Abstract
Background
Postoperative infection increases cancer recurrence and worsens survival for colorectal cancer, but the relationship after esophagectomy for esophagogastric adenocarcinoma is not well-defined. We aimed to determine whether recurrence and survival after minimally invasive esophagectomy for esophagogastric adenocarcinoma were influenced by postoperative infection using propensity-matched analysis.
Methods
We abstracted data for 810 patients (1997–2010) and defined exposure as at least one in-hospital/30-day infectious complication (n=206; 25%). Using 29 pretreatment/intraoperative variables, patients were propensity score matched (caliper=0.05). Time-to-cancer recurrence and survival (Kaplan-Meier curves, Breslow test), and associated factors (Cox regression with shared frailty) were assessed.
Results
After propensity-matching (n=167 pairs), median bias across propensity score variables was reduced from 12.9% (p<0.001) to 4.4% (p=1.000). Postoperative infection was not associated with rate (n=60 versus 63; McNemar’s p=0.736) or time to recurrence in those who recurred (median 10.7 versus 11.1 months; Wilcoxon signed-rank p-value=0.455), but was associated with shorter overall survival (n=124 versus 102 deaths; median 26 versus 41 months, Breslow p=0.002). After adjusting for age, body mass index, neoadjuvant therapy, sex, comorbidity score, positive resection margins, pathologic stage, R0 resection and recurrence, postoperative infection was associated with a 44% greater hazard for death (HR 1.44; 95% CI 1.10–1.89).
Conclusions
In patients with esophagogastric adenocarcinoma, post-esophagectomy infections were not associated with increased rate or earlier time to recurrence when baseline characteristics associated with infection risk were balanced using propensity-matching. Despite this, overall survival was shorter in patients with infectious complications. After adjusting for other important survival predictors, post-esophagectomy continued to be independently associated with worse survival.
Keywords: Esophageal cancer, Infection, Outcomes, Propensity Matching, Survival Analysis
Studies examining the impact of postoperative infection on long-term colorectal cancer outcomes report improved cancer-specific and overall five-year survival in patients without a postoperative infection independent prognostic impact on recurrence and survival.(1–3) The mechanistic explanations include enhanced postoperative systemic inflammatory response in the setting of infection,(4) which compromises natural immunity and allows residual tumor cells to evade immune detection,(5) and adjuvant therapy delays or avoidance, which increases the likelihood of recurrent disease and decreased survival.(2) The relationship between infection and recurrence and survival outcomes may also be biased by significant differences in baseline characteristics between the groups with and without infection (e.g. age, functional status, disease stage, intraoperative blood transfusions).(1) These same factors are associated with worse cancer outcomes and, when not balanced between groups, introduce bias into the analysis, obscuring precise determination of the associations between the exposure (infectious complications) and the outcome.(6) Methods to balance baseline characteristics, such as propensity-score matching, can be used to create similar groups, thus minimizing bias and allowing for a more precise estimate of the risk associated with the exposure.
The impact of infection on long-term cancer outcomes following esophagectomy for esophagogastric adenocarcinoma is not well understood, although several authors have reported associations between postoperative complications and timing of recurrence and death from recurrent cancer.(7, 8) Given the colorectal data, we hypothesized that postoperative infections would be associated with differential recurrence outcomes and overall survival after esophagectomy. Our study aim was to compare recurrence, disease-free survival, time to recurrence and overall survival following minimally invasive esophagectomy for esophagogastric adenocarcinoma in propensity matched patients 1) with and without postoperative infection; and 2) with and without anastomotic and/or conduit leak.
PATIENTS AND METHODS
Data were reviewed for 810 patients who underwent elective minimally invasive esophagectomy (MIE; our primary approach to esophagectomy) for esophagogastric adenocarcinoma (1997–2010). Open or hybrid esophagectomy (planned open approach to either abdomen or chest) was excluded to minimize bias of approach on recurrence and survival outcomes. Patients with squamous cell histology, metastasis from other sites, low-grade dysplasia or non-elective operation were also excluded. Exposure was defined as at least one infectious event within 30 days postoperatively (n=206; 25%): including sepsis (n=62), Grade II–IV(9) anastomotic or conduit leak (n=106), pneumonia (n=106), or empyema (n=52). The analysis was repeated using only anastomotic or conduit leak as the exposure. This study received Institutional Review Board approval.
Statistical Analysis
Statistical analysis was performed using Stata® version 14.(10) Frequencies and percentages for categorical variables and median with interquartile range (IQR) for continuous variables were determined. Overall survival was calculated from date of MIE to date of death from any cause and censored at date of most recent alive follow-up. Disease-free survival was calculated from date of MIE to date of first recurrence and censored at last clinical assessment for recurrence. In patients with recurrent cancer, time to recurrence was calculated from date of MIE to date of first recurrence.
Propensity Score Matching
To create two groups evenly balanced for their baseline propensity for postoperative infection, propensity scores were generated using 29 pre-operative/intra-operative variables. Seventeen patients with prolonged post-operative ventilation (greater than 48 hours), were excluded due to near-perfect association with infection (16/17), as these patients would have produced poor propensity matches. Patients missing data for any propensity variable were excluded (n=54; 6.8%). The logistic regression dependent variable was post-operative infection. Patients were matched 1:1 by nearest-neighbor matching (caliper size=0.05), without replacement (psmatch2 command).(11) If no suitable match within the 0.05 caliper remained in the control group, the exposed patient was excluded from the matched dataset.
