Abstract
Background
Chronic heart failure (CHF) is a leading cause of death worldwide. A long noncoding RNA (lncRNA) named urothelial carcinoma associated 1 (UCA1) is important in multiple diseases. However, the role of UCA1 in CHF is still unknown. Our study investigated whether UCA1 could be applied as an ideal marker to diagnose and evaluate prognosis in CHF.
Material/Methods
Total plasma RNA was extracted from 67 CHF patients and 67 controls. Quantitative real-time polymerase chain reaction was used to determine the plasma level of UCA1. Correlations between UCA1 and clinical parameters were analyzed by Pearson correlation. Receiver operating characteristic curves (ROC) were obtained to analyze the predictive power of UCA1 and BNP for CHF. Kaplan-Meier survival curves were used to evaluate prognosis of CHF within 1 year.
Results
There was no significant difference in elementary data between CHF and controls. Plasma UCA1 was much higher in CHF patients compared with controls. Plasma UCA1 was positively and negatively correlated with brain natriuretic peptide (BNP) and left ventricle ejection fraction (LVEF), respectively. Plasma UCA1 diagnosed CHF with a diagnostic power of 0.89 and a sensitivity and specificity of 100% [95% CI (0.9464–1)] and 76.12% [95%CI (0.6414–0.8569)] (P<0.05), respectively. CHF patients with higher plasma UCA1 had a lower survival rate than those with a lower level, and survival rate predicted by UCA1 had a similar tendency with BNP. However, there was no significant difference between these 2 markers in predicting the prognosis of CHF (P>0.05).
Conclusions
Plasma UCA1 might be an excellent indicator to diagnose CHF and it might predict poor outcomes of CHF.
MeSH Keywords: Heart Failure; Prognosis; RNA, Long Noncoding
Background
Heart failure (HF), a terminal stage of multiple heart diseases, is a leading cause of death all over the world. It is estimated that HF contributed to 1 out of 9 deaths in the United States during 2009, and approximately 284 000 people died of HF in United States in 2013 [1]. Most people diagnosed with HF die within 5 years [1]. It is estimated that chronic heart failure (CHF) treatment costs about 35 billion US dollars or 2% of the total UK national budget, and is an extremely heavy economic burden on the whole society [2,3]. Currently, about 20 million people worldwide have HF. The morbidity of HF is predicted to sharply increase in the next few decades due to various risk factors such as hypertension, hypercholesterolemia, and diabetes mellitus. Traditional markers like brain natriuretic peptide (BNP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) are widely applied to diagnose or exclude HF, and they are ideal markers to predict prognosis of HF. However, these 2 markers are also significantly increased in other conditions, such as kidney failure and hepatic cirrhosis [4,5]. Although left ventricle ejection fraction (LVEF) is often used to evaluate cardiac function in HF patients, many may have normal LVEFs. Thus, there is an urgent need to discover novel markers for HF.
Long noncoding RNAs (lncRNAs) are a class of RNAs seldom encoding proteins. They participate in many biological and pathological processes. A lncRNA called urothelial carcinoma-associated 1 (UCA1) has generated great interest because it is abundantly expressed in the heart and plays critical roles in many diseases [6]. UCA1 is highly expressed in human bladder cancer, and exerts a role in regulating cancer cell proliferation [7]. It can promote migration and invasion of tumor cells in prostate cancer, gastric cancer, lung cancer, and hepatocellular carcinoma [8–11]. Recent research indicates that circulating UCA1 can be used as a biomarker for myocardial infarction, suggesting that UCA1 can be detected in the plasma [12]. However, the relationship between UCA1 and CHF is largely unknown. The purpose of this study was to determine plasma levels of UCA1 in CHF patients and controls to determine whether UCA1 can be applied as an ideal marker for CHF.
Material and Methods
Study design and population recruitment
In this study, we recruited 67 CHF patients and 67 age- and sex-matched controls without CHF from our hospital. Before experiments, every participant was informed and required to sign informed consent. Diagnosis of CHF was made according to the criteria listed in the 2013 ACCF/AHA Guideline for the Management of Heart Failure and the 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure [13,14]. HF was characterized by typical clinical symptoms, such as breathlessness, fatigue, and ankle swelling, and may be accompanied by clinical signs like elevated jugular venous pressure, peripheral edema, and pulmonary crackles, which are caused by a structural and/or functional cardiac abnormality, leading to reduced cardiac output and/or elevated intracardiac pressures at rest or during stress. Patients were clinically stable and ranged from class II to IV according to NYHA class. Each of them had received optical and fixed therapeutic treatment. Included patients had a left ventricular ejection fraction (LVEF) less than 40%. BNP levels of CHF were more than 35 pg/ml. Participants were excluded if they had: (i) severe infection; (ii) surgery within 1 year; (iii) cancers; (iv) liver or renal failure; (v) cerebral vascular events in 6 months; or (vi) heart assist devices. Controls were defined as the population without CHF and none of the diseases mentioned above. Our study was approved by our hospital Ethics Committee and all procedures conformed to the Declaration of Helsinki.
