Abstract
Background
Experiments on porcine heart scaffold represent significant assays in development of immunoneutral materials for cardiac surgery. Characterization of cell-cell and cell-scaffold interactions is essential to understand the homing process of cardiac cells into the scaffolds.
Material/Methods
In the present study, the highly sensitive and real-time impedimetric technique of xCELLigence SP was used to monitor cell adhesion, which is the key process of recellularization in heart scaffolds. Our objectives were: (i) to characterize the effect of decellularized porcine heart scaffold on cell adhesion of human cardiovascular cells potentially used in the recellularization process; and (ii) to investigate cell-extracellular matrix element interactions for building artificial multi-layer systems, applied as cellular models of recellularization experiments. Human fibrosarcoma, endothelial, and cardiomyocyte cells were investigated and the effect of decellularized porcine heart scaffold (HS) and fibronectin on cell adhesion was examined. Adhesion was quantified as slope of curves.
Results
Heart scaffold had neutral effect on cardiomyocytes as well as on endothelial cells. Adhesion of cardiomyocytes was increased by fibronectin (1.480±0.021) compared to control (0.745±0.029). The combination of fibronectin and HS induced stronger adhesion of cardiomyocytes (2.407±0.634) than fibronectin alone. Endothelial and fibrosarcoma cells showed similarly strong adhesion profiles with marked enhancer effect by fibronectin.
Conclusions
Decellularized porcine HS does not inhibit adhesion of human cardiovascular cells at the cell biological level, while fibronectin has strong cell adhesion-inducer effect, as well as an enhancer effect on activity of HS. Consequently, decellularized porcine hearts could be used as scaffolds for recellularization with cardiomyocytes and endothelial cells with fibronectin acting as a regulator, leading to construction of working bioartificial hearts.
MeSH Keywords: Endothelial Cells; Myocytes, Cardiac; Tissue Engineering
Background
Currently, the only curative treatment of end-stage heart failure is heart transplantation. However, due to strict transplantation regulations and lack of public awareness, shortage of transplantable organs persists worldwide, and waiting list mortality is still unacceptably high [1,2]. The human heart lacks the ability to regenerate after tissue necrosis. Although many advances have been made in the development stem cell-based regeneration therapy, a number of unsolved problems have yet to be resolved [3]. Loss of contractile cardiomyocytes leads to decrease in myocardial pump function and symptomatic heart failure. Tissue engineering has been applied in research to treat heart failure and localized myocardial necrosis with the construction of beating myocardial patches [4]. Whole-heart tissue engineering programs have been created to circumvent the worldwide shortage of organs for transplantation. Whole-heart decellularization by organ perfusion has already been described in porcine [5,6] and rat [7] hearts. After perfusion decellularization, an intact, cell-free, 3-dimensional (3D) heart scaffold remains, consisting of extracellular matrix components, which allows recellularization with human cells: cardiomyocytes, endothelial cells, or other cell types. In 3D organ tissue engineering research, besides constructing suitable scaffolds, finding appropriate cellular compounds is a key part of the process [8]. Potential usage of organ recellularization is discussed frequently [9–12] as a successful clinical translation that could represent a new era in heart failure therapy.
Tissue engineering research exhibits a variety of approaches. Arshi et al. focused on the effect of matrix rigidity on cardiac differentiation using human and murine embryonic stem cells with the intention to manipulate proliferation of functional cardiomyocytes [13]. For patch implantation, different methods have also been described. Besides research on tissue engineering, an artificial tissue substitute consisting of poly(ɛ-caprolactone) (PCL) and poly(ethylene glycol) (PEG) has also been developed [14].
Research on the components of extracellular matrix (ECM) has been progressing for decades. Seif-Naraghi et al. used a pericardial matrix in heart surgery for septal reconstruction in congenital atrial septum defects, consisting of collagen type I, V, and VIII. In addition, elastin and fibrillin were retained in all samples. In a few experiments, collagen type III and IV, as well as microfibrillar-associated protein 4 (MFAP4) and procollagen type I, were recorded with tandem mass spectrometry [15]. Badylak [16] reported that growth factors, fibronectin, laminin, and glycosaminoglycans were measured as ECM components. Fibronectin had an even greater role in the embryological phase.
Fibronectin is a dimeric, high-molecular weight molecule that is rich in Arg-Gly-Asp (RGD) sequence. Soluble and tissue isoforms have been identified. Cell adhesion facilitating capabilities of fibronectin have been described [17,18]. Fibronectin is mainly located in submucosal structures and basement membranes. Because of its adhesive properties, fibronectin is often used to enable biocompatibility [19]. The RGD amino acid sequence is important in cell adhesions through α5β1 integrin, playing the main role in cell-cell and cell-matrix adhesion [18].
