Abstract
Natural killer (NK) cells are an emerging cellular immunotherapy for patients with acute myeloid leukemia (AML); however, the best approach to maximize NK cell antileukemia potential is unclear. Cytokine-induced memory-like NK cells differentiate after a brief preactivation with interleukin-12 (IL-12), IL-15, and IL-18 and exhibit enhanced responses to cytokine or activating receptor restimulation for weeks to months after preactivation. We hypothesized that memory-like NK cells exhibit enhanced antileukemia functionality. We demonstrated that human memory-like NK cells have enhanced interferon-γ production and cytotoxicity against leukemia cell lines or primary human AML blasts in vitro. Using mass cytometry, we found that memory-like NK cell functional responses were triggered against primary AML blasts, regardless of killer cell immunoglobulin-like receptor (KIR) to KIR-ligand interactions. In addition, multidimensional analyses identified distinct phenotypes of control and memory-like NK cells from the same individuals. Human memory-like NK cells xenografted into mice substantially reduced AML burden in vivo and improved overall survival. In the context of a first-in-human phase 1 clinical trial, adoptively transferred memory-like NK cells proliferated and expanded in AML patients and demonstrated robust responses against leukemia targets. Clinical responses were observed in five of nine evaluable patients, including four complete remissions. Thus, harnessing cytokine-induced memory-like NK cell responses represents a promising translational immunotherapy approach for patients with AML.
INTRODUCTION
Acute myeloid leukemia (AML) is a hematologic malignancy primarily of older individuals that remains a substantial clinical challenge (1). Currently, less than 30% of AML patients are cured with standard therapies, and relapsed/refractory (rel/ref) AML patients who are not candidates for hematopoietic cell transplantation (HCT) have a poor prognosis and no curative treatment options (2, 3). Cellular immunotherapy mediated by alloreactive T and NK cells administered in the context of an allogeneic HCT is an effective treatment for AML; however, most AML patients are not candidates for this procedure because it is associated with substantial treatment-related morbidity and mortality (4, 5). An alternative approach that provides the immunotherapeutic benefits of allogeneic HCT without severe toxicity is the adoptive transfer of allogeneic lymphocytes that mediate the “graft versus leukemia” effect. This strategy may expand the option of cellular immunotherapy to most AML patients.
Natural killer (NK) cells are innate lymphoid cells that are important for host defense against pathogens and mediate antitumor immune responses (6, 7). Major histocompatibility complex (MHC)–haploidentical NK cells exhibit antileukemia responses without causing “graft versus host disease” (GVHD) after HCT (8), providing evidence of their utility as a cellular effector for leukemia patients. Allogeneic NK cell adoptive transfer is safe and can induce remissions in patients with leukemia (9–12); however, these studies have been limited by inadequate persistence, expansion, and in vivo antileukemia activity of the adoptively transferred NK cells. Thus, one key barrier in the field is the need for biology-driven approaches to enhance NK cell antitumor functionality before adoptive transfer.
Although NK cells have traditionally been considered members of the innate immune system, paradigm-shifting studies in mice have identified memory-like properties after hapten exposure, virus infection, or combined interleukin-12 (IL-12), IL-15, and IL-18 cytokine pre-activation (13, 14). Cytokine-induced memory-like NK cells were defined by briefly preactivating murine NK cells with IL-12, IL-15, and IL-18, followed by adoptive transfer into syngeneic mice. Weeks to months later, memory-like NK cells had proliferated and exhibited enhanced restimulation responses to cytokines or triggering via activating receptors (15, 16). This preactivation approach also resulted in anti-tumor responses to murine NK cell–sensitive cell lines after adoptive transfer in mice (17). The potential translation of these findings as immunotherapy was established by the identification of human IL-12, IL-15, and IL-18–induced memory-like NK cells (18). Key properties of human memory-like NK cells include enhanced proliferation, expression of the high-affinity IL-2 receptor αβγ (IL-2Rαβγ), and increased interferon-γ (IFN-γ) production after restimulation with cytokines or via activating receptors (19, 20). However, the ability of human memory-like NK cells to respond to cancer target cells has not been extensively reported. We hypothesized that human cytokine-induced memory-like NK cells exhibit enhanced antileukemia properties. This was tested in vitro against primary AML blasts and in vivo in nonobese diabetic (NOD)/severe combined immunodeficient (SCID)/common gamma chain−/− (γc−/−) (NSG) mouse xenograft models and in AML patients who were administered memory-like NK cells as part of a first-in-human clinical trial. Our results demonstrate that combined preactivation with IL-12, IL-15, and IL-18 differentiates cytokine-induced memory-like NK cells that have potent antileukemia functionality in vitro and in vivo and thus represent a promising immunotherapy strategy for AML patients.
RESULTS
Memory-like NK cells exhibit enhanced functional responses against leukemia target cells
Although the enhanced recall response of human IL-12, IL-15, and IL-18–induced memory-like NK cells was well established after cytokine receptor restimulation (18), their response to leukemia target cells, including primary AML blasts, was not extensively studied. To address this issue, we preactivated human NK cells with IL-12, IL-15, and IL-18 or control conditions (low-dose IL-15 only) overnight and then differentiated memory-like or control NK cells from the same individual for 7 days (Fig. 1A). Memory-like NK cells exhibited enhanced IFN-γ production in response to restimulation with both K562 leukemia cells (Fig. 1, A and B) and primary AML blasts (Fig. 1, A and C). In addition, memory-like NK cells demonstrated significantly higher cytotoxicity compared to control NK cells from the same donors, which was dependent on NKG2D and DNAM-1 (P = 0.02; Fig. 1D and fig. S1). Consistent with augmented cytotoxicity, we observed increased expression of the cytotoxic effector protein granzyme B (Fig. 1, E and F). To further define the differences between memory-like and control NK cells, we established a mass cytometry panel that included functionally relevant proteins to deeply immunophenotype NK cells.
Fig. 1. Memory-like NK cells exhibit enhanced functional responses against leukemia targets.
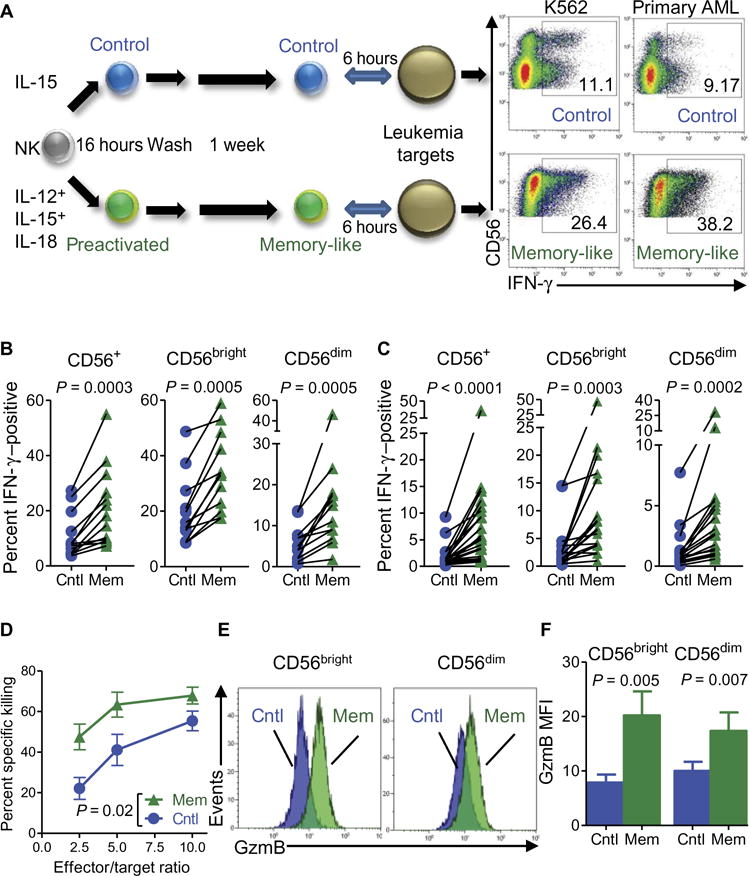
Purified NK cells were preactivated with IL-12, IL-15, and IL-18 or control (cntl; IL-15) for 16 hours, washed, and then rested in low concentrations of IL-15 to allow for differentiation. (A) Schema of memory-like (mem) NK cell in vitro experiments and representative flow plots showing enhanced IFN-γ production by NK cells after K562 (left) and primary AML (right) triggering. Inset numbers are the percentages of IFN-γ –positive NK cells within the indicated regions. (B and C) Summary of data showing enhanced IFN-γ production by all memory-like NK cells (CD56+) as well as each of the two major human NK cell subsets (CD56dim and CD56bright) restimulated with K562 (B) or primary allogeneic AML blasts (C). (D) Increased killing of K562 leukemia target cells by purified memory-like NK cells as compared to control NK cells from the same individuals. (E) Representative flow cytometry data showing increased granzyme B (GzmB) protein in memory-like compared to in control NK cells. (F) Summary of data from (E) showing granzyme B median fluorescence intensity (MFI). Data represent two to six independent experiments and were compared using Wilcoxon signed-rank test with means ± SEM displayed in all graphs.