Endpoints
The primary endpoint was death related to cardiac diseases, including cardiac death, or death associated with heart failure, cardiac infarction, severe arrhythmia, hypertension, stroke, or cardiac rupture. No patient dropped out during the 1-year follow-up.
Plasma isolation
About 5 ml of fresh fasting blood was drawn intravenously into ethylene diamine tetra-acetic acid (EDTA)-anticoagulant tubes. The whole blood was centrifuged at 3000 rpm at 4°C for 10 min. Plasma was separated and placed into nuclease-free Eppendorf tubes.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
RNA from plasma was extracted by miRNeasy Serum/Plasma Kit (Qiagen, German) according to the manufacturer’s recommendation. The total RNA was eluted in 10 ul of pre-heated nuclease-free water. The quality and quantity were determined using a NanoDrop 2000 spectrophotometer (Thermo Scientific, USA). RNA was immediately reverse-transcribed into cDNA. Reverse transcription (RT) and qRT-PCR were performed by use of the GoScript™ Reverse Transcription System (Promega, USA) and GoTaq® qPCR Master Mix (Promega, USA) following the manufacturer’s protocols in order to determine the quantity of UCA1 expression in the plasma. RT reactions were performed with a total volume of 20 ul with random primers. RT procedures were: 25°C for 5 min, 42°C for 1 h, 72°C for 15 min, followed by storage at 4°C. During the qRT-PCR, 1 ul of cDNA was added and blended with 10 ul of SYBR Green I dye Premix (Promega, USA), 1 ul of specific forward and reverse primers (10uM), and 8 ul of nuclease-free water, with a following procedure: 95°C for 2 min, 40 cycles of 95°C for 15 s, and 60°C for 30 s, followed by 55°C for 30 min. The relative expression of UCA1 in different groups was calculated by 2−ΔCt method. U6 was obtained as an internal control [12]. Values of Ct over 40 were considered to be negative.
Forward primer of UCA1 was: ACGCTAACTGGCACCTTGTT, reverse primer of was UCA1: TGGGGATTACTGGGGTAGGG.
Forward primer of was U6: GCTTCGGCAGCACATATACTAAAAT, and reverse primer of U6 was: CGCTTCACGAATTTGCGTGTCAT.
Statistical analysis
Data are presented as means ± SD. Statistical analysis was performed using SPSS 22 and GraphPad 6. The independent samples t test was used to analyze differences in quantitative parameters between 2 groups. Welch’s correction was used if SD was not equal. Pearson correlation was obtained to analyze correlations between each quantitative parameter. Receiver operating characteristic curves (ROC) and the area under ROC curves (AUC) were used to evaluate prognostic power of UCA1 and BNP for CHF. Thresholds of these markers in ROC curves were demonstrated by maximums of Youden indexes (Youden index=specificity+sensitivity−1). Sensitivities and specificities to responding maximum Youden indexes were listed to indicate the best diagnostic power. In addition, Kaplan-Meier survival curves were used to evaluate survival rates of CHF and controls after 1-year observation. The median concentration of UCA1 and BNP was used to divide the CHF and controls into high- and low-concentration groups. P value less than 0.05 was considered to be a statistically significant difference.
Results
The baseline of clinical parameters in the whole participants
In this study, 67 CHF patients and 67 matched controls were enrolled to test relative UCA1 concentration in the plasma. Their basic information is listed in Table 1. We found that there was no significant difference in age, sex, body mass index (BMI), total cholesterol (TC), total triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), uric acid (UA), alcohol and cigarette consumption, or proportions of diabetes mellitus and hypertension between these 2 groups (P>0.05). However, BNP and LVEF were significantly different in the CHF group compared with those in controls (P<0.05).
Table 1.