The basics of impedimetry applied to cellular systems were developed by Giaever and Keese [20], and their electric cell-substrate impedance sensing (ECIS) system was the starting equipment in the field. A great advantage of these cell physiological recordings is their real-time character, which provides even longer tracking periods. For high-sensitivity monitoring of cell adhesion, the xCELLigence system has been developed [19,21]. The xCELLigence SP device measures impedance changes on network of gold electrodes caused by adhering cells, allowing label-free assessment of cell adhesion and non-invasive measurements of cell impedances alterations. The measured impedance depends on the number of adhering cells, as well as the level and type of adhesion.
Our aims in the present study were: (i) to characterize the effect of decellularized porcine heart scaffold homogenates on cell adhesion of human cells (cardiomyocytes and endothelial cells) used as the chief constituents of recellularization; and (ii) to investigate cell-ECM element interactions as guiding processes in development of artificial multi-layer systems – cellular models of recellularization of heart scaffolds.
Material and Methods
Cell culture
We performed experiments with fibrosarcoma, endothelial cells, and cardiomyocytes. HT-1080 human connective tissue-derived fibrosarcoma cell line (ATCC, Manassas, VA) was cultured in Minimum Essential Medium (ATCC, Manassas, VA) and 10% fetal calf serum (FCS). HMEC-1 human dermal microvascular endothelial cells (Invitrogen, Life Technologies, Carlsbad, CA) were cultured in MCDB-131 (Sigma Ltd. St. Louis, MO), 10% FCS (Lonza Group Ltd. Basel, Switzerland), L-glutamine (4 mmol/mL) (Gibco®/Invitrogen Corporation, NY), penicillin/streptomycin (100 I.U./mL, 100 μg/mL) (Gibco®/Invitrogen Corporation, NY), human recombinant epidermal growth factor (10 ng/ml) (Sigma, Ltd., St. Louis, MO), and hydrocortisone (1 μg/mL) (Sigma, Ltd.). HCM primary human cardiac myocytes (PromoCell, Biomedica, Wien, Austria) were cultured in Myocyte Growth Medium (PromoCell, Biomedica).
Decellularized porcine heart scaffold
Decellularized porcine heart scaffold was produced by perfusion technique as previously described by Weymann et al. [5]. Briefly, the hearts were perfused through the aorta in a perfusion circuit containing a pressure control module and a heat exchanger. The hearts were perfused on a constant pressure of 100 Hgmm and temperature of 37°C with a 4% solution of sodium dodecyl sulfate in PBS (phosphate buffer solution) at 2 L/min for 12 h. Every 3 h, the hearts were washed with PBS for 15 min. After 12 h of perfusion, the hearts were washed with PBS at 1.5 L/min for 24 h. For our experiments, an approximately 1-cm3 patch of the heart scaffold’s apex was obtained and homogenized by an ultrasound-based approach (pulse mode, 25 pulse/min, amplitude=60, tune=50, Vibra Cell VC40, Sonics & Materials Inc., USA), after which the sample was suspended in distilled water.
Impedimetry measurements
For measurement of cell adhesion, we used the xCELLigence SP (Roche Applied Science, Indianapolis, IN) system, which uses an impedance-based approach. In this technique adhesion surfaces are intricate gold microelectrode arrays placed at the bottom of 96-well of E-plates, covering about 80% of each well [22,23]. Adhering cells generate changes in electric impedance measured on the electrode, which directly correlate to surface coverage by cells, as determined by 2 factors: (i) the number of adhering cells and (ii) the spreading of individual cells [21]. Impedance changes are monitored in real-time.
Data are expressed in the form of Cell Index (CI), a dimensionless value calculated from impedance measured at a given time point (Zt), background impedance (Zb), and a in-built factor depending on the frequency of the alternating current (F) according to the following formula [24]:
| (1) |
The advantage of this technique is automated continuous measurement and label-free detection. We used real-time monitoring RTCA Software version 1.2.0.0909 and an Analyzer model W380.
Single-cell layout experiments
The bottoms of the wells were coated with either 25 μL of: (a) heart scaffold homogenate (HS), (b) fibronectin (Human Plasma Fibronectin Purified Protein, Merck, Whitehouse Station, NJ) diluted in distilled water to an end concentration of 16 μg/mL (FN), (c) a compound of fibronectin and heart scaffold homogenate (fibronectin diluted in heart scaffold homogenate to an end concentration of 16 μg/mL) (FN + HS), or (d) distilled water (Control). After 30 min of incubation in room temperature, the remaining solution was gently aspirated and the electrodes were air dried. For the measurement of baseline, 100 μL of medium (according to each cell line) was added to the wells and incubated for 1 h at 37°C and 5% CO2. After the measurement of baseline impedance, 100 uL of cell suspension was added to the wells and changes in impedance were recorded for 72 h at 37°C and 5% CO2 incubation. Three different cell densities were measured each cell line (10 000/well, 15 000/well, and 20 000/well for HT-1080 fibrosarcoma cells and HMEC-1 endothelial cells, and 5000/well, 10 000/well, and 15 000/well for HCM cardiomyocytes). In each sample, 4 parallels were measured (Figure 1).