Mass cytometry defines the differences between memory-like and control NK cells
Mass cytometry allows for high-throughput analysis of a large number of parameters on single cells, and it has been used to deeply immunophenotype and track the diversity of human NK cells (21, 22). We developed custom mass cytometry panels that include NK cell lineage, maturation, receptor repertoire, and functional capacity (tables S1 and S2), and using the viSNE clustering and visualization strategy (23), we identified the differences between control and memory-like NK cells (Fig. 2, A and B, and figs. S2 and S3). Control and memory-like NK cells were localized within separate areas of the viSNE map, indicative of distinct cellular islands (Fig. 2A). We confirmed significant differences in the expression of a number of markers that were previously reported (18, 19), including CD94 (P = 0.002), NKG2A (P=0.004), NKp46(P=0.04),and CD25 (P = 0.03) on the viSNE gated control and memory-like populations. Leveraging mass cytometry’s expanded view of NK cell proteins, we observed increases in NKp30, NKp44, CD62L, CD27, and TRAIL and in the cytotoxic molecules perforin and granzyme B and also decreases in NKp80, compared to controls (Fig. 2B and fig. S3). Thus, memory-like and control NK cells were identifiable as distinct populations when tracked with high NK cell marker dimensionality by mass cytometry. In addition, mass cytometry confirmed flow cytometry findings of increased cytotoxic effector proteins, which likely contribute to the enhanced cytotoxicity of memory-like NK cells against leukemia targets.
Fig. 2. Multidimensional analyses define the differences between memory-like and control NK cells.
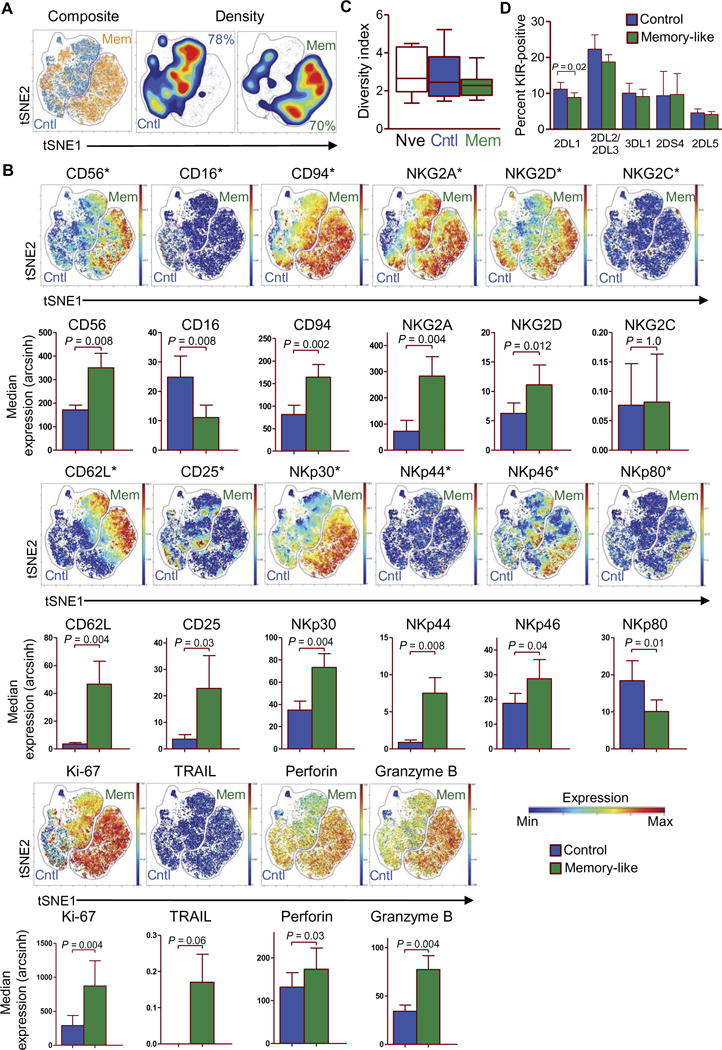
NK cells were assessed at baseline or after in vitro control and memory-like differentiation at day 7 (Fig. 1A) for the expression of 36 markers using mass cytometry. (A and B) Comparison of control and memory-like NK cells from a representative healthy individual using viSNE, clustered on 21 NK cell phenotypic markers. (A) Memory-like (orange) and control NK cell (blue) events overlaid in the tSNE1/tSNE2 fields (left) show their differential localization within the viSNE map. Density plots of control and memory-like NK cells in the tSNE1/tSNE2 fields (right). Inset values indicate the frequency of cells that fall within the control or memory-like gate. (B) The composite of the control and memory-like populations from (A), displaying the median expression of the indicated markers. Data are representative of nine individuals. Color scale indicates the intensity of expression of each marker signal. Minimum (min) and maximum (max) correspond to the 2nd and the 98th percentile values for each indicated marker, respectively. For additional summary data and statistical comparisons, see fig. S3. (C) Inhibitory KIR receptor inverse Simpson diversity index of baseline [naïve (nve)], control, and memory-like NK cells from nine individuals; box with whiskers displaying minimum to maximum. (D) Summary of data showing the percent positive of each indicated KIR for control and memory-like NK cells. All summary graphs display means ± SEM, unless otherwise indicated. Comparisons were made using Wilcoxon signed-rank test.
Memory-like NK cells respond to AML regardless of inhibitory KIR to KIR-ligand interactions
Because allogeneic NK cell responses to AML blasts are influenced by the interaction of the inhibitory killer cell immunoglobulin-like receptors (KIRs) with their cognate human leukocyte antigen (HLA) ligands (24–26), we next examined whether memory-like differentiation altered KIR diversity. We used mass cytometry to examine NK cells at baseline (naïve) and after control or memory-like differentiation in vitro at day 7 (table S1 and Fig. 1A). The inverse Simpson diversity index of KIR expressed by naïve, control (day 7), and memory-like (day 7) NK cells was unaltered (Fig. 2C) (21, 27), demonstrating that inhibitory KIR phenotypes present at baseline were stable in vitro. Consistent with this, the percent of KIR-positive NK cells was similar at day 7 between control and memory-like NK cell populations (Fig. 2D).
In the setting of allogeneic HCT, donor KIR repertoire affects patient prognosis, highlighting the importance of the KIR to KIR-ligand mismatch for conventional NK cell anti-AML activity (24–26, 28). To experimentally identify NK cell subsets that preferentially respond to primary AML blasts, we restimulated control and memory-like NK cells for 6 hours with primary AML blasts and assessed the expression of 36 parameters via mass cytometry (tables S2 to S4). Memory-like NK cells again produced more IFN-γ when triggered with primary AML blasts compared to controls (Fig. 3, A and B). We used spanning-tree progression analysis of density-normalized events (SPADE) to cluster the NK cells according to the expression of core NK cell markers and assessed each node (representing a distinct NK cell specificity) for IFN-γ expression (Fig. 3C) (29, 30). The KIR-ligand interaction status of each node was defined to investigate their impact on memory-like and control NK cell responses. For a representative individual, we assigned the SPADE nodes as matched if they expressed KIR2DL2/KIR2DL3, which recognizes HLA-C1 present on the AML blasts. KIR2DL1 and KIR3DL1 did not have ligands present, and nodes that lacked KIR2DL2/KIR2DL3 were categorized as mismatched. Based on this approach, we observed the expected increased response in KIR-mismatched versus KIR-matched node in IFN-γ production in the control cells when triggered with AML blasts (Fig. 3, D to F). However, memory-like NK cells produced increased IFN-γ compared to control cells in both the KIR-ligand–matched and KIR-ligand–mismatched situations. Moreover, the IFN-γ production by memory-like NK cells was similar in the KIR-matched and KIR-mismatched setting (Fig. 3, D and E), which was reproducible across the seven individuals tested (Fig. 3E). Furthermore, when we expanded our analysis to four different primary AML blasts, we observed a consistent increase in IFN-γ production in KIR-matched memory-like NK cells compared to KIR-mismatched control-treated cells (Fig. 3F). Because most memory-like NK cells express NKG2A, we assessed IFN-γ production by NKG2A− or NKG2A+ KIR-ligand–matched or KIR-ligand–mismatched NK cells and observed increased IFN-γ production by memory-like NK cells compared to controls in both subpopulations (figs. S4, A to C). Cell surface CD107a, tumor necrosis factor, and macrophage inflammatory protein-1α were also increased on memory-like NK cells in these assays, compared to controls (fig. S4D). Thus, memory-like differentiation enhances IFN-γ production in response to primary AML, regardless of whether memory-like NK cells expressed inhibitory KIR with a ligand present on the target AML blast.
