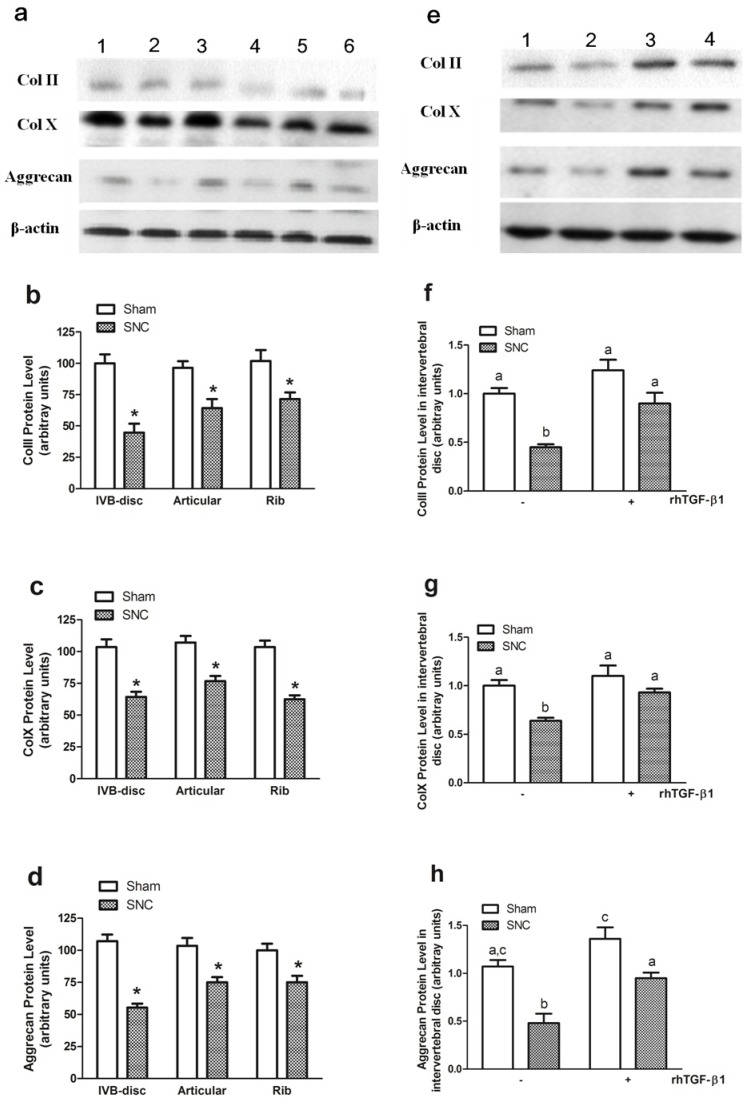
The western blot analysis was applied to detect the protein levels of ColII, ColX and aggrecan. The results showed that the protein levels were significantly decreased after sciatic nerve crush in all cartilage samples (Figs. 5a, 5b, 5c, and 5d), with the greatest changes of all proteins again seen in the intervertebral disc. In addition, rhTGF-β1 treatment prevented sciatic nerve crush-induced reduction of ColII, ColX and aggrecan protein levels (Fig. 5e, 5f, 5g, and 5h), which was similar to what we observed in mRNA levels. β-actin protein levels were not altered by any of the treatment and thus served as internal controls. These results suggest that the reduction in phenotypic markers of endochondral differentiation in mouse cartilage may be the result of decreased production of ColII, ColX and aggrecan, possibly regulated at the transcriptional level.
Figure 5.
ColII, ColX and aggrecan protein levels. (a) A representative autoradiograph of Western blots signals in intervertebral disc (lane a1 and a2), rib cartilage (lane a3 and a4) and articular cartilage (lane a5 and a6) in Sham (lane a1, a3 and a5 for ColII and aggrecan; lane a1, a3 and a6 for ColX) and SNC (lane a2, a4 and a6 for ColII and aggrecan; lane a2, a4 and a5 for ColX ) mice.(b) ColII, (c) ColX and (d) Aggrecan protein level after sciatic nerve crush. (e) A representative autoradiograph of Western blots signals in intervertebral disc of mice with treatment of sham (lane e1), SNC-vehicle (lane e2), sham-rhTGF-β1 (lane e3 for ColII and aggrecan; lane e4 for ColX) and SNC-rhTGF-β1 (lane e4 for ColII and aggrecan; lane e3 for ColX). (f) ColII, (g) ColX and (h) Aggrecan protein level in intervertebral disc of mice with treatment rhTGF-β1. Data are means ± SEM (n = 7) with * p < 0.05, sham vs SNC by Student's t-test for (b-d) and with different letter among all groups by ANOVA for (f-h).