Figure 1.
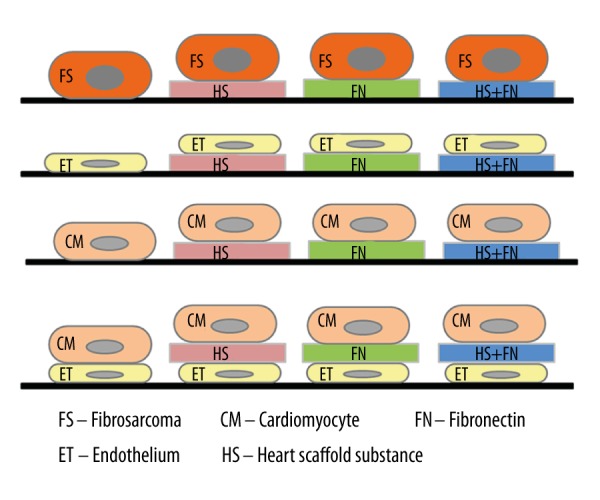
Experimental protocols. In the experiments, cells were investigated: (i) as single-cell layers on non-coated or on FN, HS, or FN+HS coated surfaces, and (ii) as “sandwich” cell combinations of ET and CM cell layers with or without interconnecting layer of FN, HS, or FN+HS. HS – decellularized heart scaffold homogenate; FN – fibronectin; FN + HS – combination of fibronectin and decellularized heart scaffold homogenate; ET – HMEC-1 human microvascular endothelial cell; CM – HCM human cardiac myocytes.
“Sandwich” layout experiment
We added 100 μL medium to the wells, and baseline impedance was measured for 1 h at 37°C and 5% CO2 incubation. After that, 100 μL HMEC-1 cell suspension (cell count 20 000/well) was added to the wells and incubated for 24 h (37°C and 5% CO2) until an endothelial monolayer was formed, as confirmed by impedimetry curves reaching plateau phase. Then, 100 μL of medium was gently aspirated and replaced with 100 μL of either HS, FN, or FN + HS treatment solution as described in the previous section. In the control wells, no treatment solution was added (Control), and incubated for 30 min at 37°C and 5% CO2. Subsequently, 100 μL solution was gently aspirated and 150 μL cardiomyocyte suspension was added to the wells in 5000/well, 10 000/well, and 15 000/well cell densities. After that, impedance changes were recorded for 72 h at 37°C and 5% CO2 incubation. In each sample, 4 parallels were measured (Figure 1).
Statistical analysis
Cell adhesion was quantified as slope values (the rise of a line laid on a defined interval of the curve) (1/h) as calculated by RTCA software. In every experiment, 2 slope values depicting short-term and long-term adhesion activity were analyzed (as given below). For statistical analysis, one-way ANOVA was used. For marking the level of significance, the following symbols were used: x – p<0.05, y – p<0.01 and z – p<0.001. In graphical representation of impedimetry data curves, the Delta Cell Index is shown, and the zero point is the first measured data point for the examined cell line in the well.
Results
In cell adhesion experiments, calculation of slope values provides one of the best opportunities to evaluate dynamics of cell adhesion in short- and long-term experiments, as well as to compare responsiveness of identical experimental schedules. Our pilot experiments in all 4 types of model cells showed that completion of adhesive processes is rather different. In the short-term, it lasts from 1: 09 to 2: 25 h, while in the long-term it lasts from 16 to 40 h. These data show good correlation with the cell physiology, as short-term experiments describe the rapid adhesion of the cells, while the long-term adhesion experiments investigated how the initial adhesion continues to spread or other time-consuming cellular processes (e.g., micromotion and haptotaxis).
In the data presented below, values of slope calculated by RTCA (for details see 2.4) are given to characterize effects of the substances investigated. The calculated slope values approximate the rise or fall of specific intervals, and, as such, they are relative values and can vary between experiments, cell types, and different time ranges. Trends, direction of change, and rough measure of effect are comparable between experiments, but exact values are not. Consequently, graphs below showing slope values do not have the same scales. All time values and intervals are given in Delta Time, where zero point is chosen as the first measurement point after addition of the measured cells (cardiomyocytes in the “sandwich” experiment. In the text, time intervals are shown in ‘TI: hh: mm’ format.