The basic information about recruiters.
| Parameters | Control (N=67) | CHF (N=67) | p-value |
|---|---|---|---|
| Age (years) | 65.66±0.6173 | 68.13±1.228 | 0.0745 |
| Male/Female | 34/33 | 37/30 | 0.3650 |
| BMI (kg/m2) | 25.23±0.3278 | 24.94±0.3223 | 0.5209 |
| TC (mmol/L) | 4.980±0.1428 | 4.639±0.1646 | 0.1195 |
| LDL-C(mmol/L) | 2.985±0.1474 | 2.938±0.1569 | 0.8275 |
| HDL-C (mmol/L) | 1.215±0.03200 | 1.151±0.02996 | 0.1465 |
| UA (umol/L) | 345.7±10.52 | 357.6±14.17 | 0.5004 |
| Smoking | 21 (31.3%) | 26 (38.3%) | 0.2350 |
| Alcohol | 23 (34.3%) | 33 (49.3%) | 0.0570 |
| BNP (ng/L) | 64.18±15.87 | 1684±122.7 | 0.0001*** |
| LVEF (%) | 59.85±0.5821 | 30.52±0.7751 | 0.0001*** |
| Hypertension | 38 (56.7%) | 46 (68.7%) | 0.1050 |
| Diabetes | 37 (55.2%) | 43 (64.2%) | 0.1890 |
| Types (systolic/diastolic) | 0/0 | 67/0 | – |
Values were presented as mean ±SD. P<0.05 was considered to be statistically significant.
P<0.001. There was no significant difference between these two groups except levels of BNP and LVEF.
BMI – body mass index; BNP – brain natriuretic peptide; CHF – chronic heart failure; HDL-C – high density lipoprotein cholesterol; LDL-C – low density lipoprotein cholesterol; LVEF – left ventricle ejection fraction; TC – total cholesterol; UA – uric acid.
Relative expression of UCA1 was higher in CHF compared with controls
Plasma UCA1 in the CHF group was much higher than in controls (P<0.05) (Figure 1). Thus, we speculated that UCA1 plays important roles in CHF, or it could be synthesized and released into circulation when cardiomyocytes are damaged, killed, or stretched by the accumulated volume of blood during CHF.
Figure 1.
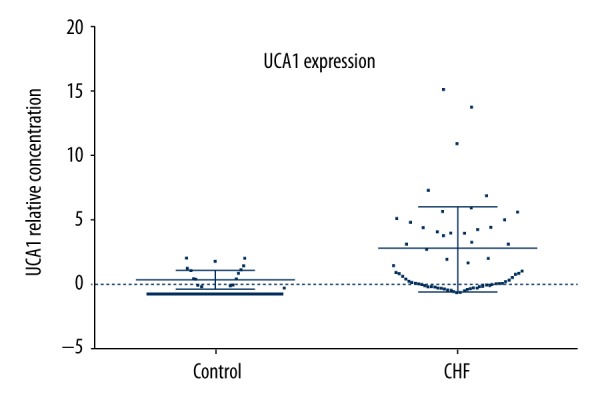
The plasma level of UCA1 in the CHF patients and controls. Plasma level of UCA1 was determined by qRT-PCR in 67 CHF patients and 67 controls. P<0.05 was considered to be a significant difference.
Correlations between UCA1, BNP, and LVEF
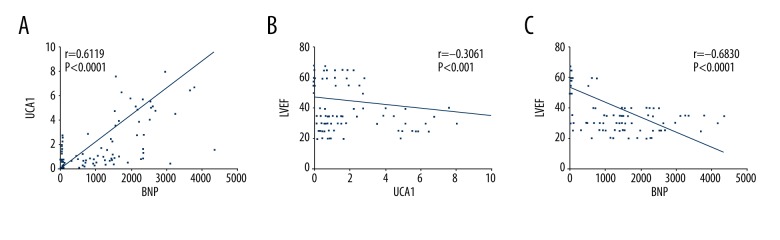
UCA1 and BNP were positively correlated, with a correlation coefficient r of 0.6119 (P<0.0001) (Figure 2A). Plasma UCA1 was negatively correlated with LVEF, with a correlation coefficient r of −0.3061 (P=0.0003) (Figure 2B). Moreover, BNP and LVEF were negatively correlated with each other, with a correlation coefficient r of −0.6830 (P<0.0001) (Figure 2C).
Figure 2.