Using murine rib cartilage-derived chondrocytes in primary cultures, we found on day 7 of culture that control cells (without rhTGF-β1 treatment) displayed flattened fibroblastic cell morphology with a nonrefractile contour (likely due to lack of a proteoglycan-rich pericellular matrix). In contrast, cells grown in rhTGF-β1 containing medium displayed a polygonal morphology and a highly refractile contour indicative of abundant pericellular matrix accumulation (data not shown). These cytological traits are characteristic of differentiated chondrocytes in monolayer culture.
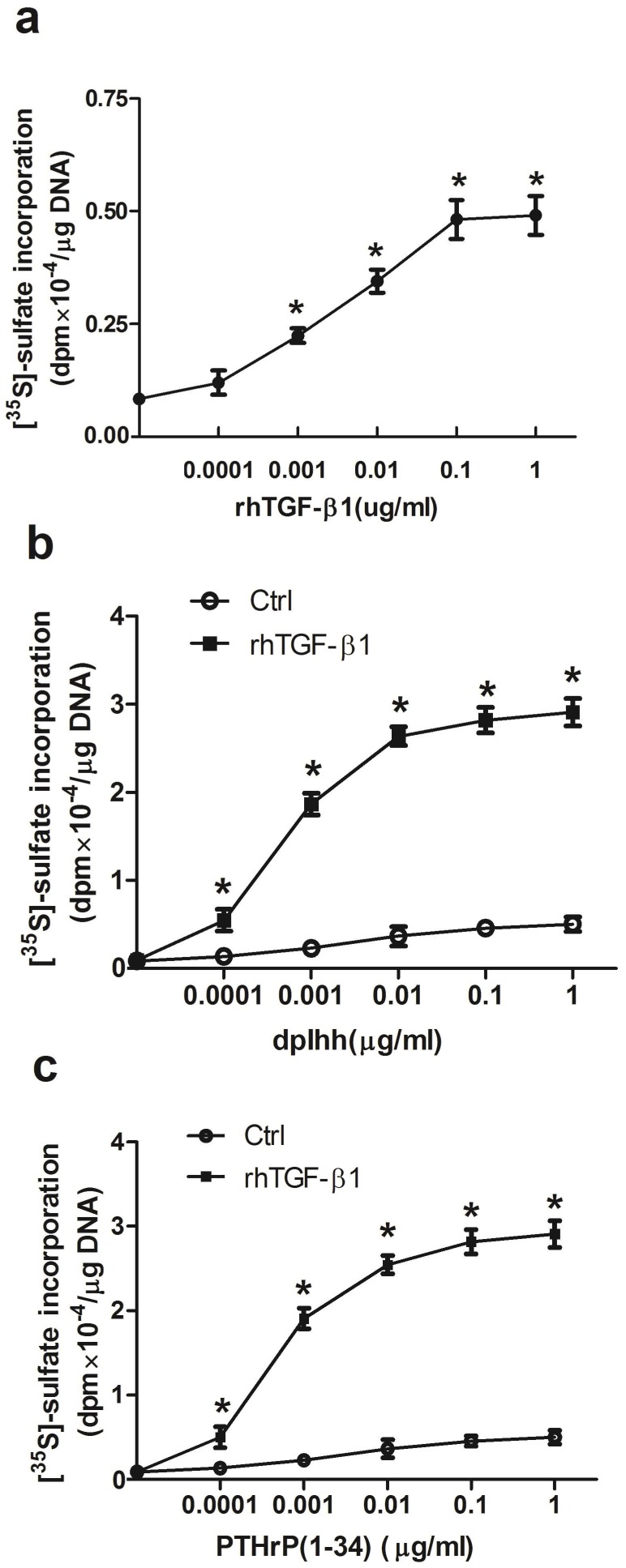
Cells grown in control medium failed to stain with Alcian blue; while rhTGF-β1 treated cultures were stained positively (data not shown). Proteoglycan synthesis, measured by incorporation of [35S]-sulfate into GAG, was limited in control cells and stimulated by rhTGF-β1. To determine whether rhTGF-β1 stimulated proteoglycan synthesis in a dose-dependent manner, cell cultures were treated with increasing amounts of rhTGF-β1 for 7 days (Fig. 6a). Proteoglycan synthesis was progressively stimulated by increasing amounts of rhTGF-β1, with stimulatory effects seen at concentrations as low as 0.1 ng/ml and up to 100 ng/ml. Co-treatments with dpIhh or PTHrP (1-34) and rhTGF-β1 stimulated proteoglycan synthesis (Fig. 6b and 6c), and mouse rib cartilage cultures exhibited positive Alcian blue staining (data not shown).