Short-term effects
Fibrosarcoma cell line (HT-1080) (TI: 0:00–1:21)
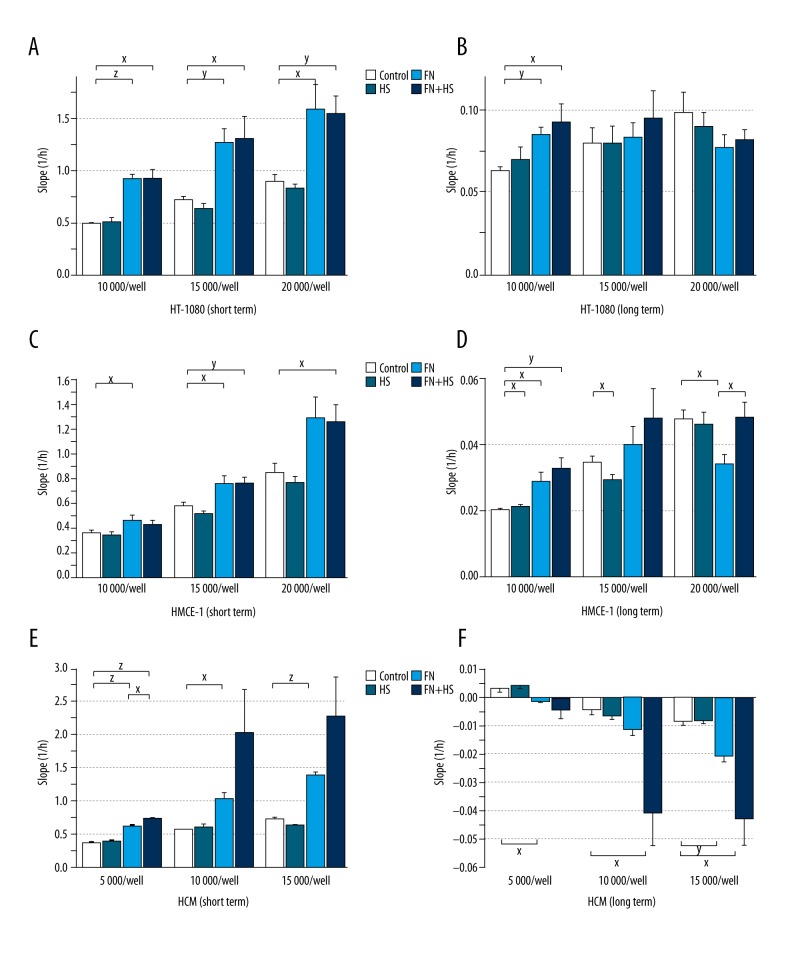
HT-1080 cells showed strong adhesion activity that was not influenced by the HS at any cell density (10 000/well – Control 0.492±0.012 vs. HS 0.514±0.034; 15 000/well – Control 0.721±0.029 vs. HS 0.634±0.040; 20 000/well – Control 0.900±0.057 vs. HS 0.831±0.037). In contrast HS, fibronectin (FN) increased cell adhesion significantly in all the examined cell densities (10 000/well – FN 0.923±0.044, p<0.001; 15 000/well – FN 1.267±0.137, p=0.01; 20 000/well – FN 1.591±0.233, p=0.05). The combination of fibronectin and heart scaffold homogenate (FN+HS) also had a significant adhesion-inducer activity in every tested cell density (10 000/well – FN + HS 0.925±0.083, p=0.01; 15 000/well – FN + HS 1.309±0.203, p=0.05; 20 000/well – FN + HS 1.545±0.162, p=0.01). The inducer effects elicited by fibronectin alone and in combination with fibronectin showed no significant difference (Figure 2A).
Figure 2.
Impedimetric measurements – Single-cell layout protocols. Effect of decellularized heart scaffold homogenate and fibronectin electrode coating on adhesion of fibrosarcoma (A: short-term, B: long-term), endothelial (C: short-term, D: long-term), and cardiomyocyte (E: short-term, F: long-term) cell lines. HS – decellularized heart scaffold homogenate; FN – fibronectin, FN + HS – combination of fibronectin and decellularized heart scaffold homogenate; HT-1080 human fibrosarcoma cell line; HMEC-1 human microvascular endothelial cell line; HCM human cardiac myocytes; x: p<0.05; y: p<0.01; z: p<0.001.
Human microvascular endothelial cell line (HMEC-1) (TI: 0:00–2:25)
HMEC-1, similarly to fibrosarcoma cells, expressed strong adhesion that was not changed by HS applied in different cell densities (10 000/well – Control 0.369±0.016 vs. HS 0.347±0.019, 15 000/well – Control 0.581±0.031 vs. HS 0.520±0.032; 20 000/well – Control 0.856±0.080 vs. HS 0.780±0.042). FN increased adhesion significantly in 10 000/well and 15 000/well cell densities (10 000/well – FN 0.473±0.033, p=0.05 and 15 000/well – FN 0.770±0.056, p=0.05) and tendentially in 20 000/well cell density (20 000/well – FN 1.298±0.164). Combination of FN and HS increased adhesion tendentially in 10 000/well cell density (10,000/well – FN + HS 0.431±0.030) and significantly on 2 higher cell densities (15 000/well – FN + HS 0.772±0.040, p=0.01 and 20 000/well – FN + HS 1.263±0.138, p=0.05). No significant difference was observed between the positive effect of FN in combination with HS and responses elicited by FN alone (Figure 2C).