In propensity-matched datasets, disease-free and overall survival were compared using Kaplan-Meier curves, and differences assessed using the Breslow test. Rate of recurrence and time to recurrence in those who recurred were compared between matched pairs using McNemar’s and Wilcoxon signed-rank test, respectively. Factors associated with overall survival were analyzed using multivariable Cox proportional hazards regression with shared frailty to account for matched pairs. Two-sided p-values < 0.05 were considered statistically significant. The analysis was repeated using only anastomotic or conduit leak as the exposure for propensity-score matching (caliper size=0.10 in order to ensure a reasonable matched sample size).
RESULTS
Propensity-score for postoperative infection was generated for 763 patients (94%; n=179 with and n=584 patients without postoperative infection), yielding 167 matched pairs (n=334 patients; Figure 1). Prior to propensity score matching, significant baseline imbalances (defined as absolute standardized differences >15%) were identified; (Table 1) patients who developed post-operative infections were significantly more likely to be female, older and have greater co-morbid burden. (Table 1) Conversion from MIE to thoracotomy and intraoperative blood transfusion were also associated with increased infection while fewer infections were seen with history of gastroesophageal reflux disease and with our chief surgeon (JDL versus all other surgeons). In hospital and/or 30 day mortality for the overall cohort with propensity scores was 2.8%. In the patients with postoperative infection, in hospital and/or 30-day mortality was 8.9% (n=16/179) compared with 0.86% (n=5/584) in patients without postoperative infection. Median absolute standardized bias across all 29 propensity-score variables in the unmatched cohort was 12.9% (p<0.001).
Figure 1.
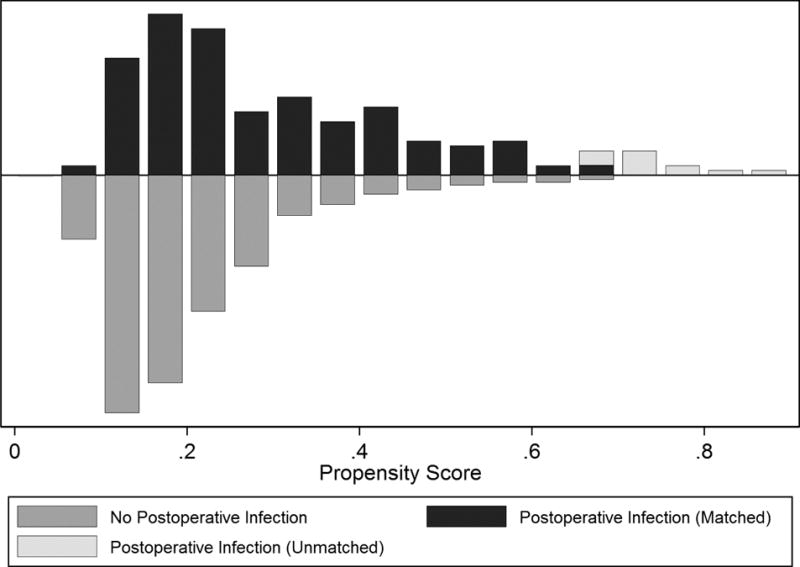
Mirrored histogram depicting balance of patients with and without post-operative infection across propensity scores. (x = propensity score ~ risk of developing an infection, y= number of patients)
Table 1.
Propensity score variables stratified by presence of infection, unmatched but eligible for matching and matched cohorts
| Variable | Unmatched1 | Matched | |||||
|---|---|---|---|---|---|---|---|
| Infection n = 179 (23%) |
No infection n = 584 (77%) |
Absolute Standardized % Bias | Infection n = 167 (%) |
No infection n = 167 (%) |
Absolute Standardized % Bias | ||
| Male sex | 143 (80) | 501 (86) | 15.7 | 133 (80) | 130 (78) | 4.8 | |
| Age, median (IQR) | 68 (59–75) | 64 (56–71) | 28.7 | 66 (59–74) | 65 (59–74) | 7.1 | |
| BMI, median (IQR) | 28 (25–33) | 28 (25–32) | 5.1 | 28 (26–33) | 30 (25–33) | 1.7 | |
| Race, Caucasian | 174 (97) | 573 (98) | 6.0 | 163 (98) | 163 (98) | 0.0 | |
| Smoking history | 129 (72) | 438 (75) | 6.6 | 123 (74) | 122 (73) | 1.4 | |
| Daily alcohol use | 27 (15) | 85 (15) | 1.5 | 26 (16) | 28 (17) | 3.4 | |
| Comorbid conditions | |||||||
| Age-adjusted Charlson comorbidity index score (median; IQR) | 3 (0–6) | 2 (0–4) | 44.2 | 3 (0–5) | 4 (0–4) | 5.8 | |
| History of: | |||||||
| Gastroesophageal reflux | 123 (69) | 442 (76) | 15.6 | 116 (70) | 119 (71) | 4.0 | |
| Barrett’s metaplasia | 121 (68) | 393 (67) | 0.6 | 112 (67) | 115 (69) | 3.8 | |
| Myocardial infarction/coronary artery disease | 63 (35) | 116 (20) | 34.8 | 52 (31) | 53 (32) | 1.4 | |