Fig. 3. Response of memory-like NK cells to primary AML blasts is enhanced regardless of KIR-ligand interactions.
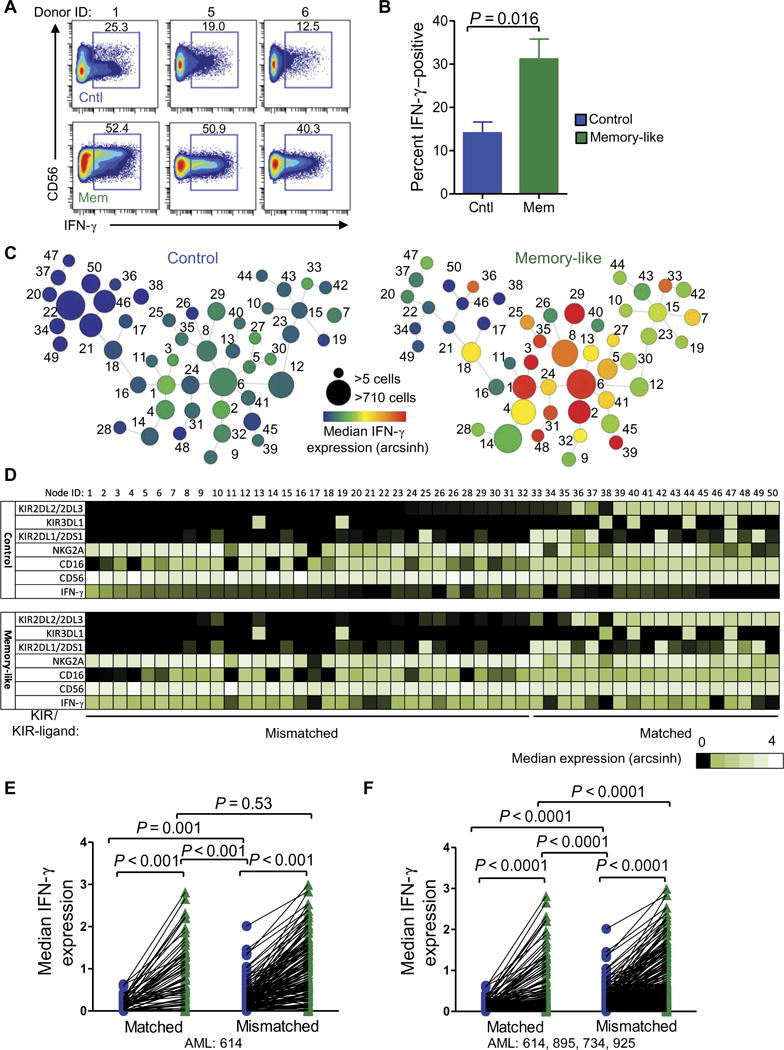
Control and memory-like NK cells were stimulated with primary AML blasts for 6 hours and assessed for the expression of 36 markers using mass cytometry. (A) Representative bivariate mass cytometry plots of IFN-γ production by control and memory-like NK cells stimulated at the bulk population level. Numbers depict percentages of cells within the indicated regions. (B) Summary of data (means ± SEM) from seven individuals showing percentages of IFN-γ –positive NK cells. (C to E) NK cells were further analyzed on 21 clustering parameters using SPADE. (C) Representative SPADE diagram of in vitro–differentiated control and memory-like NK cells from a healthy individual. Node size depicts relative number of cells per node, and color indicates median IFN-γ expression for each node. Numbers next to each node represent the node ID. (D) Heat map of the nodes from (C). KIR to KIR-ligand matched and mismatched status was assigned on the basis of the presence or absence of KIR2DL2/KIR2DL3, which recognizes HLA-C1 expressed by the primary AML. (E) Summary of data from seven individuals analyzed as in (C). (F) Summary of data from four to seven different individuals stimulated with four different AML blasts showing reproducibility of these findings. Control and memory-like data were compared using the Wilcoxon signed-rank test. Matched and mismatched data were compared using the Mann-Whitney test.
Memory-like NK cells exhibit potent antileukemia functionality after transfer into NSG mice
To assess whether human memory-like NK cells maintain memory-like function in vivo, we used an adoptive transfer model with NSG mice (Fig. 4A). After 7 days, memory-like NK cells were found to localize in key hematopoietic tissues, including bone marrow (BM), spleen, and blood (Fig. 4B), and were found in similar numbers compared to control NK cells (Fig. 4C). The expression of selected chemokine receptors was minimally changed after IL-12, IL-15, and IL-18 pre-activation, with the exception of reduced expression of CX3CR1 (fig. S7). Memory-like NK cells also maintained their enhanced functionality as evidenced by increased IFN-γ production in response to ex vivo restimulation with K562 leukemia cells after 7 days in NSG mice (Fig. 4, D and E).
Fig. 4. Human memory-like NK cells control human leukemia in an NSG xenograft model.
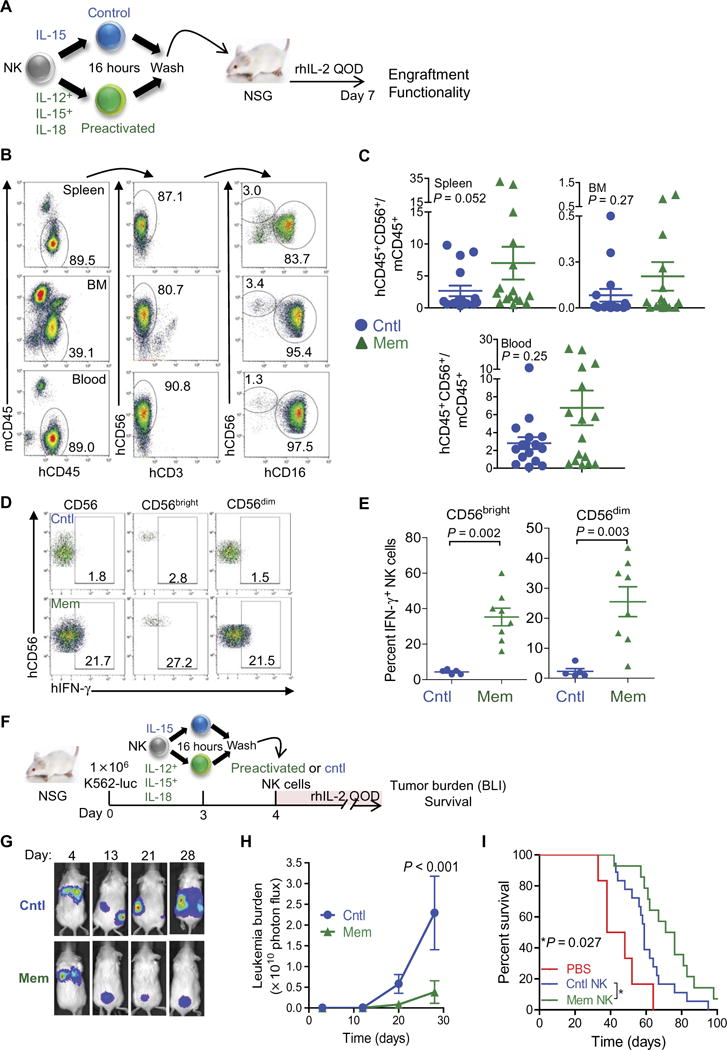
(A) Experimental design for (B) to (E). rhIL-2, recombinant human IL-2; QOD, every other day. (B to E) NSG mice received human NK cell adoptive transfers as indicated in (A). Representative flow cytometry at day 7 after transfer shows engraftment of human memory-like NK cells in the indicated tissues from a representative donor. Both CD56bright and CD56dim subsets are detectable. (C) Summary of data from (B) demonstrating the engraftment of control and memory-like NKcells, with abundance identified as the ratio to murine CD45+ mononuclear cells. (D) Control or memory-like NK cells were administered to NSG mice as in (A). After 7 days, splenocytes were isolated and restimulated with K562 for 6 hours, followed by assessment of human NK cells for IFN-γ production. Numbers depict the percentages of cells within the indicated regions. (E) Summary of data from (D) showing the means ± SD of percent IFN-γ –positive NK cells from the indicated NK cell subsets. Statistical analysis was performed with Mann-Whitney test. (F) Experimental design for (G) to (I). (G to I) K562-luc was injected intravenously into NSG mice. After 4 days, BLI was performed to ensure leukemia engraftment, and control or memory-like NK cells were administered to the mice. The mice were treated with rhIL-2 every other day and monitored for tumor burden (BLI) and survival. (G) Representative BLI of recipient mice engrafted with K562-luc on the indicated day after tumor administration. (H) Summary of serial BLI measurements that show reduced tumor burden in mice receiving memory-like NK cells compared to control NK cells. Differences were determined using analysis of variance (ANOVA). (I) Mice were treated as in (F), monitored for survival, and analyzed using the log-rank test. PBS, phosphate-buffered saline. Summary data are from two to three experiments with n = 12 to 24 mice per group represented as means ± SEM.