HCM human cardiomyocyte cell line (TI: 00:05–01:09)
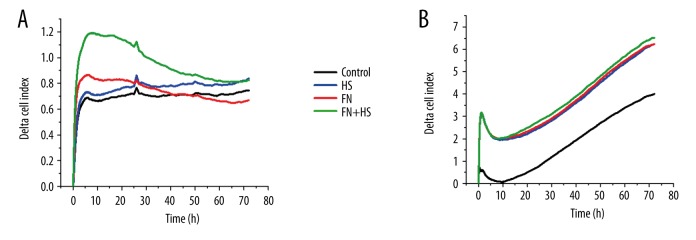
HCM cardiac cells showed good adhesion behavior that was not changed by HS at any cell density (5000/well – Control 0.368±0.016 vs. HS 0.390±0.025, 10 000/well – Control 0.571±0.009 vs. HS 0.613±0.031 and 15 000/well – Control 0.724±0.025 vs. HS 0.640±0.022). FN significantly increased cell adhesion at all cell densities (5000/well – FN 0.627±0.010, p<0.001, 10 000/well – FN 1.032±0.082, p=0.05 and 15 000/well – FN 1.394±0.021, p<0.001). The combination of FN and HS increased cell adhesion significantly in 5000/well cell count (5000/well – FN + HS 0.731±0.026, p<0.001) and tendentially in the 2 higher cell densities (10 000/well – FN + HS 2.035±0.630; 15 000/well – FN + HS 2.278±0.583). The positive effect of FN applied alone was significantly enhanced in combination with HS at 5000/well cell density (FN 0.627±0.010 vs. FN + HS 0.731±0.026, p=0.05) and a tendentially increased effect was registered in the 2 higher cell counts (10 000/well – FN 1.032±0.082 vs. FN + HS 2.035±0.630 and 15 000/well – FN 1.394±0.021 vs. FN + HS 2.278±0.583) (Figure 2E, Figure 3A).
Figure 3.
Impedimetric detection of cell adhesion. Representative experiments showing effect of electrode coating on adhesion of HCM human cardiac myocytes (5000/well) (A) and effect of coating treatment on adhesion of HCM human cardiac myocytes (5000/well) to HMEC-1 human microvascular endothelial cell monolayer (20 000/well) (B).
“Sandwich” layout (endothelial and cardiomyocyte) (TI: 00:00–00:23)
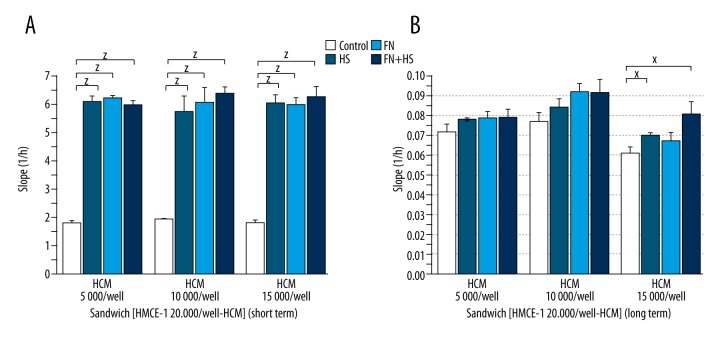
In short-term experiments, cardiomyocytes adhered strongly to the endothelial cell layer in all cardiomyocyte cell densities and was significantly increased by HS treatment (5000/well – Control 1.815±0.043 vs. HS 6.104±0.192, p<0.001, 10 000/well – Control 1.943±0.019 vs. HS 5.774±0.506, p<0.001 and 15 000/well – Control 1.794±0.120 vs. HS 6.048±0.299, p<0.001). FN increased significantly adhesion at all cell densities as well (5000/well – FN 6.215±0.095, p<0.001, 10 000/well – FN 6.107±0.520, p<0.001 and 15 000/well – FN 6.001±0.258, p<0.001). The combination of FN and HS increased significantly adhesion at all cell counts as well (5000/well – FN + HS 6.006±0.115, p<0.001, 10 000/well – FN + HS 6.391±0.208, p < 0.001 and 15 000/well – FN + HS 6.297±0.344, p<0.001). Adhesion-inducer effects of FN and HS applied alone or in combination showed no difference in volume or dynamics of HCM cell adhesion (Figure 3B, Figure 4A).
Figure 4.
Impedimetric measurements – “Sandwich” layout protocols. Effect of decellularized heart scaffold homogenate and fibronectin coating treatment on adhesion of cardiomyocyte cell line to endothelial cell monolayer (A: short-term, B: long-term). HS – decellularized heart scaffold homogenate; FN – fibronectin, FN + HS – combination of fibronectin and decellularized heart scaffold homogenate; HMEC-1 human microvascular endothelial cell line; HCM human cardiac myocytes; x: p<0.05; y: p<0.01; z: p<0.001.