| Peripheral vascular disease | 28 (16) | 27 (5) | 37.1 | 20 (12) | 20 (12) | 0.0 | |
| Diabetes requiring medical therapy | 39 (22) | 95 (16) | 14.1 | 37 (22) | 41 (25) | 6.1 | |
| Pulmonary disease | 48 (27) | 114 (20) | 17.3 | 41 (25) | 44 (26) | 4.3 | |
| Any prior malignancy in last 5 years | 23 (13) | 47 (8) | 15.7 | 21 (13) | 17 (10) | 7.8 | |
| Peptic ulcer disease | 20 (11) | 51 (9) | 8.1 | 16 (10) | 19 (11) | 6.0 | |
| Any neurologic event or disorder | 18 (10) | 26 (5) | 21.7 | 14 (8) | 16 (10) | 4.6 | |
| Coronary revascularization | 44 (25) | 87 (15) | 24.5 | 35 (21) | 37 (22) | 3.0 | |
| Renal insufficiency or Cerebral vascular accident (composite) | 27 (15) | 31 (5) | 32.7 | 17 (10) | 22 (13) | 10.0 | |
| Neoadjuvant therapy received | 60 (34) | 178 (31) | 6.5 | 56 (34) | 51 (31) | 6.4 | |
| Prior esophageal surgery | 20 (11) | 38 (7) | 16.5 | 17 (10) | 17 (10) | 0.0 | |
| Adenocarcinoma (vs. high grade dysplasia) | 158 (88) | 504 (86) | 5.9 | 146 (87) | 143 (86) | 5.4 | |
| Year of operation* | 2006 | 2006 | 8.6 | 2006 | 2006 | 4.5 | |
| Hospital (Presbyterian vs all others) | 132 (74) | 435 (75) | 1.7 | 45 (27) | 41 (25) | 5.5 | |
| Surgeon (JDL vs all others) | 125 (70) | 427 (73) | 7.3 | 47 (28) | 40 (24) | 9.3 | |
| Thoracic anastomosis (vs cervical) | 95 (53) | 309 (53) | 0.3 | 86 (52) | 91 (55) | 6.0 | |
| Number of lymph nodes examined, median (IQR) | 21 (15–29) | 21 (15–29) | 5.5 | 21 (15–27) | 22 (15–27) | 1.4 | |
| Intraoperative blood transfusion | 52 (29) | 90 (15) | 33.2 | 42 (25) | 45 (27) | 4.4 | |
| Conversion to thoracotomy | 5 (3) | 6 (1) | 12.9 | 4 (2) | 4 (2) | 0.0 | |
| Conversion to laparotomy | 4 (2) | 11 (2) | 2.5 | 3 (2) | 3 (2) | 0.0 | |
median year; trend across timespan 1997–2010; IQR = Interquartile range; JDL = James D. Luketich
After propensity matching, the absolute standardized % bias was 10% or less for all perioperative variables; (Table 1) median absolute standardized bias across all 29 propensity-score variables decreased to 4.4% (p=1.00). (Figure 2) There was notable reduction in % bias for clinically relevant predictors of overall survival, including age at operation (75% reduction in bias), age-adjusted CCI score (87% reduction in bias), and individual comorbid diseases. (Figure 2)
Figure 2.
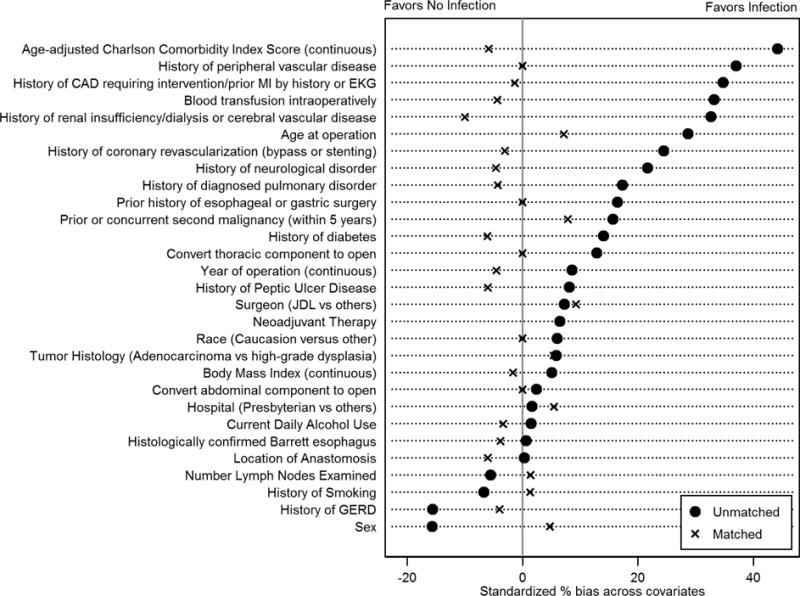
Standardized % bias between patients with and without post-operative infection for pretreatment variables. (MI=myocardial infarction; EKG=electrocardiogram; JDL=James D. Luketich; GERD=gastroesophageal reflux disease)
Recurrence rates prior to propensity matching were similar between the two groups (n=64 [35.8%] versus n=224 [38.4%]; p=0.530) as was disease-free survival (Breslow p-value = 0.359) and median time to cancer recurrence in those who recurred (9.96 versus 10.98 months with and without infection, respectively; Wilcoxon rank-sum test p-value=0.398). In the propensity-matched cohort, 37% recurred (123/334); 36% (n=60) of patients with infection, versus 38% (n=63) of patients without infection (McNemar’s p-value=0.736). There was no difference in disease-free survival. (Figure 3; Breslow p-value 0.458) Median time to recurrent disease in patients who recurred was 10.7 months (IQR 6.3–18 months) versus 11.1 months (IQR 5.8–19.4 months) in patients with and without infection, respectively (Wilcoxon signed-rank p-value=0.455).