To test the ability of human memory-like NK cells to control leukemia in vivo, we engrafted K562-luc (luciferase-expressing) leukemia cells in NSG mice and adoptively transferred human IL-12, IL-15, and IL-18–preactivated or control NK cells into groups of NSG mice on day 4 (Fig. 4F). Memory-like NK cells were significantly more effective in controlling K562 tumor cell growth in vivo using whole-body bio-luminescence imaging (BLI) (P < 0.001; Fig. 4, G and H). The enhanced leukemia control afforded by a single injection of human memory-like NK cells also resulted in improved survival of these mice (Fig. 4I).
Donor memory-like NK cells proliferate and expand after adoptive transfer into patients with AML
On the basis of these preclinical findings with human memory-like NK cells and those from mouse models evaluating NK cell–sensitive tumor cell line challenge (17), we initiated a first-in-human phase 1 clinical trial of allogeneic, HLA-haploidentical, IL-12, IL-15, and IL-18–preactivated NK cells in patients with rel/ref AML (fig. S5). Here, we report memory-like NK cell biology and antileukemia activity within the first nine evaluable patients treated at three different dose levels (Table 1). Donor NK cells were purified by CD3 depletion followed by CD56-positive selection (10), pre-activated for 12 to 16 hours with rhIL-12, rhIL-15, and rhIL-18 in a good manufacturing practice (GMP) laboratory, washed, and infused into AML patients who were preconditioned with fludarabine/cyclophosphamide (9) on day 0. After adoptive transfer, low-dose rhIL-2 was administered to sup-portmemory-likeNKcellsthroughtheirinducedhigh-affinityIL-2Rαβγ (19).
Table 1. Summary of clinical characteristics of evaluable patients treated with cytokine-induced memory-like NK cells.
All patients were Caucasian. KIR-ligand mismatch is reported in the NK cell donor versus patient vector. Patient 012 was reenrolled at a higher dose level as patient 019. UPN, universal patient number; WHO, World Health Organization; DLT, dose-limiting toxicity; M, male; F, female; IWG, International Working Group; TF-PD, treatment failure due to progressive disease; CR, complete remission; MLFS, morphologic leukemia-free state; CRi, CR with incomplete blood count recovery; MDS, myelodysplastic syndrome.
| UPN | Dose level | Gender | Age (years) | WHO diagnosis | Number of previous therapies | Pretreatment BM blast (%) | KIR-ligand mismatch | IWG response | DLT | GVHD |
|---|---|---|---|---|---|---|---|---|---|---|
| 001 | 1 | M | 73 | M2 | 2 | 16 | Yes | TF-PD | No | No |
| 006 | 1 | M | 70 | M0 | 3 | 28 | Yes | TF-PD | No | No |
| 007 | 1 | M | 77 | M0 | 1 | 47 | Yes | CR | No | No |
| 008 | 2 | M | 76 | t-AML | 3 | 17 | Yes | TF-PD | No | No |
| 009 | 2 | F | 73 | M1 | 3 | 80 | No | MLFS | No | No |
| 012 | 2 | F | 71 | M5 | 3 | 15 | Yes | CR | No | No |
| 017 | 3 | M | 64 | t-AML | 3 | 69 | Yes | TF-PD | No | No |
| 019 | 3 | F | 71 | M5 | 4 | 15 | Yes | CR | No | No |
| 020 | 3 | M | 60 | MDS-AML | 1 | 13 | Yes | CRi | No | No |
Three patients at dose level 1 (0.5 × 106/kg), three patients at dose level 2 (1.0 × 106/kg), and three patients at dose level 3 (all NK cells generated, capped at 10 × 106/kg) were evaluable. Donor memory-like NK cells were tracked in the blood of all patients with informative HLA [using donor- or patient-specific anti-HLA monoclonal antibodies (mAbs)], peaked in frequency at 7 to 14 days after infusion and, as expected (9), decreased in number after recipient T cell recovery (Fig. 5, A to C). Memory-like NK cells comprised >90% of blood NK cells at day 7 (Fig. 5, A and B), with an average of 419 ± 166–fold increase (range, 39 to 1270) comparing day 1 and day 7 counts (Fig. 5C). Donor memory-like NK cells had increased proliferation (Ki-67+) at days 3 and 7 (Fig. 5, D and E). Similarly, assessments of the BM at day 8 after infusion revealed large percentages and absolute numbers of donor memory-like NK cells (Fig. 5, F to H), although there was heterogeneity between donor and recipient pairs, especially between NK cell dose levels (fig. S6).
Fig. 5. Donor memory-like NK cells expand and proliferate in vivo in AML patients.
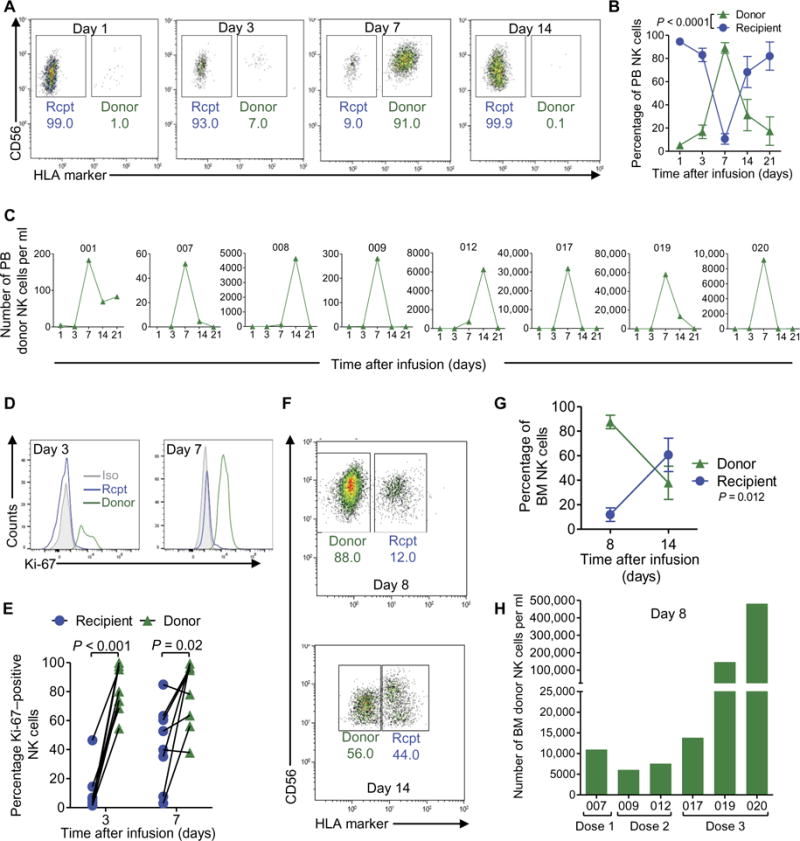
(A) Representative flow cytometry data of donor (HLA+) versus recipient (rcpt) (HLA−) NK cells on the indicated day after infusion. (B) Percentages of the peripheral blood (PB) NK cell compartment composed of donor versus recipient NK cells at the indicated time points after infusion, summarizing the eight patients with informative HLA mAbs available for all time points. (C) Absolute numbers of donor NK cells per milliliter of peripheral blood at the indicated times after infusion for each patient (UPN above individual graphs). Absolute numbers were calculated by multiplying the number of mono-nuclear cells obtained per milliliter of blood by the fraction of CD45+ cells that consisted of donor NK cells. (D) Representative flow cytometry data showing Ki-67 staining in donor and recipient NK cells from the peripheral blood on the indicated day after infusion. Isotype control (iso) staining for Ki-67 is indicated by the gray histogram. (E) Summary of data of the percentages of Ki-67–positive donor versus recipient peripheral blood NK cells at days 3 and 7 after infusion. (F) Representative flow cytometry data of donor (HLA−) versus recipient (HLA+) NK cells on the indicated day after infusion. (G) Percentages of the BM NK cell compartment that were donor versus recipient NK cells at the indicated time point after infusion, summarizing the seven patients with informative HLA mAbs available for all time points. (H) Absolute numbers of donor and recipient NK cells per milliliter of BM at day 8 after infusion. Absolute numbers were obtained as in (C). Statistical comparison was performed by two-way ANOVA. In representative bivariate flow plots (A and F), numbers indicate the frequency of cells within the indicated gate.