Long-term effects
HT-1080 fibrosarcoma cell line (TI: 2:54–31:00)
In long-term experiments, we found no significant change of the aforementioned effects, and in 10 000/well cell density, the positive effect of FN was significantly increased (10 000/well – Control 0.063±0.002 vs. FN 0.085±0.004, p=0.01), similar to the positive effect of FN + HS (10 000/well – Control 0.063±0.002 vs. FN + HS 0.092±0.011, p=0.05) (Figure 2B).
HMEC-1 human microvascular endothelial cell line (TI: 07:06–47:45)
At 10 000/well cell density, HS increased adhesion slightly but significantly (10 000/well – Control 0.020±0.0004 vs. HS 0.021±0.0001, p=0.05). The effect of FN alone and the combination of FN + HS was significantly increased (10 000/well – FN 0.029±0.003, p=0.05 and FN + HS 0.033±0.003, p=0.01). At 15 000/well cell density, a weak but significantly decreased adhesion was observed with HS (15 000/well – Control 0.035±0.001 vs. HS 0.029±0.001, p=0.05), but with FN alone and in FN +HS combination, only positive tendencies (15 000/well – FN 0.040±0.006 and FN + HS 0.048±0.009) were registered. At 20,000/well cell density, FN had a significant adhesion-decreasing effect (20 000/well – Control 0.048±0.003 vs. FN 0.034±0.003, p=0.05), which was completely counteracted in the combination FN + HS (20 000/well FN + HS 0.048±0.005, p=0.05) (Figure 2D).
HCM human cardiomyocyte cell line (TI: 07:52–24:00)
In the long-term experiment, a backlash of the positive short-term adhesion was found. Compared to Control, responses were significantly stronger in FN alone in 5000/well and 15 000/well cell densities (5000/well – Control 0.004±0.001 vs. FN -0.001±0.0004, p=0.05 and 15 000/well – Control −0.008±0.002 vs. FN −0.021±0.002, p=0.01), and in combinations FN + HS in 10 000/well and 15 000/well cell densities (10 000/well – Control −0.004±0.002 vs. FN + HS −0.040±0.012, p=0.05 and 15 000/well – Control −0.008±0.002 vs. FN + HS −0.043±0.009, p=0.05) (Figure 2F, Figure 3A).
“Sandwich” layout of endothelins and cardiomyocytes (TI: 17:01–66:00)
At 15 000/well cell density, the adhesion was significantly enhanced by HS alone (15 000/well – Control 0.061±0.003 vs. HS 0.070±0.002, p=0.05) and also in combination with FN (15 000/well – Control 0.061±0.003 vs. FN + HS 0.081±0.006, p=0.05) (Figure 3B, Figure 4B).
Discussion
In the last decade great strides have been made towards the construction of a tissue-engineered whole heart; however, unsolved challenges still remain, particularly in recellularization of decellularized, whole-organ scaffolds [25]. Endothelialization of human pulmonary and aortic valve grafts has been described [26], but recellularization of 3D myocardial scaffolds with cardiomyocytes has only been achieved in thin myocardial patches with low cell density [27]. Advances have been made with re-cellularized rat heart, showing a fraction of adult contractile function [7]; however, construction of a recellularized, functional whole heart is still a goal to be achieved.
For tissue engineering of human-sized whole hearts, cadaveric human hearts could constitute optimal scaffolds, but the scarcity of organ donors limits this option. Porcine hearts are easily obtainable from slaughterhouse pigs, and, after proper decellularization, cell-free, potentially immuno-neutral scaffolds can be produced [5]. By recellularization with human cells and human regulatory molecules, an optimal, implantable whole-heart allograft could be achieved.
Cellular-level characterization of the adhesive properties of decellularized heart scaffolds and their relationship with human cells can provide valuable insight for future recellularization research. The HT-1080 human fibrosarcoma cell line is a tumor cell line with strong adhesive properties used, among others, as the model cell for adhesion studies [28]. In our experiments with HT-1080 cells, characteristic cell density-dependent, strong adhesion profile was measured, which was further increased by fibronectin (FN) precoating. On porcine heart scaffold homogenate coating, a similarly strong adhesion of HT-1080 cells was measured and the positive effect of FN could be observed in this case as well. According to these results, decellularized heart scaffold has no adhesion-inhibiting effect per se. Endothelial cells are unique to the cardiovascular system that covers the luminal, vascular part of the endocardium. Characterization of the effect of porcine decellularized heart scaffold on the behavior of this essential part of cardiac anatomy can aid the development of a usable recellularization protocol. In our assessment, the adhesion activity of HMEC-1 endothelial cells was maintained on precoating with porcine decellularized heart scaffold (HS) homogenate with or without the addition of FN. Besides endothelial cells, cardiomyocytes are the other function-specific cell types essential to include in the whole-heart recellularization process. Therefore, it is necessary to characterize their reaction to decellularized heart scaffold. In our experiments, adhesion activity of HCM cardiomyocytes was not decreased by HS. Combined with FN coating, an enhancer effect was observed, especially at low cell densities (5000/well). Even though a decrease in this enhancement was observed in the long term, the initial level of adhesion persisted. At higher cell densities (10 000/well and 15 000/well), this decline was more strongly expressed, which suggests that technical factors, such as static experimental setup and no disruption of experiment with medium change, might also influence the development of this phenomenon. In the “sandwich” experimental layout, HS significantly increased cardiomyocyte adhesion to the preloaded and preseeded (24 h) confluent (signified by curves reaching plateau phase) endothelial layer, which shows the positive effect of FN. This strong positive effect proved to be constant through the whole period of the long-term measurement (72 h). These results establish that the decellularized porcine HS has a neutral or enhancer effect on human cardiovascular cells at the cellular adhesion level. In addition, our experiments show that HMEC-1 microvascular-origin endothelial cells and HCM cardiomyocytes are promising candidates for further recellularization experiments on tissue-engineered heart scaffolds.