Figure 3.
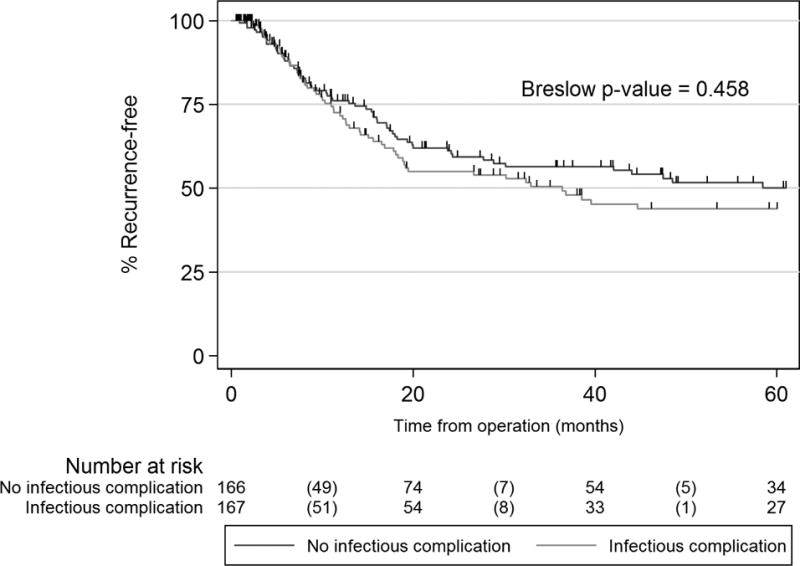
Disease-free survival in propensity-matched cohorts with and without postoperative infection
Postoperative infection was associated with a significantly shorter median overall survival time (21.9 [IQR 7.7–74.3] versus 45.9 months [IQR 18.3–128.4]; Breslow p-value <0.0001) and an increased hazard for death during follow-up prior to propensity- score matching. (Table 2) Patients in the matched dataset had a median time to follow-up of 33.1 months; 74% of matched patients with infection died during follow-up (n=124) compared to 62% (n=103) of patients without infection. Median overall survival was 25.9 months (IQR 8.3–81 months) versus 40.6 months (IQR 18–112 months) in the non-infection group (Figure 4; Breslow p=0.002). Patients with postoperative infection were 44% more likely to be dead at each time-point in follow-up. (Table 2)
Table 2.
Multivariable Cox regression analysis of survival (each variable adjusted for all other variables)
| Unmatched Cohort (n=763) |
Propensity-matched cohort (n=167 pairs) |
|||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% Confidence Interval | p-value | Hazard Ratio | 95% Confidence Interval | p-value1 |
|
|
|
|||||
| Infectious complication | 1.53 | 1.24–1.89 | <0.001 | 1.44 | 1.10–1.89 | 0.008 |
|
|
|
|||||
| Cancer recurrence | 2.26 | 1.81–2.83 | <0.001 | 1.62 | 1.16–2.26 | 0.005 |
|
|
|
|||||
| R0 resection | 0.32 | 0.20–0.52 | <0.001 | 0.26 | 0.10–0.65 | 0.004 |
|
|
|
|||||
| Positive circumferential margins | 1.63 | 1.17–2.29 | 0.004 | 2.50 | 1.34–4.65 | 0.004 |
|
|
|
|||||
| Age2 | 1.03 | 1.02–1.04 | <0.001 | 1.03 | 1.01–1.05 | <0.001 |
|
|
|
|||||
| Age-adjusted Charlson Comorbidity Index Risk Score | 0.0293 | 0.2323 | ||||
| Score 0–1 | Ref | Ref | Ref | Ref | ||
| Score 2–5 | 1.03 | 0.84–1.26 | 0.772 | 1.11 | 0.81–1.52 | 0.506 |
| Score 6 or greater | 1.49 | 1.10–2.03 | 0.011 | 1.44 | 0.94–2.18 | 0.090 |
|
|
|
|||||
| Male sex | 0.99 | 0.77–1.27 | 0.916 | 1.06 | 0.76–1.50 | 0.724 |
|
|
|
|||||
| Neoadjuvant chemotherapy | 1.46 | 1.20–1.78 | <0.001 | 1.40 | 1.02–1.91 | 0.036 |
|
|
|
|||||
| Body mass index2 | 0.99 | 0.98–1.01 | 0.444 | 0.99 | 0.97–1.01 | 0.488 |
|
|
|
|||||
| AJCC 7th edition Pathologic stage (collapsed) | <0.00012 | 0.0342 | ||||
| Stage 0 | Ref | Ref | Ref | Ref | ||
| Stage 1 | 1.01 | 0.70–1.47 | 0.969 | 0.99 | 0.59–1.67 | 0.981 |
| Stage 2 | 1.34 | 0.94–2.00 | 0.121 | 1.30 | 0.76–2.22 | 0.337 |
| Stage 3 | 2.24 | 1.56–3.22 | <0.001 | 1.83 | 1.08–3.08 | 0.024 |
| Stage 4 | 3.18 | 1.59–6.33 | 0.001 | 2.59 | 0.94–7.13 | 0.066 |
|
|
|
|||||
Analysis accounts for shared frailty for matched pairs
Per 1 year or point increase
Overall p-value for multi-category variables
Figure 4.