Figure 6.
Induction of chondrogenic differentiation. Proteoglycan synthesis was determined in parallel cultures that: (a) Dose dependent effects of rhTGF-β1 on proteoglycan synthesis; Treatment with the indicated concentrations of dpIhh (b) or PTHrP(1-34) (c) and in presence or absence of 1 ng/ml of rhTGF-β1 for 7 days. During the last 6 h, cultures were labeled with [35S]-sulfate and incorporation into proteoglycan was measured. Values represent means ± SEM from 3 individual experiments with * p < 0.05, rhTGF-β1 (a), or dpIhh (b) or PTHrP(1-34) (c) treatment vs control at each concentration point by Student's t-test.
Discussion
In this study, denervation decreased mRNA expression of ColII, ColX and aggrecan in mouse rib cartilage, articular cartilage and intervertebral disc, suggesting that endochondral bone formation is inhibited by denervation. Furthermore, reduced ColII, ColX and aggrecan protein levels in all three cartilage sites (rib, articular, and intervertebral disc) were similar to mRNA levels, indicating that denervation-inhibited endochondral bone formation is not specific for any particular area mouse cartilage, an effect not limited to the sciatic nerve crush area. The mRNA and protein levels of ColII, ColX and aggrecan recovered after treatment with rhTGF-β1, suggesting a therapeutic effect on endochondral bone formation. Stimulated proteoglycan synthesis and a highly refractile contour in primary cultured chondrocyte derived from mouse rib cartilage indicates that rhTGF-β1 enhanced endochondral bone formation may be due to stimulated chondrogenic cell differentiation. Ihh and PTHrP gene expression was also recovered, and rhTGF-β1 had a synergistic effect on chondrogenic cell differentiation, suggesting that chondrogenic cell differentiation stimulated by TGF-β1 may act as a relay signal between Ihh and PTHrP. TGF-β1 was chosen in this study because it expresses the highest level in skeletal tissue among all the isoforms 19.
As bone-forming cells differentiate into chondrocytes through cellular condensation, synthesis of ColII and a marked increase in ColII mRNA level occur. The continuous and progressive accumulation of ColII and aggrecan mRNA after treatment with rhTGF-β1 in denervation mice suggests that TGF-β1 stimulates chondrogenesis in denervation mice, in line with previous reports 12, 36 and supported in the present study by increased proteoglycan synthesis.
The results in the present study that rhTGF-β1 treatment promoted the denervation-induced increase of ColX mRNA and protein levels was unexpected, since TGF-β typically down-regulates gene expression of ColX and chondrocyte differentiation 37. Thereout, rhTGF-β1 treatment in denervated-mice increased ColX expression and endochondral differentiation, suggesting a variance in these cell types and tissues under different experimental conditions.
Factors involved in regulating endochondral differentiation are yet to be elucidated. PTHrP was first identified as the causative agent of humoral hypercalcemia of malignancy 38, and is expressed in normal adult and embryonic cells including osteoblasts and chondrocytes 39-41. PTHrP signals through the same receptor as parathyroid hormone (PTH), a G-protein coupled receptor with seven membrane-spanning domains expressed early in endochondral bone formation and in transition zone chondrocytes 42. Thus, PTHrP is a clear marker for cartilage differentiation. In the present study, we observed that denervation decreased PTHrP mRNA levels, similar to a recent report in which chronic metabolic acidosis (a condition with denervation-like catabolic effects) also down-regulated endochondral differentiation 43.
Ihh, a secreted protein expressed early in hypertrophic chondrocytes, may be involved in regulation of hypertrophic conversion via a feedback loop through the perichondrium 23, 25, 39, 44. However, the mechanism by which Ihh regulates endochondral differentiation is unclear. It is unlikely that Ihh regulates expression of PTHrP directly, but rather is part of a larger pathway. In the present study, denervation reduced gene expression levels of Ihh, supporting the hypothesis that denervation inhibits endochondral differentiation. The present results agree with a previous report in which mouse recombinant N-terminal protein of Ihh upregulated the gene expression of ColX 45, and suggest that Ihh may act through a mechanical signal during cartilage growth and repair.