Regardless of these encouraging results about the relationship between cardiovascular cells and decellularized heart scaffold, discovering potential regulator molecules for the recellularization process could provide substantial help. FN Fibronectin is a well-known enhancer and modifier of cell adhesion [18]. Ott et al. reported the presence of FN in decellularized heart scaffold [7]. In our experiment, FN treatment increased the positive effect of FN on adhesion of HT-1080 fibrosarcoma, HMEC-1 endothelial and HCM cardiomyocyte cell lines, and the cell-cell adhesion between endothelial cells and cardiomyocytes. Coating of decellularized aortic grafts with FN was shown to increase in vivo endothelialization of grafts in rats [24]. Our experiments with porcine decellularized heart scaffold showed a similar enhancer effect of addition of FN + HS surface coating on adhesion of endothelial cells and even cardiomyocytes. Therefore, our present results support the proposal [24] that FN could be employed as both an enhancer and regulator molecule in the recellularization process in heart engineering.
Conclusions
Our experiment shows for the first time that real-time impedimetry assay is a strong de/recellularization model of cardiac heart scaffold. Decellularized porcine heart scaffold had neutral or enhancer effect on the adhesion of human endothelial cells and cardiomyocytes, while no inhibiting effect was observed. On the cellular level, decellularized porcine heart scaffold homogenates (HS) provided a compatible and attractive environment for human cardiovascular cells. Fibronectin elicited a strong positive effect on cell adhesion, so in the future this extracellular compound could be a valuable regulator and enhancer factor of the recellularization process. Consequently, homogenates of decellularized porcine heart scaffolds, as well as impedimetry, are good candidate systems to investigate recellularization as a part of research in whole-heart tissue engineering.
Footnotes
Conflict of interest
None declared.
Source of support: The present research was funded by the Heart and Vascular Center, Semmelweis University, Budapest, Hungary and OTKA – Hungarian Scientific Research Fund – 105555
References
- 1.Colvin-Adams M, Smith JM, Heubner BM, et al. OPTN/SRTR 2011 Annual Data Report: Heart. Am J Transplant. 2013;13(Suppl 1):119–48. doi: 10.1111/ajt.12023. [DOI] [PubMed] [Google Scholar]
- 2.Koch A, Tochtermann U, Remppis A, et al. The Eurotransplant High-Urgency Heart Transplantation Program: An option for patients in acute heart failure? Thorac Cardiovasc Surg. 2006;54(6):414–17. doi: 10.1055/s-2006-924245. [DOI] [PubMed] [Google Scholar]
- 3.Tang X-L, Rokosh DG, Guo Y, Bolli R. Cardiac progenitor cells and bone marrow-derived very small embryonic-like stem cells for cardiac repair after myocardial infarction. Circ J. 2010;74(3):390–404. doi: 10.1253/circj.cj-09-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patra C, Talukdar S, Novoyatleva T, et al. Silk protein fibroin from Antheraea mylitta for cardiac tissue engineering. Biomaterials. 2012;33(9):2673–80. doi: 10.1016/j.biomaterials.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Weymann A, Loganathan S, Takahashi H, et al. Development and evaluation of a perfusion decellularization porcine heart model – generation of 3-dimensional myocardial neoscaffolds. Circ J. 2011;75(4):852–60. doi: 10.1253/circj.cj-10-0717. [DOI] [PubMed] [Google Scholar]
- 6.Wainwright JM, Czajka CA, Patel UB, et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng Part C Methods. 2010;16(3):525–32. doi: 10.1089/ten.tec.2009.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ott HC, Matthiesen TS, Goh S-K, et al. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 8.Lundberg MS. Cardiovascular tissue engineering research support at the National Heart, Lung, and Blood Institute. Circ Res. 2013;112(8):1097–103. doi: 10.1161/CIRCRESAHA.112.300638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karikkineth BC, Zimmermann W-H. Myocardial tissue engineering and heart muscle repair. Curr Pharm Biotechnol. 2013;14(1):4–11. doi: 10.2174/138920113804805322. [DOI] [PubMed] [Google Scholar]