Donor memory-like NK cells exhibit enhanced functionality after adoptive transfer into patients with AML
We next assessed donor and recipient blood NK cell IFN-γ production from these patients after a short-term ex vivo restimulation triggered by K562 leukemia cells (Fig. 6, A to D). These analyses revealed an increased frequency of IFN-γ –positive donor, compared to recipient, NK cells (Fig. 6A). More strikingly, these experiments revealed that the absolute number of donor IFN-γ –producing NK cells was markedly increased, compared to the recipient NK cells in these samples (Fig. 6, B to D). Similarly, the number of IFN-γ –positive donor memory-like NK cells was greater than recipient NK cells in BM, although fewer patients had BM samples with adequate cell numbers for functional analyses (Fig. 6, E to G).
Fig. 6. Donor memory-like NK cells display enhanced antileukemia responses at 1 week after adoptive transfer.
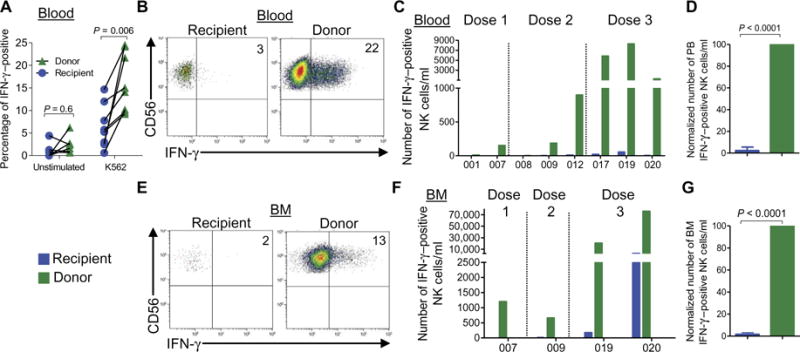
Freshly isolated peripheral blood mononuclear cells (PBMCs) from patient blood or BM were stimulated with K562 leukemia cells at an effector/target ratio of 10:1 for 6 hours and assessed for IFN-γ production by flow cytometry. (A) Percentage of IFN-γ – positive donor versus recipient NK cells in the peripheral blood for all patients. Data were compared using paired t test. (B) Representative flow cytometric data showing donor and recipient NK cell IFN-γ responses from theperipheralblood.(C)Absolutenumbersofrecipient(blue)anddonor(green) NK cells producing IFN-γ in the blood. (D) Summary of data from (C) depicting relative IFN-γ production by recipient and donor NK cells from the blood; the data were normalized to donor IFN-γ –positive NK cells, which were set to 100%. (E) Representative flow cytometry data showing donor and recipient NK cell IFN-γ responses from the BM. (F) Absolute numbers of recipient and donor NK cells producing IFN-γ in the BM. (G) Summary of data from (F) depicting relative IFN-γ production by recipient and donor NK cells from the BM; the data were normalized to donor IFN-γ –positive NK cells, which were set to 100%. Normalized control NK cell responses were tested against 100% (donor) using a one-sample t test. Numbers represent percentage of cells within the indicated quadrant. All summary data depict means ± SEM.
Complete remissions were observed after IL-12, IL-15, and IL-18–preactivated donor NK cell infusion in patients with rel/ref AML
Performing a phase 1 study of memory-like NK cell adoptive immunotherapy in older patients with active rel/ref AML is a clinical challenge, but it does permit investigation of anti-AML responses. Thirteen patients who had progressed after multiple previous treatments were administered with memory-like NK cells at dose levels 1, 2, and 3. Four patients were not evaluable because of inadequate donor cell collection caused by apheresis technical failure (n = 1) or death resulting from bacteremia with septic shock before day 14 (n = 1) or before day 35 (n = 2). Of the nine evaluable patients (Table 1), there were four CR/CRi and one MLFS by the IWG response criteria (31), yielding an overall response rate of 55% and a CR/CRi rate of 45%. The changes in leukemia blast percentages in the BM of responding and nonresponding patients are shown in fig. S6. There was no detectable correlation between pre-therapy BM blast percentage, donor KIR haplotype, the presence of predicted KIR-ligand mismatch, and the frequency of donor NK cells in the blood or BM and clinical response, but the number of patients was small. A complete set of clinical vignettes describing each patient and his or her clinical course is included in the Supplementary Materials and Methods. Additional parameters of relevant NK cell receptor biology are shown in table S5. Thus, allogeneic human IL-12, IL-15, and IL-18–induced memory-like NK cells proliferate, expand, and exhibit anti-leukemia function after adoptive transfer into rel/ref AML patients with active disease.
DISCUSSION
Motivated by the need to develop innovative treatment options for AML patients (4, 5), we investigated the ability of a recently defined functional class of NK cells, cytokine-induced memory-like NK cells, to mediate antileukemia responses (20). Here, we demonstrated that primary human NK cells, differentiated in vitro into memory-like NK cells via brief preactivation with IL-12, IL-15, and IL-18, exhibit potent antileukemia responses in the form of IFN-γ production and cytotoxicity. Using mass cytometry and multidimensional analyses to assess a large number of NK cell relevant proteins simultaneously, we found that memory-like NK cells were clearly distinguishable from control NK cells from the same individual. Despite stable inhibitory KIR receptor expression, memory-like NK cells responded more robustly to primary AML blasts, regardless of KIR-ligand interactions. Moreover, human memory-like NK cells were superior, in terms of both controlling leukemia burden and prolonging survival, in an in vivo NSG xenograft model. Translating this to the clinic, we examined the biology of the MHC-haploidentical donor IL-12, IL-15, and IL-18–preactivated NK cells administered to patients with active rel/ref AML in the context of a first-in-human NK cell trial. After transfer, memory-like NK cells were detectable in the blood and BM of patients for weeks, proliferated extensively, and expanded in vivo. Further, memory-like NK cells that differentiated in patients in vivo exhibited enhanced functionality against leukemia target cells. Notably, five of the nine evaluable AML patients receiving IL-12, IL-15, and IL-18–preactivated NK cells had clinical responses, suggesting preliminary evidence of in vivo antileukemia activity. Thus, preactivation of NK cells with IL-12, IL-15, and IL-18 that results in memory-like NK cell differentiation represents a promising approach to enhancing adoptive allogeneic NK cell therapy.