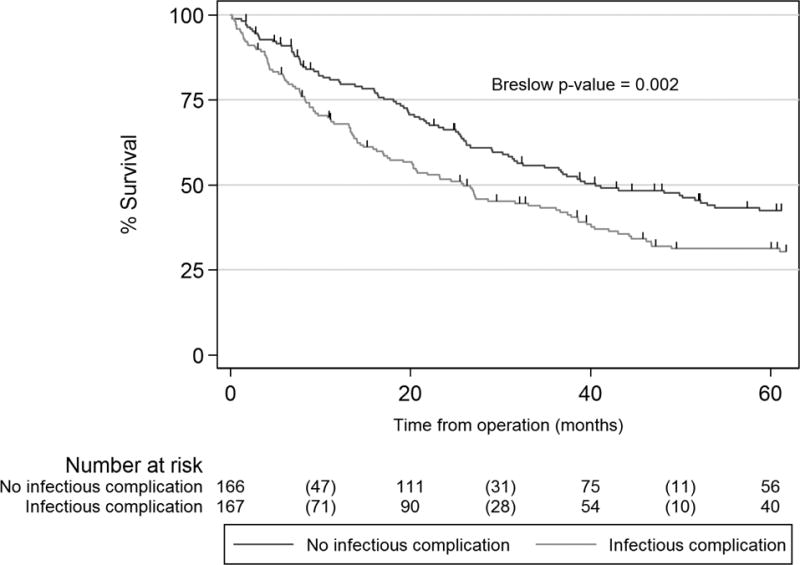
Overall survival in propensity-matched cohorts with and without postoperative infection
Analysis using clinically significant anastomotic or gastric conduit leak as exposure
We repeated the analysis using clinically significant anastomotic or conduit leak as the exposure. Patients in whom the decision to convert to laparotomy was made were excluded from the analysis because of perfect separation among leak patients, with no leaks identified in the 16 patients who had conversion to laparotomy. Following propensity-score generation, 99 of 106 patients with leak were available for matching; 95 matched pairs were generated. The median absolute standardized % bias across 28 propensity-score variables in the unmatched cohort was 14.4% (p=0.003). After matching, the median absolute standardized % bias was 5.8% (p=1.000). (Figure 5) There was no statistically significant difference in recurrence rates between propensity-matched cohorts with and without clinically significant leak (n=38 versus 30; p=0.228), disease-free survival (Breslow p-value 0.158) or median time to recurrence in those who recurred (14.14 versus 15.2 months; Wilcoxon signed-rank test p-value=0.552).
Figure 5.
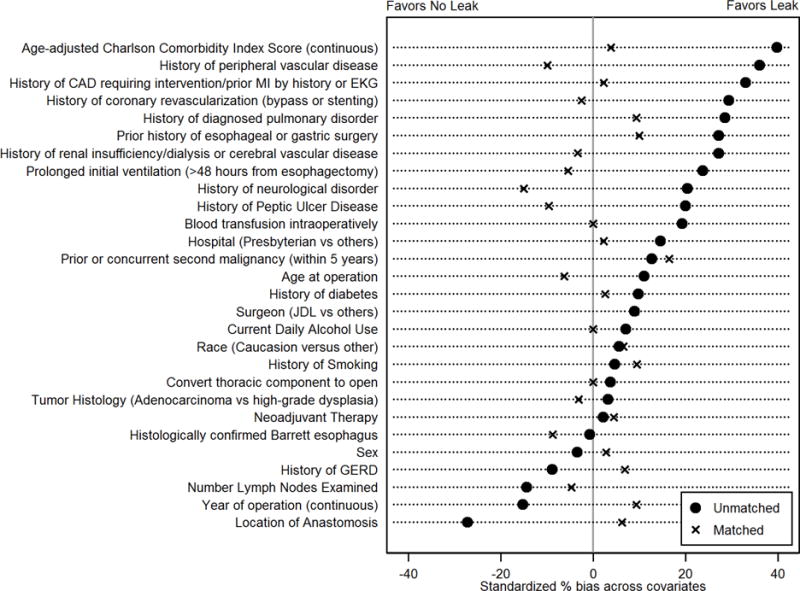
Standardized % bias between patients with and without post-operative leak for pretreatment variables. (MI=myocardial infarction; EKG=electrocardiogram; JDL=James D. Luketich; GERD=gastroesophageal reflux disease)
In the propensity-matched leak dataset, median time to follow-up was 35.6 months; 75% of matched patients with leak died during follow-up (n=71) compared to 64% (n=61) of patients without leak. Median overall survival was 26.8 months (IQR 9.2–99 months) versus 43.1 months (IQR 18–178 months) in the non-leak group (Breslow p=0.014). After adjusting for recurrence, R0 resection status, age at operation, positive circumferential margin, age-adjusted CCI risk score, sex, body mass index, induction therapy, and AJCC 7th edition pathologic stage, postoperative leak was independently associated with a 60% increased hazard of death during follow-up compared to patients without leak (Cox proportional hazard ratio=1.60; 95% CI 1.12–2.89).