- 10.Udelsman BV, Maxfield MW, Breuer CK. Tissue engineering of blood vessels in cardiovascular disease: moving towards clinical translation. Heart. 2013;99(7):454–60. doi: 10.1136/heartjnl-2012-302984. [DOI] [PubMed] [Google Scholar]
- 11.Le Huu A, Paul A, Xu L, et al. Recent advancements in tissue engineering for stem cell-based cardiac therapies. Ther Deliv. 2013;4(4):503–16. doi: 10.4155/tde.13.13. [DOI] [PubMed] [Google Scholar]
- 12.Lakshmanan R, Krishnan UM, Sethuraman S. Living cardiac patch: The elixir for cardiac regeneration. Expert Opin Biol Ther. 2012;12(12):1623–40. doi: 10.1517/14712598.2012.721770. [DOI] [PubMed] [Google Scholar]
- 13.Arshi A, Nakashima Y, Nakano H, et al. Rigid microenvironments promote cardiac differentiation of mouse and human embryonic stem cells. Sci Technol Adv Mater. 2013;14(2) doi: 10.1088/1468-6996/14/2/025003. pii: 025003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silvestri A, Sartori S, Boffito M, et al. Biomimetic myocardial patches fabricated with poly(varepsilon-caprolactone) and polyethylene glycol-based polyurethanes. J Biomed Mater Res B Appl Biomater. 2014;102(5):1002–13. doi: 10.1002/jbm.b.33081. [DOI] [PubMed] [Google Scholar]
- 15.Seif-Naraghi SB, Horn D, Schup-Magoffin PA, et al. Patient-to-patient variability in autologous pericardial matrix scaffolds for cardiac repair. J Cardiovasc Transl Res. 2011;4(5):545–56. doi: 10.1007/s12265-011-9293-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Badylak SF. Xenogeneic extracellular matrix as a scaffold for tissue reconstruction. Transpl Immunol. 2004;12(3–4):367–77. doi: 10.1016/j.trim.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzbauer JE. Fibronectin: from gene to protein. Curr Opin Cell Biol. 1991;3(5):786–91. doi: 10.1016/0955-0674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto S, Katz BZ, Lafrenie RM, Yamada KM. Fibronectin and integrins in cell adhesion, signaling, and morphogenesis. Ann NY Acad Sci. 1998;857:119–29. doi: 10.1111/j.1749-6632.1998.tb10112.x. [DOI] [PubMed] [Google Scholar]
- 19.Jonsson MKB, Wang Q-D, Becker B. Impedance-based detection of beating rhythm and proarrhythmic effects of compounds on stem cell-derived cardiomyocytes. Assay Drug Dev Technol. 2011;9(6):589–99. doi: 10.1089/adt.2011.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giaever I, Keese CR. Monitoring fibroblast behavior in tissue culture with an applied electric field. Proc Natl Acad Sci USA. 1984;81(12):3761–64. doi: 10.1073/pnas.81.12.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing JZ, Zhu L, Jackson JA, et al. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem Res Toxicol. 2005;18(2):154–61. doi: 10.1021/tx049721s. [DOI] [PubMed] [Google Scholar]
- 22.Roche Diagnostics GmbH. RTCA SP intrument operator’s manual: Introduction of the RTCA SP instrument. ACEA Biosciences; Indianapolis: 2008. pp. 14–18. [Google Scholar]
- 23.Urcan E, Haertel U, Styllou M, et al. Real-time xCELLigence impedance analysis of the cytotoxicity of dental composite components on human gingival fibroblasts. Dent Mater. 2010;26:51–58. doi: 10.1016/j.dental.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Assmann A, Delfs C, Munakata H, et al. Acceleration of autologous in vivo recellularization of decellularized aortic conduits by fibronectin surface coating. Biomaterials. 2013;34(25):6015–26. doi: 10.1016/j.biomaterials.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 25.Ikada Y. Challenges in tissue engineering. J R Soc Interface. 2006;3(10):589–601. doi: 10.1098/rsif.2006.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weymann A, Schmack B, Okada T, et al. Reendothelialization of human heart valve neoscaffolds using umbilical cord-derived endothelial cells. Circ J. 2013;77(1):207–16. doi: 10.1253/circj.cj-12-0540. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Borazjani A, Tahai M, et al. Fabrication of cardiac patch with decellularized porcine myocardial scaffold and bone marrow mononuclear cells. J Biomed Mater Res A. 2010;94(4):1100–10. doi: 10.1002/jbm.a.32781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lucena SE, Jia Y, Soto JG, et al. Anti-invasive and anti-adhesive activities of a recombinant disintegrin, r-viridistatin 2, derived from the Prairie rattlesnake (Crotalus viridis viridis) Toxicon. 2012;60(1):31–39. doi: 10.1016/j.toxicon.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]