Most existing therapies in the treatment of denervation patients act primarily through stimulation of bone formation, but inhibition of bone resorption has been relatively neglected. TGF-β has a powerful modulatory effect in bone formation and resorption, and evidence documents a role for TGF-β in chondrogenesis and osteogenesis 46, 47. In the present study, we describe for the first time that TGF-β prevents denervation-reduced bone formation in mice through its effect on chondrogenesis. The data strongly suggests that TGF-β exhibits a therapeutic effect in denervation-induced reduction of endochondral bone formation. The mechanism by which this effect occurs requires further study, so that a complete understanding of the interactions among the signaling pathways may lead to therapeutic strategies for prevention of bone loss after denervation.
Acknowledgments
This research was supported by the Grants of Guangdong Natural Science Fund (9151031701000002), Guangzhou scientific support fund (2009Z1-E351) and in part by National Natural Science Foundation of China Grant (81401920) as well as by awards the Doctoral Program of Higher Education of China Grant (20134433120020).
References
- 1.Lerner UH. Deletions of genes encoding calcitonin/alpha-CGRP, amylin and calcitonin receptor have given new and unexpected insights into the function of calcitonin receptors and calcitonin receptor-like receptors in bone. J Musculoskelet Neuronal Interact. 2006;6(1):87–95. [PubMed] [Google Scholar]
- 2.Chenu C. Role of innervation in the control of bone remodeling. J Musculoskelet Neuronal Interact. 2004;4(2):132–134. [PubMed] [Google Scholar]
- 3.Chen G, Deng C, Li YP. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. INT J BIOL SCI. 2012;8(2):272–288. doi: 10.7150/ijbs.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ripamonti U, Tsiridis E, Ferretti C. et al. Perspectives in regenerative medicine and tissue engineering of bone. Br J Oral Maxillofac Surg. 2011;49(7):507–509. doi: 10.1016/j.bjoms.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Mendias CL, Gumucio JP, Davis ME. et al. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. MUSCLE NERVE. 2012;45(1):55–59. doi: 10.1002/mus.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang SC, Hoang B, Thomas JT. et al. Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development. J BIOL CHEM. 1994;269(45):28227–28234. [PubMed] [Google Scholar]
- 7.Horner A, Kemp P, Summers C. et al. Expression and distribution of transforming growth factor-beta isoforms and their signaling receptors in growing human bone. BONE. 1998;23(2):95–102. doi: 10.1016/s8756-3282(98)00080-5. [DOI] [PubMed] [Google Scholar]
- 8.Loeser RF, Carlson CS, McGee MP. Expression of beta 1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. EXP CELL RES. 1995;217(2):248–257. doi: 10.1006/excr.1995.1084. [DOI] [PubMed] [Google Scholar]
- 9.Engstrand T. Molecular biologic aspects of cartilage and bone: potential clinical applications. Ups J Med Sci. 2003;108(1):25–35. [PubMed] [Google Scholar]
- 10.Merino R, Ganan Y, Macias D. et al. Bone morphogenetic proteins regulate interdigital cell death in the avian embryo. Ann N Y Acad Sci. 1999;887:120–132. doi: 10.1111/j.1749-6632.1999.tb07927.x. [DOI] [PubMed] [Google Scholar]
- 11.Kulyk WM, Rodgers BJ, Greer K. et al. Promotion of embryonic chick limb cartilage differentiation by transforming growth factor-beta. DEV BIOL. 1989;135(2):424–430. doi: 10.1016/0012-1606(89)90191-7. [DOI] [PubMed] [Google Scholar]
- 12.Ripamonti U, Crooks J, Matsaba T. et al. Induction of endochondral bone formation by recombinant human transforming growth factor-beta2 in the baboon (Papio ursinus) GROWTH FACTORS. 2000;17(4):269–285. doi: 10.3109/08977190009028971. [DOI] [PubMed] [Google Scholar]
- 13.Schofield JN, Wolpert L. FEffect of TGF-beta 1, TGF-beta 2, and bFGF on chick cartilage and muscle cell differentiation. EXP CELL RES. 1990;191(1):144–148. doi: 10.1016/0014-4827(90)90048-f. [DOI] [PubMed] [Google Scholar]