COMMENT
Our study sought to determine whether postoperative infections and anastomotic/conduit leaks were associated with differential rates of cancer recurrence, time to recurrence, disease-free survival and overall survival after esophagectomy for the treatment of esophagogastric adenocarcinoma. Propensity-matching for both postoperative infection and for leak resulted in relatively large, well-balanced cohorts. Importantly, significant baseline differences that were highly associated with postoperative infection and leak and, therefore, bias analysis of survival outcomes, were eliminated in both matched datasets. We found no association between infectious complication or anastomotic leak and rates of tumor recurrence following MIE. Disease-free survival and time to tumor recurrence in patients who recurred were similar between groups. We did, however, find that patients with post-operative infection and anastomotic leak had worse overall survival. In multivariable analysis, postoperative infection and anastomotic leak remained independently associated with increased hazard for death during follow-up, after adjusting for important survival predictors.
It is worth noting several studies examining the impact of postoperative complications on post-esophagectomy outcomes. Similar to our study, Lagarde and colleagues found that complications were not associated with increased hazard of cancer-related death in 351 patients. In contrast to our findings, however, patients with tumor recurrence and complications (n=121) had an increased hazard of death compared to those with recurrence but without complications (n=70).(7) Another study from Lerut and colleagues found that Clavien Grade 2–4 complications were associated with greater odds of recurrence and hazard of death from recurrence during follow-up.(8) Both studies differ from ours in that they included all complications rather than infectious/anastomotic complications only, which may partially explain differences in our results. Neither study balanced baseline covariates for risk of complications. Interestingly, we found decreased overall survival after infection, despite similar time to recurrence and disease-free survival in the propensity matched groups. This difference occurs within the first year after operation, with survival curves diverging until approximately 10 months. Post-operative infections may be taking their toll most heavily in the first year post-operatively; if patients survive past this period, their long-term survival mirrors that of patients without post-operative infection.
Not unexpectedly, we found a significantly increased hazard of death in patients with postoperative infectious complications and anastomotic leak, after adjusting for other important survival predictors. These findings are consistent with some reports in the literature, which have shown that postoperative complications are associated with worse short- and long-term survival,(12, 13) but not others.(14) Markar and colleagues reported on the association between Clavien-Dindo(15) III–IV anastomotic leaks and disease-free and overall survival in nearly 3000 patients from 30 university hospitals. In contrast to our study, they found that both disease-free and overall survival were negatively impacted by postoperative leak, with a 28% increased hazard of death and 35% increased hazard of tumor recurrence during follow-up.(16) They did not balance baseline covariates, which may have influenced their recurrence and survival outcomes.
Our findings are in direct contrast to data for colorectal cancer resection. In a meta-analysis that included over 21,000 patients, the odds of local recurrence were more than 2 times higher after a leak at the rectal anastomosis and nearly 3 times higher when both colon and rectal anastomotic leaks were considered,(3) and overall and cancer specific survival is significantly reduced.(1–4, 12) There are several possible explanations for the difference in our findings. First, our study balanced baseline covariates with propensity-matching, thus minimizing important biases in the data with regard to recurrence risk. It is also biologically plausible that the patient’s natural immunity is already impaired, given the prolonged inflammation-metaplasia-dysplasia-carcinoma sequence necessary for development of esophagogastric adenocarcinoma.(17–19)
While our study did not directly examine the role of inflammatory mediators on survival after esophagectomy for esophagogastric adenocarcinoma, this relationship is increasingly recognized, independent of postoperative complications. Esophagectomy, in and of itself, appears to have a heighted influence on the postoperative inflammatory cascade compared to other cancer operations,(20–22) independent of infectious or inflammatory complications.(23) In addition, several studies have shown that baseline upregulation of tumor inflammation-associated genes, inflammatory markers, and inflammation-based preoperative prognostic scores are predictive of worse prognosis.(24–31) This increase in cytokines and inflammatory molecules is present as much as 2 years prior to diagnosis.(32) Together, these studies suggest that esophagogastric adenocarcinomas and esophagectomy induce a significant inflammatory response in the majority of patients which may mask or eliminate the impact of infectious complications and anastomotic leak on tumor recurrence after esophagectomy.