Published studies have identified comparable IL-12, IL-15, and IL-18–induced memory-like NK cell biology in mice and humans (15–19). In mouse models, preactivation with IL-12, IL-15, and IL-18 has been shown to improve NK cell responses to lymphoma and melanoma cell lines in vivo, which required T cell–derived IL-2 (17). Although some phenotypic differences have been identified (18), a clear approach to distinguishing memory-like from conventional NK cells from the same donor was lacking. Through mass cytometry and multidimensional data reduction algorithms, control and memory-like NK cells were identified in separate areas of viSNE maps, indicating a distinct phenotype and providing an approach to tracking memory-like NK cells in vivo. Currently, memory-like NK cells that emerge after IL-12, IL-15, and IL-18 preactivation are thought to result from a differentiation process that yields a long-term alteration of functional capacity; however, the molecular mechanisms that control this process are yet to be defined. This concept of differentiation is consistent with the distinct phenotype tracked by multidimensional analyses, and the observations that enhanced memory-like NK cell responses to restimulation are retained after extensive cell division and persisted for weeks to months after the initial preactivation (15, 18). The phenotypic changes observed after IL-12, IL-15, and IL-18 preactivation include increased expression of inhibitory, activating, and cytokine receptors (CD94/NKG2A, NKp30, NKp44, NKp46, NKG2D, CD62L, and CD25), whereas other receptors appear unchanged (KIR, CD57, NKG2C, DNAM-1, CD137, and CD11b) or decreased (NKp80). Such dynamic changes in activating, inhibitory, cytokine, and adhesion receptors are also consistent with differentiation. Further, prolonged IL-12, IL-15, and IL-18 activation (for 5 days) resulted in reduced methylation of the IFN-γ conserved noncoding sequence 1 locus in human NK cells, indicating that cytokines can affect epigenetic control mechanisms (32). One alternative hypothesis to the differentiation theory is that IL-12, IL-15, and IL-18–preactivated NK cells exist at an enhanced activation state without a fundamental change in their molecular programs. Although most published data are consistent with the idea of differentiation rather than activation, elucidation of molecular mechanisms controlling cytokine-induced memory would provide additional clarification of this issue.
Earlier work has set the stage for the use of allogeneic NK cells as adoptive immunotherapy for AML patients (9–12), harnessing the leukemia-targeting potential of NK cell allorecognition of MHC-mismatched AML first identified after HCT (8). Initial pioneering adoptive NK cell therapy clinical trials identified that an available lymphoid niche was essential for donor NK cell engraftment and expansion; however, clinical responses occurred in a minority of patients with limited duration (9). This modest effect is consistent with the concept that combined modulatory approaches will be required to fully optimize NK cell adoptive immunotherapy for cancer patients, including (i) enhancement of NK cell targeting of cancer cells, (ii) augmentation of NK cell functional capacity, and (iii) elimination of inhibitory checkpoints or cellular negative regulators (33S). In the context of AML, NK cell allorecognition of MHC-haploidentical blasts has provided an established mode of targeting NK cells to myeloid leukemia via inhibitory receptor mismatch and activating receptor ligation. We discovered that IL-12, IL-15, and IL-18–induced memory-like NK cells exhibit enhanced triggering against AML regardless of KIR to KIR-ligand interactions, resulting in an expanded NK cell pool of AML-reactive effector cells. Because NKG2A is expressed on most memory-like NK cells, the interaction with HLA-E on AML blasts or bystander cells in the micro-environment remains a potentially important inhibitory pathway for memory-like NK cells. The functionality of memory-like NK cells is also enhanced in terms of cytotoxicity and IFN-γ production. Thus, through brief combined cytokine stimulation, two aspects of the NK cell anti-tumor response have been improved.
Most adoptive NK cell therapy studies to date use IL-2 or IL-15 to activate NK cells overnight before transfer. This strategy results in a short-term priming signal that increases NK cell functional capacity (33), but the effect is rapidly lost after removal from the in vitro cytokine milieu and transfer into the patient. Here, we reasoned that the longer-lasting increase in functional capacity afforded by memory-like NK cell differentiation, combined with improved AML recognition, would enhance in vivo expansion and antileukemia responses, resulting in a several week “window of opportunity” to attack AML blasts. Donor memory-like NK cells consistently expanded to become >90% of blood NK cells, as well as most BM NK cells. Because of concerns for cytokine-release syndrome, our phase 1 study was intentionally initiated with a starting NK cell dose of 1/10 to 1/20 of the typical adoptive NK cell dose (9–12). Considering the relatively low doses of highly purified IL-12, IL-15, and IL-18–preactivated NK cells administered to patients, the donor NK cell frequencies and numbers observed here in the blood and BM are remarkable. Two published studies have transferred doses of CD56+CD3−-purified NK cells similar to our dose level 3 as adoptive immunotherapy for patients with active AML (11, 12). The overall response rate of 55% (5 of 9) and CR/CRi rate of 45% (4 of 9) in our study compare favorably to 7% (1 of 15) observed within cohort 2 of Bachanova et al. (0 of 10) (12) and patients with active AML on the work of Curti et al. (1 of 5) (11). Because our sample size was limited, this finding is hypothesis-generating and will need to be studied in more patients treated with IL-12, IL-15,andIL-18–preactivated NK cells. In our small sample set treated to date, these responses did not segregate with any KIR-based rules for NK cell allorecognition, although most patients studied had a KIR to KIR-ligand mismatch in the donor versus recipient direction. Similarly, there were no associations with NK cell number or IFN-γ production measurements, which had substantial variability within patients who received different doses of NK cells. Thus, this report provides proof of concept that human IL-12, IL-15, and IL-18–preactivated NK cells may be translated into the cancer immunotherapy clinic, with consistent memory-like NK cell biology in vitro, in xenograft models in vivo and in AML patients in vivo.
MATERIALS AND METHODS
Study design
Patients treated on an open-label, nonrandomized, first-in-human phase 1 dose escalation trial (NCT01898793) are included in this study. The primary objective of the clinical trial was to identify the maximum tolerated or tested dose of memory-like NK cells administered to patients with rel/ref AML. Sample size was based on a standard 3 + 3 dose escalation design. Patients with rel/ref AML who were not candidates for immediate HCT were eligible to participate. Patients were treated with fludarabine/cyclophosphamide between days −7 and −2 for immunosuppression, followed on day 0 by allogeneic donor IL-12, IL-15, and IL-18–preactivated NK cells in escalating doses: 0.5 × 106/kg (dose level 1), 1.0 × 106/kg (dose level 2), and all NK cells that could be generated from a single leukapheresis capped at 10 × 106/kg (dose level 3). After donor NK cell adoptive transfer, patients received low-dose rhIL-2 (1 × 106 IU/m2) subcutaneously every other day for a total of six doses. Donor NK cell products were generated by purifying NK cells from a nonmobilized leukapheresis product using CD3 depletion followed by CD56-positive selection on a CliniMACS device (>90% CD56+CD3−). Purified NK cells were preactivated with IL-12 (10 ng/ml), IL-15 (50 ng/ml), and IL-18 (50 ng/ml) for 12 to 16 hours under current GMP conditions. Samples were obtained from the peripheral blood (before treatment and at days 1, 3, 7, 8, 14, 21, 30, 60, and 100) and BM (before treatment and at days 8, 14, 30, 60, and 100) after the NK cell infusions. Clinical responses were defined by the revised IWG criteria for AML (31). If cell numbers were limiting, analyses of NK cell number and phenotype were prioritized. All patients provided informed consent before participating and were treated at the Washington University Institutional Review Board (IRB)–approved clinical trial (Human Research Protection Office #201401085).
Reagents, mice, and cell lines
Antihuman mAbs were used for flow and mass cytometry (tables S1, S2, and S6), including previously reported anti-HLA mAbs (34). Endotoxin-free, recombinant human cytokines are described in the Supplementary Materials and Methods. NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (4 to 8 weeks old) were obtained from The Jackson Laboratory, maintained under specific pathogen–free conditions, and used in accordance with our animal protocol approved by the Washington University Animal Studies Committee. K562 cells [American Type Culture Collection (ATCC), CCL-243] were obtained in 2008, viably cryopreserved and stored in liquid nitrogen, thawed for use in these studies, and maintained for <2 months at a time in continuous culture according to ATCC instructions. K562 cells were authenticated in 2015 using single-nucleotide polymorphism analysis and were found to be exactly matched to the K562 cells from the Japanese Collection of Research Bioresources, German Collection of Microorganisms and Cell Cultures (DSMZ), and ATCC databases (2015, Genetic Resources Core Facility at Johns Hopkins University).