- 14.Centrella M, Massague J, Canalis E. Human platelet-derived transforming growth factor-beta stimulates parameters of bone growth in fetal rat calvariae. ENDOCRINOLOGY. 1986;119(5):2306–2312. doi: 10.1210/endo-119-5-2306. [DOI] [PubMed] [Google Scholar]
- 15.Cutroneo KR. Gene therapy for tissue regeneration. J CELL BIOCHEM. 2003;88(2):418–25. doi: 10.1002/jcb.10357. [DOI] [PubMed] [Google Scholar]
- 16.Panagakos FS. Transforming growth factor-alpha stimulates chemotaxis of osteoblasts and osteoblast-like cells in vitro. Biochem Mol Biol Int. 1994;33(4):643–650. [PubMed] [Google Scholar]
- 17.Pfeilschifter J, Wolf O, Naumann A. et al. Chemotactic response of osteoblastlike cells to transforming growth factor beta. J BONE MINER RES. 1990;5(8):825–830. doi: 10.1002/jbmr.5650050805. [DOI] [PubMed] [Google Scholar]
- 18.Komarcevic A. The modern approach to wound treatment. Med Pregl. 2000;53(7-8):363–368. [PubMed] [Google Scholar]
- 19.Cho TJ, Gerstenfeld LC, Einhorn TA. Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J BONE MINER RES. 2002;17(3):513–520. doi: 10.1359/jbmr.2002.17.3.513. [DOI] [PubMed] [Google Scholar]
- 20.Kusafuka K, Luyten FP, De Bondt R. et al. Immunohistochemical evaluation of cartilage-derived morphogenic protein-1 and -2 in normal human salivary glands and pleomorphic adenomas. VIRCHOWS ARCH. 2003;442(5):482–490. doi: 10.1007/s00428-003-0761-y. [DOI] [PubMed] [Google Scholar]
- 21.O'Driscoll SW, Fitzsimmons JS. The role of periosteum in cartilage repair. Clin Orthop Relat Res. 2001;391(Supp l):S190–S207. doi: 10.1097/00003086-200110001-00019. [DOI] [PubMed] [Google Scholar]
- 22.Valcourt U, Gouttenoire J, Aubert-Foucher E. et al. Alternative splicing of type II procollagen pre-mRNA in chondrocytes is oppositely regulated by BMP-2 and TGF-beta1. FEBS LETT. 2003;545(2-3):115–119. doi: 10.1016/s0014-5793(03)00510-6. [DOI] [PubMed] [Google Scholar]
- 23.Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. DEV BIOL. 1995;172(1):126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- 24.Koyama E, Leatherman JL, Noji S. et al. Early chick limb cartilaginous elements possess polarizing activity and express hedgehog-related morphogenetic factors. Dev Dyn. 1996;207(3):344–354. doi: 10.1002/(SICI)1097-0177(199611)207:3<344::AID-AJA11>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Vortkamp A, Lee K, Lanske B. et al. Regulation of rate of cartilage differentiation by Indian hedgehog and PTH-related protein. SCIENCE. 1996;273(5275):613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- 26.Enomoto-Iwamoto M, Nakamura T, Aikawa T. et al. Hedgehog proteins stimulate chondrogenic cell differentiation and cartilage formation. J BONE MINER RES. 2000;15(9):1659–1668. doi: 10.1359/jbmr.2000.15.9.1659. [DOI] [PubMed] [Google Scholar]
- 27.Tsukazaki T, Ohtsuru A, Enomoto H. et al. Expression of parathyroid hormone-related protein in rat articular cartilage. Calcif Tissue Int. 1995;57(3):196–200. doi: 10.1007/BF00310258. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa Y, Seyedin SM. Purification of transforming growth factors beta 1 and beta 2 from bovine bone and cell culture assays. Methods Enzymol. 1991;198:317–327. doi: 10.1016/0076-6879(91)98032-2. [DOI] [PubMed] [Google Scholar]
- 29.Lefebvre V, Garofalo S, Zhou G. et al. Characterization of primary cultures of chondrocytes from type II collagen/beta-galactosidase transgenic mice. MATRIX BIOL. 1994;14(4):329–335. doi: 10.1016/0945-053x(94)90199-6. [DOI] [PubMed] [Google Scholar]
- 30.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. ANAL BIOCHEM. 1987;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 31.Nemeth GG, Heydemann A, Bolander ME. Isolation and analysis of ribonucleic acids from skeletal tissues. ANAL BIOCHEM. 1989;183(2):301–304. doi: 10.1016/0003-2697(89)90483-1. [DOI] [PubMed] [Google Scholar]