Study strengths and limitations
Prior to propensity-matching, patients who developed infections tended to be older with greater co-morbidities, introducing significant bias against postoperative infections and leaks with regard to subsequent outcomes which are also influenced by those covariates. Following propensity-matching, these covariates were well-balanced for both the postoperative infection exposure and anastomotic leak exposure, allowing a more precise point estimate of the relationship between these two exposures and our outcomes. Propensity-matching mitigates the usual limitations of observational studies by creating balance across multiple variables, which is otherwise lacking in non-randomized studies and greatly strengthens our study, despite the retrospective nature of the study design. Our analysis is limited by missing data for exposure to adjuvant chemo- and/or radiation; these variables were not able to be analyzed as a result; adjuvant therapy likely influences recurrence and overall survival and will require analysis in future studies. The fact that the analysis for postoperative infection does not apply to the small percentage of patients who had prolonged initial ventilation after esophagectomy is also limiting. To consider a patient for propensity-matching, they must, theoretically, be assignable to either group; this was not the case given that 16 of 17 patients with prolonged ventilation had postoperative infection. When patients are excluded as outliers who never had any possible matches (the extremes of the propensity score) or have near-perfect separation into one exposure or another, generalizability of findings to those patients is reduced.
Conclusions
In summary, using propensity-matched cohorts, we found that post-operative infections and anastomotic leak are not associated with worse cancer-specific outcomes. Not surprisingly, these adverse postoperative outcomes are associated with worse survival following minimally invasive esophagectomy for esophagogastric adenocarcinoma. Strategies to prevent post-operative complications will likely improve overall, non-cancer related morbidity and mortality.
Acknowledgments
This project was supported by National Cancer Institute Award Number K07CA151613 (KSN) and National Institutes of Health Grant Number UL1-TR-000005.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the Fifty-second Annual Meeting of the Society of Thoracic Surgeons, Phoenix, AZ, Jan 23–27, 2016.
References
- 1.Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: A study of 12,075 patients. Ann Surg. 2015;261(3):497–505. doi: 10.1097/SLA.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 2.Eberhardt JM, Kiran RP, Lavery IC. The impact of anastomotic leak and intra-abdominal abscess on cancer-related outcomes after resection for colorectal cancer: A case control study. Dis Colon Rectum. 2009;52(3):380–386. doi: 10.1007/DCR.0b013e31819ad488. [DOI] [PubMed] [Google Scholar]
- 3.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: Systematic review and meta-analysis. Ann Surg. 2011;253(5):890–899. doi: 10.1097/SLA.0b013e3182128929. [DOI] [PubMed] [Google Scholar]
- 4.McArdle CS, McMillan DC, Hole DJ. Impact of anastomotic leakage on long-term survival of patients undergoing curative resection for colorectal cancer. Br J Surg. 2005;92(9):1150–1154. doi: 10.1002/bjs.5054. [DOI] [PubMed] [Google Scholar]
- 5.McMillan DC, Canna K, McArdle CS. Systemic inflammatory response predicts survival following curative resection of colorectal cancer. Br J Surg. 2003;90(2):215–219. doi: 10.1002/bjs.4038. [DOI] [PubMed] [Google Scholar]
- 6.McMurry TL, Hu Y, Blackstone EH, Kozower BD. Propensity scores: Methods, considerations, and applications in the journal of thoracic and cardiovascular surgery. J Thorac Cardiovasc Surg. 2015;150(1):14–19. doi: 10.1016/j.jtcvs.2015.03.057. [DOI] [PubMed] [Google Scholar]
- 7.Lagarde SM, de Boer JD, ten Kate FJ, Busch OR, Obertop H, van Lanschot JJ. Postoperative complications after esophagectomy for adenocarcinoma of the esophagus are related to timing of death due to recurrence. Ann Surg. 2008;247(1):71–76. doi: 10.1097/SLA.0b013e31815b695e. [DOI] [PubMed] [Google Scholar]
- 8.Lerut T, Moons J, Coosemans W, et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: Role of systematic grading of complications using the modified clavien classification. Ann Surg. 2009;250(5):798–807. doi: 10.1097/SLA.0b013e3181bdd5a8. [DOI] [PubMed] [Google Scholar]
- 9.Schuchert MJ, Abbas G, Nason KS, et al. Impact of anastomotic leak on outcomes after transhiatal esophagectomy. Surgery. 2010;148(4):831–838. doi: 10.1016/j.surg.2010.07.034. discussion 838–840. [DOI] [PubMed] [Google Scholar]
- 10.StataCorp, editor. Stata statistical software: Release 14. College Station, TX: StataCorp LP; 2015. [Google Scholar]
- 11.Leuven E, Sianesi B. Psmatch2: Stata module to perform full mahalanobis and propensity score matching, common support graphing, and covariate imbalance testing. 2015 [Google Scholar]
- 12.Dhungel B, Diggs BS, Hunter JG, Sheppard BC, Vetto JT, Dolan JP. Patient and peri-operative predictors of morbidity and mortality after esophagectomy: American college of surgeons national surgical quality improvement program (acs-nsqip), 2005–2008. J Gastrointest Surg. 2010;14(10):1492–1501. doi: 10.1007/s11605-010-1328-2. [DOI] [PubMed] [Google Scholar]