NK cell purification and cell culture
Normal donor PBMCs were obtained from anonymous healthy platelet donors and isolated by Ficoll centrifugation (18, 19, 35). NK cells were purified using RosetteSep (STEMCELL Technologies; routinely ≥95% CD56+CD3−). KIR genotyping of donor PBMC was performed by Kashi Clinical Laboratories. Memory-like and control NK cells were generated as previously described (see the Supplementary Materials and Methods for more details) (18).
Patient samples
Patients with newly diagnosed AML provided informed consent under the Washington University IRB-approved protocol (2010-11766) and were the source of primary AML blasts for in vitro resimulation experiments (table S4). After informed consent, patient samples were also obtained from the first-in-human phase 1 clinical study of allogeneic donor memory-like NK cells in rel/ref AML (fig. S5). Patient peripheral blood or BM aspirate was obtained at the indicated time point after NK cell infusion, and PBMCs were isolated by Ficoll centrifugation and immediately used in experiments.
Functional assays to assess cytokine production
Control and memory-like NK cells were harvested after a rest period of 7 days to mimic transfer into a patient and to allow memory-like NK cell differentiation to occur. Cells were then restimulated in a standard functional assay (18, 19, 35). Cells were stimulated with K562 leukemia targets or freshly thawed primary AML blasts (effector/target ratio of 5:1, unless otherwise indicated). Further details are provided in the Supplementary Materials and Methods.
Flow-based killing assay
Flow-based killing assays were performed by co-incubating memory-like or control NK cells with carboxyfluorescein diacetate succinimidyl ester–labeled K562 cells for 4 hours and assaying 7-aminoactinomycin D uptake as described (18, 19, 35).
Adoptive transfer of human memory-like NK cells into NSG mice
Purified control (5 × 106; ≥95% CD56+CD3−) or IL-12, IL-15, and IL-18–preactivated NK cells were injected retro-orbitally into NSG mice. Mice received rhIL-2 (50,000 IU) through intraperitoneal injection every other day to support the adoptively transferred NK cells. After 7 days, the mice were sacrificed, and organs (spleen, blood, and BM) were assessed for the presence of transferred cells. NSG splenocytes were also examined for intracellular IFN-γ production after restimulation with K562 leukemia targets.
In vivo BLI
For BLI experiments, mice were treated as described in the Supplementary Materials and Methods. For imaging, mice were injected intraperitoneally with D-luciferin (150 μg/g) (Biosynth) in PBS and imaged using the IVIS 100 (Caliper). BLI was performed at the indicated time points.
Flow cytometric analysis
Cell staining was performed as described (18, 19, 35), and data were acquired on a Gallios flow cytometer (Beckman Coulter) and analyzed using Kaluza (Beckman Coulter) or FlowJo (Tree Star) software.
Mass cytometry
All mass cytometry data were collected on a CyTOF2 mass cytometer (Fluidigm) and analyzed using Cytobank (36). Mass cytometry data were analyzed using previously described methods (37). For detailed information, refer to the Supplementary Materials and Methods. Diversity was assessed on eight individual donors at baseline (freshly thawed PBMCs or thawed pure NK cells), after control treatment or memory-like differentiation (described above) using the Boolean gating strategy described in fig. S2 (21, 22). Event counts were used to generate inverse Simpson index for KIR2DL1, KIR2DL1/KIR2DL2, KIR3DL1, KIR2DS4, and KIR2DL5 expression.
Statistical analysis
Before statistical analyses, all data were tested for normal distribution (D’Agostino-Pearson omnibus normality test). If data were not normally distributed, the appropriate nonparametric tests were used (GraphPad Prism v5.0), with all statistical comparisons indicated in the figure legends. Uncertainty is represented in the figures as SEM, except as indicated in the figure legends. All comparisons used a two-sided α of 0.05 for significance testing.
Supplementary Material
Fig. S1. Reduction of memory-like NK cell cytotoxicity against K562 leukemia cells by blockade of NKG2D or DNAM-1.
Fig. S2. Mass cytometry gating strategies.
Fig. S3. Phenotypic marker expression on viSNE gated control and memory-like NK cells.
Fig. S4. Enhanced effector responses of memory-like NK cells compared to control-treated NK cells.
Fig. S5. Schema of allogeneic memory-like NK cell phase 1 clinical trial (NCT01898793).
Fig. S6. Distribution of BM blast percentages and donor NK cell numbers sorted by clinical outcomes.
Fig. S7. Chemokine receptor expression on control versus IL-12, IL-15, and IL-18–preactivated human NK cells.
Table S1. NK cell phenotypic mass cytometry panel design, reagents, and clustering usage.
Table S2. NK cell functional mass cytometry panel design, reagents, and clustering usage.
Table S3. Characteristics of normal donors used in mass cytometry functional experiments.
Table S4. Characteristics of AML samples used for in vitro NK cell functional assays.
Table S5. Patient HLA and donor KIR characteristics for evaluable donor-patient pairs treated in the phase 1 clinical trial.
Table S6. Flow cytometry mAbs.
Acknowledgments
We would like to thank W. Yokoyama, A. French, T. Ley, P. Westervelt, and J. DiPersio for insightful discussion. We thank C. Keppel, K. Shah, and A. Ireland for technical assistance. We also thank our patient volunteers and the BM transplant/leukemia physician and nurse coordinator teams at the Washington University School of Medicine (WUSM).
Funding: This work was supported by the American Society of Hematology Foundation, the Conquer Cancer Foundation of the American Society of Clinical Oncology, the WUSM Siteman Cancer Center Developmental Research Award and Team Science Award, the WUSM Institute of Clinical and Translational Research Award, the Leukemia Specialized Program of Research Excellence (P50 CA171963) Development Research Award, the Howard Hughes Medical Institute Medical Fellow Award, the Translational TL1 program, NIH/National Cancer Institute (NCI) grant F32 CA200253, the V Foundation for Cancer Research, and the Gabrielle’s Angel Foundation for Cancer Research. Technical support was provided by the Immunomonitoring Laboratory (also supported by the Center for Human Immunology and Immunotherapy Programs), the Biological Therapy Core, and the Small Animal Cancer Imaging Core (also supported by P50 CA94056), which are supported by the NCI Cancer Center Support grant P30CA91842. We acknowledge the use of the Protein Production and Purification Facility for CyTOF mAb conjugation (P30 AR048335).