- 32.Kellner K, Lang K, Papadimitriou A. et al. Effects of hedgehog proteins on tissue engineering of cartilage in vitro. TISSUE ENG. 2002;8(4):561–572. doi: 10.1089/107632702760240481. [DOI] [PubMed] [Google Scholar]
- 33.Kato Y, Hiraki Y, Inoue H. et al. Differential and synergistic actions of somatomedin-like growth factors, fibroblast growth factor and epidermal growth factor in rabbit costal chondrocytes. Eur J Biochem. 1983;129(3):685–690. doi: 10.1111/j.1432-1033.1983.tb07103.x. [DOI] [PubMed] [Google Scholar]
- 34.Johnson-Wint B, Hollis S. A rapid in situ deoxyribonucleic acid assay for determining cell number in culture and tissue. ANAL BIOCHEM. 1982;122(2):338–344. doi: 10.1016/0003-2697(82)90292-5. [DOI] [PubMed] [Google Scholar]
- 35.Lev R, Spicer SS. Specific staining of sulfate groups with Alcian blue at low pH. J Histochem. Cytochem. 1964;12:309. doi: 10.1177/12.4.309. [DOI] [PubMed] [Google Scholar]
- 36.Kock LM, Ravetto A, van Donkelaar CC. et al. Tuning the differentiation of periosteum-derived cartilage using biochemical and mechanical stimulations. Osteoarthritis Cartilage. 2010;18(11):1528–1535. doi: 10.1016/j.joca.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Kim KO, Sampson ER, Maynard RD. et al. Ski inhibits TGF-beta/phospho-Smad3 signaling and accelerates hypertrophic differentiation in chondrocytes. J CELL BIOCHEM. 2012;113(6):2156–2166. doi: 10.1002/jcb.24089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu H, Monden T, Tomotsune T. et al. A case of myeloma with hypercalcemia caused by high serum concentrations of both parathyroid hormone-related peptide (PTHrP) and macrophage inflammatory protein-1alpha (MIP-1alpha) Intern Med. 2011;50(24):2993–2996. doi: 10.2169/internalmedicine.50.6096. [DOI] [PubMed] [Google Scholar]
- 39.Pacheco M, Valencia M, Caparros-Martin JA. et al. Evc works in chondrocytes and osteoblasts to regulate multiple aspects of growth plate development in the appendicular skeleton and cranial base. BONE. 2012;50(1):28–41. doi: 10.1016/j.bone.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 40.Suva LJ, Winslow GA, Wettenhall RE. et al. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. SCIENCE. 1987;237(4817):893–896. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]
- 41.Lee K, Deeds JD, Segre GV. Expression of parathyroid hormone-related peptide and its receptor messenger ribonucleic acids during fetal development of rats. ENDOCRINOLOGY. 1995;136(2):453–463. doi: 10.1210/endo.136.2.7835276. [DOI] [PubMed] [Google Scholar]
- 42.Kronenberg HM. PTHrP and skeletal development. Ann N Y Acad Sci. 2006;1068:1–13. doi: 10.1196/annals.1346.002. [DOI] [PubMed] [Google Scholar]
- 43.Arnott JA, Lambi AG, Mundy C. et al. The role of connective tissue growth factor (CTGF/CCN2) in skeletogenesis. Crit Rev Eukaryot Gene Expr. 2011;21(1):43–69. doi: 10.1615/critreveukargeneexpr.v21.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burdan F, Szumilo J, Korobowicz A. et al. Morphology and physiology of the epiphyseal growth plate. Folia Histochem Cytobiol. 2009;47(1):5–16. doi: 10.2478/v10042-009-0007-1. [DOI] [PubMed] [Google Scholar]
- 45.Akiyama H, Shigeno C, Iyama K. et al. Indian hedgehog in the late-phase differentiation in mouse chondrogenic EC cells, ATDC5: upregulation of type X collagen and osteoprotegerin ligand mRNAs. Biochem Biophys Res Commun. 1999;257(3):814–820. doi: 10.1006/bbrc.1999.0494. [DOI] [PubMed] [Google Scholar]
- 46.Taipaleenmaki H, Harkness L, Chen L. et al. The crosstalk between transforming growth factor-beta1 and delta like-1 mediates early chondrogenesis during embryonic endochondral ossification. STEM CELLS. 2012;30(2):304–313. doi: 10.1002/stem.792. [DOI] [PubMed] [Google Scholar]
- 47.Grimes R, Jepsen KJ, Fitch JL. et al. The transcriptome of fracture healing defines mechanisms of coordination of skeletal and vascular development during endochondral bone formation. J BONE MINER RES. 2011;26(11):2597–2609. doi: 10.1002/jbmr.486. [DOI] [PubMed] [Google Scholar]