- 13.Luc G, Durand M, Chiche L, Collet D. Major post-operative complications predict long-term survival after esophagectomy in patients with adenocarcinoma of the esophagus. World J Surg. 2015;39(1):216–222. doi: 10.1007/s00268-014-2754-1. [DOI] [PubMed] [Google Scholar]
- 14.Hii MW, Smithers BM, Gotley DC, et al. Impact of postoperative morbidity on long-term survival after oesophagectomy. Br J Surg. 2013;100(1):95–104. doi: 10.1002/bjs.8973. [DOI] [PubMed] [Google Scholar]
- 15.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markar S, Gronnier C, Duhamel A, et al. The impact of severe anastomotic leak on long-term survival and cancer recurrence after surgical resection for esophageal malignancy. Ann Surg. 2015;262(6):972–980. doi: 10.1097/SLA.0000000000001011. [DOI] [PubMed] [Google Scholar]
- 17.Rieder F, Biancani P, Harnett K, Yerian L, Falk GW. Inflammatory mediators in gastroesophageal reflux disease: Impact on esophageal motility, fibrosis, and carcinogenesis. Am J Physiol Gastrointest Liver Physiol. 2010;298(5):G571–581. doi: 10.1152/ajpgi.00454.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hardikar S, Onstad L, Song X, et al. Inflammation and oxidative stress markers and esophageal adenocarcinoma incidence in a barrett’s esophagus cohort. Cancer Epidemiol Biomarkers Prev. 2014;23(11):2393–2403. doi: 10.1158/1055-9965.EPI-14-0384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kavanagh ME, O’Sullivan KE, O’Hanlon C, O’Sullivan JN, Lysaght J, Reynolds JV. The esophagitis to adenocarcinoma sequence; the role of inflammation. Cancer Lett. 2014;345(2):182–189. doi: 10.1016/j.canlet.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 20.Aosasa S, Ono S, Mochizuki H, et al. Activation of monocytes and endothelial cells depends on the severity of surgical stress. World J Surg. 2000;24(1):10–16. doi: 10.1007/s002689910003. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi Y, Hihara J, Hironaka K, et al. Postoperative immunosuppression cascade and immunotherapy using lymphokine-activated killer cells for patients with esophageal cancer: Possible application for compensatory anti-inflammatory response syndrome. Oncol Rep. 2006;15(4):895–901. [PubMed] [Google Scholar]
- 22.Yamamoto K, Takiguchi S, Miyata H, et al. Reduced plasma ghrelin levels on day 1 after esophagectomy: A new predictor of prolonged systemic inflammatory response syndrome. Surg Today. 2013;43(1):48–54. doi: 10.1007/s00595-012-0342-2. [DOI] [PubMed] [Google Scholar]
- 23.Tsujimoto H, Ono S, Takahata R, et al. Systemic inflammatory response syndrome as a predictor of anastomotic leakage after esophagectomy. Surg Today. 2012;42(2):141–146. doi: 10.1007/s00595-011-0049-9. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen GH, Schetter AJ, Chou DB, et al. Inflammatory and microrna gene expression as prognostic classifier of barrett’s-associated esophageal adenocarcinoma. Clin Cancer Res. 2010;16(23):5824–5834. doi: 10.1158/1078-0432.CCR-10-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutta S, Crumley AB, Fullarton GM, Horgan PG, McMillan DC. Comparison of the prognostic value of tumour- and patient-related factors in patients undergoing potentially curative resection of oesophageal cancer. World J Surg. 2011;35(8):1861–1866. doi: 10.1007/s00268-011-1130-7. [DOI] [PubMed] [Google Scholar]
- 26.Feng JF, Huang Y, Chen QX. A new inflammation index is useful for patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2014;7:1811–1815. doi: 10.2147/OTT.S68084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han L, Song Q, Jia Y, et al. The clinical significance of systemic inflammation score in esophageal squamous cell carcinoma. Tumour Biol. 2015 doi: 10.1007/s13277-015-4152-1. [DOI] [PubMed] [Google Scholar]
- 28.Hirahara N, Matsubara T, Hayashi H, Takai K, Fujii Y, Tajima Y. Impact of inflammation-based prognostic score on survival after curative thoracoscopic esophagectomy for esophageal cancer. Eur J Surg Oncol. 2015;41(10):1308–1315. doi: 10.1016/j.ejso.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Liu JS, Huang Y, Yang X, Feng JF. A nomogram to predict prognostic values of various inflammatory biomarkers in patients with esophageal squamous cell carcinoma. Am J Cancer Res. 2015;5(7):2180–2189. [PMC free article] [PubMed] [Google Scholar]
- 30.Shrotriya S, Walsh D, Bennani-Baiti N, Thomas S, Lorton C. C-reactive protein is an important biomarker for prognosis tumor recurrence and treatment response in adult solid tumors: A systematic review. PLoS One. 2015;10(12):e0143080. doi: 10.1371/journal.pone.0143080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li J, Zhang BZ, Qin YR, et al. Cd68 and interleukin 13, prospective immune markers for esophageal squamous cell carcinoma prognosis prediction. Oncotarget. 2016 doi: 10.18632/oncotarget.6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keeley BR, Islami F, Pourshams A, et al. Prediagnostic serum levels of inflammatory biomarkers are correlated with future development of lung and esophageal cancer. Cancer Sci. 2014;105(9):1205–1211. doi: 10.1111/cas.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]