Footnotes
SUPPLEMENTARY MATERIALS
www.sciencetranslationalmedicine.org/cgi/content/full/8/357/357ra123/DC1
Materials and Methods
Clinical vignettes
Reference (38)
Author contributions: R.R., M.R., M.M.B.-E., S.T.O., M.A.C., and T.A.F. conceived and designed the study; R.R., M.R., M.M.B.-E., J.A.W., B.A.J., T.S., S.A.-L., S.E.S., S.W., L.Y., Y.-S.L., and C.C.N. collected, analyzed, and assembled the data; A.M. and F.C. provided critical reagents; R.R., M.R., M.M.B.-E., J.W.L., M.A.C., and T.A.F. wrote the manuscript; and all authors reviewed the data and edited and approved the final version of the manuscript.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Juliusson G, Antunovic P, Derolf Å, Lehmann S, Möllgård L, Stockelberg D, Tidefelt U, Wahlin A, Höglund M. Age and acute myeloid leukemia: Real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. doi: 10.1182/blood-2008-07-172007. [DOI] [PubMed] [Google Scholar]
- 2.Estey E, Döhner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 3.Rowe JM, Tallman MS. How I treat acute myeloid leukemia. Blood. 2010;116:3147–3156. doi: 10.1182/blood-2010-05-260117. [DOI] [PubMed] [Google Scholar]
- 4.Döhner H, Estey E, Amadori S, Appelbaum FR, Büchner T, Burnett AK, Dombret H, Fenaux P, Grimwade D, Larson RA, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz MA, Sierra J, Tallman MS, Löwenberg B, Bloomfield CD. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 5.Mrózek K, Marcucci G, Nicolet D, Maharry KS, Becker H, Whitman SP, Metzeler KH, Schwind S, Wu Y-Z, Kohlschmidt J, Pettenati MJ, Heerema NA, Block AW, Patil SR, Baer MR, Kolitz JE, Moore JO, Carroll AJ, Stone RM, Larson RA, Bloomfield CD. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30:4515–4523. doi: 10.1200/JCO.2012.43.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pahl J, Cerwenka A. Tricking the balance: NK cells in anti-cancer immunity. Immunobiology. 2015 doi: 10.1016/j.imbio.2015.07.012. S0171–2985(15)30031-0. [DOI] [PubMed] [Google Scholar]
- 8.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 9.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 10.Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: A pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955–959. doi: 10.1200/JCO.2009.24.4590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curti A, Ruggeri L, D’Addio A, Bontadini A, Dan E, Motta MR, Trabanelli S, Giudice V, Urbani E, Martinelli G, Paolini S, Fruet F, Isidori A, Parisi S, Bandini G, Baccarani M, Velardi A, Lemoli RM. Successful transfer of alloreactive haploidentical KIR ligand-mismatched natural killer cells after infusion in elderly high-risk acute myeloid leukemia patients. Blood. 2011;118:3273–3279. doi: 10.1182/blood-2011-01-329508. [DOI] [PubMed] [Google Scholar]
- 12.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123:3855–3863. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Min-Oo G, Kamimura Y, Hendricks DW, Nabekura T, Lanier LL. Natural killer cells: Walking three paths down memory lane. Trends Immunol. 2013;34:251–258. doi: 10.1016/j.it.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rölle A, Pollmann J, Cerwenka A. Memory of infections: An emerging role for natural killer cells. PLOS Pathog. 2013;9:e1003548. doi: 10.1371/journal.ppat.1003548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keppel MP, Yang L, Cooper MA. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J Immunol. 2013;190:4754–4762. doi: 10.4049/jimmunol.1201742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18–preactivated NK cells against established tumors. J Exp Med. 2012;209:2351–2365. doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, Fehniger TA. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplant. 2014;20:463–473. doi: 10.1016/j.bbmt.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrien-Elliott MM, Wagner JA, Fehniger TA. Human cytokine-induced memory-like natural killer cells. J Innate Immun. 2015;7:563–571. doi: 10.1159/000382019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horowitz A, Strauss-Albee DM, Leipold M, Kubo J, Nemat-Gorgani N, Dogan OC, Dekker CL, Mackey S, Maecker H, Swan GE, Davis MM, Norman PJ, Guethlein LA, Desai M, Parham P, Blish CA. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5:208ra145. doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strauss-Albee DM, Fukuyama J, Liang EC, Yao Y, Jarrell JA, Drake AL, Kinuthia J, Montgomery RR, John-Stewart G, Holmes S, Blish CA. Human NK cell repertoire diversity reflects immune experience and correlates with viral susceptibility. Sci Transl Med. 2015;7:297ra115. doi: 10.1126/scitranslmed.aac5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amir E-aD, Davis KL, Tadmor MD, Simonds EF, Levine JH, Bendall SC, Shenfeld DK, Krishnaswamy S, Nolan GP, Pe’er D. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol. 2013;31:545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cooley S, Weisdorf DJ, Guethlein LA, Klein JP, Wang T, Le CT, Marsh SGE, Geraghty D, Spellman S, Haagenson MD, Ladner M, Trachtenberg E, Parham P, Miller JS. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood. 2010;116:2411–2419. doi: 10.1182/blood-2010-05-283051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooley S, Trachtenberg E, Bergemann TL, Saeteurn K, Klein J, Le CT, Marsh SGE, Guethlein LA, Parham P, Miller JS, Weisdorf DJ. Donors with group B KIR haplotypes improve relapse–free survival after unrelated hematopoietic cell transplantation for acute myelogenous leukemia. Blood. 2009;113:726–732. doi: 10.1182/blood-2008-07-171926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venstrom JM, Pittari G, Gooley TA, Chewning JH, Spellman S, Haagenson M, Gallagher MM, Malkki M, Petersdorf E, Dupont B, Hsu KC. HLA-C–dependent prevention of leukemia relapse by donor activating KIR2DS1. N Engl J Med. 2012;367:805–816. doi: 10.1056/NEJMoa1200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strauss-Albee DM, Horowitz A, Parham P, Blish CA. Coordinated regulation of NK receptor expression in the maturing human immune system. J Immunol. 2014;193:4871–4879. doi: 10.4049/jimmunol.1401821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanier LL. NK cell recognition. Annu Rev Immunol. 2005;23:225–274. doi: 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 29.Bendall SC, Simonds EF, Qiu P, Amir E-aD, Krutzik PO, Finck R, Bruggner RV, Melamed R, Trejo A, Ornatsky OI, Balderas RS, Plevritis SK, Sachs K, Pe’er D, Tanner SD, Nolan GP. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science. 2011;332:687–696. doi: 10.1126/science.1198704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu P, Simonds EF, Bendall SC, Gibbs KD, Jr, Bruggner RV, Linderman MD, Sachs K, Nolan GP, Plevritis SK. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat Biotechnol. 2011;29:886–891. doi: 10.1038/nbt.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Löwenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD. Revised recommendations of the international working group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 32.Luetke-Eversloh M, Hammer Q, Durek P, Nordström K, Gasparoni G, Pink M, Hamann A, Walter J, Chang H-D, Dong J, Romagnani C. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLOS Pathog. 2014;10:e1004441. doi: 10.1371/journal.ppat.1004441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Romee R, Leong JW, Fehniger TA. Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica. 2014;2014:205796. doi: 10.1155/2014/205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mulder A, Kardol MJ, Arn JS, Eijsink C, Franke MEI, Schreuder GMT, Haasnoot GW, Doxiadis IIN, Sachs DH, Smith DM, Claas FHJ. Human monoclonal HLA antibodies reveal interspecies crossreactive swine MHC class I epitopes relevant for xenotransplantation. Mol Immunol. 2010;47:809–815. doi: 10.1016/j.molimm.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Rosario M, Liu B, Kong L, Collins LI, Schneider SE, Chen X, Han K, Jeng EK, Rhode PR, Leong JW, Schappe T, Jewell BA, Keppel CR, Shah K, Hess B, Romee R, Piwnica-Worms DR, Cashen AF, Bartlett NL, Wong HC, Fehniger TA. The IL-15-based ALT-803 complex enhances FcγRIIIa-triggered NK cell responses and in vivo clearance of B cell lymphomas. Clin Cancer Res. 2016;22:596–608. doi: 10.1158/1078-0432.CCR-15-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotecha N, Krutzik PO, Irish JM. Web-based analysis and publication of flow cytometry experiments. Curr Protoc Cytom Chapter. 2010 doi: 10.1002/0471142956.cy1017s53. Chapter 10, Unit10.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diggins KE, Ferrell PB, Jr, Irish JM. Methods for discovery and characterization of cell subsets in high dimensional mass cytometry data. Methods. 2015;82:55–63. doi: 10.1016/j.ymeth.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zunder ER, Finck R, Behbehani GK, Amir E-aD, Krishnaswamy S, Gonzalez VD, Lorang CG, Bjornson Z, Spitzer MH, Bodenmiller B, Fantl WJ, Pe’er D, Nolan GP. Palladium-based mass tag cell barcoding with a doublet-filtering scheme and single-cell deconvolution algorithm. Nat Protoc. 2015;10:316–333. doi: 10.1038/nprot.2015.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Reduction of memory-like NK cell cytotoxicity against K562 leukemia cells by blockade of NKG2D or DNAM-1.
Fig. S2. Mass cytometry gating strategies.
Fig. S3. Phenotypic marker expression on viSNE gated control and memory-like NK cells.
Fig. S4. Enhanced effector responses of memory-like NK cells compared to control-treated NK cells.
Fig. S5. Schema of allogeneic memory-like NK cell phase 1 clinical trial (NCT01898793).
Fig. S6. Distribution of BM blast percentages and donor NK cell numbers sorted by clinical outcomes.
Fig. S7. Chemokine receptor expression on control versus IL-12, IL-15, and IL-18–preactivated human NK cells.
Table S1. NK cell phenotypic mass cytometry panel design, reagents, and clustering usage.
Table S2. NK cell functional mass cytometry panel design, reagents, and clustering usage.
Table S3. Characteristics of normal donors used in mass cytometry functional experiments.
Table S4. Characteristics of AML samples used for in vitro NK cell functional assays.
Table S5. Patient HLA and donor KIR characteristics for evaluable donor-patient pairs treated in the phase 1 clinical trial.
Table S6. Flow cytometry mAbs.