Correlations between UCA1, LVEF, and BNP. (A) The correlation between UCA1 and BNP. The correlation coefficient r was 0.6119, P<0.0001. (B) The correlation between UCA1 and LVEF. The correlation coefficient r was −0.3061, P<0.001. (C) The correlation between BNP and LVEF. The correlation coefficient r was −0.6830, P<0.0001. P smaller than 0.05 was considered to be a significant difference.
ROC curves of UCA1 and BNP to diagnose CHF
ROC analysis illustrated that the AUC of UCA1 was 0.89, and the sensitivity and specificity of UCA1 to diagnose CHF were 100% [95% CI (0.9464–1)] and 76.12% [95%CI (0.6414–0.8569)] (P<0.05), respectively (Figure 3). The AUC of BNP was 0.98, and the sensitivity and specificity of BNP to diagnose CHF were 95.52% [95% CI (0.8747 to 0.9907)] and 95.52% [95%CI (0.8747 to 0.9907)] (P<0.05), respectively. However, the AUC of the combination of UCA1 and BNP was 0.98, showing that this combination caused no significant increase in the diagnostic power compared with BNP (P>0.05), suggesting that UCA1 might be a good indicator to diagnose CHF, but is not superior to BNP.
Figure 3.
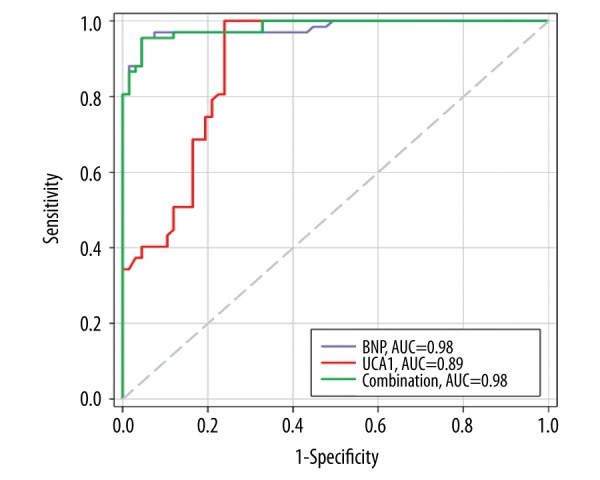
ROC curves of UCA1 and BNP to diagnose CHF. The diagnostic power (AUC) of UCA1 and BNP to diagnose CHF were 89% and 98%, respectively. However, the combination of these 2 markers seemed to have no significant increase in predictive power to diagnose CHF.
Survival rates of CHF patients and controls by determination of circulating UCA1
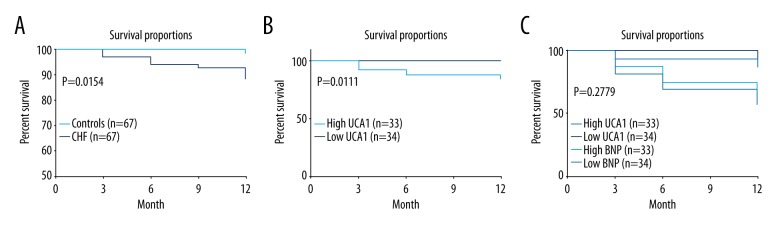
We discovered that survival rates of CHF patients and controls were 88.06% and 98.507%, respectively (P=0.0154) (Figure 4A). However, survival rate of high UCA1 in CHF patients was 84.615% compared with the low UCA1 group in CHF with a survival rate of 97.143% (P=0.011) (Figure 4B), suggesting that a high concentration of circulating UCA1 is associated with poor prognosis in CHF patients. In addition, although UCA1 had a similar survival tendency with BNP, there was no significant difference between these 2 markers in predicting prognosis in CHF patients (P=0.2779) (Figure 4C).
Figure 4.
Kaplan-Meier curves of UCA1 and BNP in prediction of survival rates of CHF patients. (A) Comparison of survival proportions between CHF and controls. Kaplan-Meier curves indicated that the survival rate of CHF patients was much lower than in controls (P=0.0154). (B) Comparison of survival proportions between high and low UCA1 in CHF patients. Kaplan-Meier curves demonstrated that the survival rate of CHF with higher circulating UCA1 was much lower than in CHF patients with lower UCA1 (P=0.0111). (C) Comparisons of survival proportions between high and low UCA1 and BNP. Kaplan-Meier curves demonstrated that the survival rates predicted by plasma UCA1 had similar survival tendencies predicted by BNP. There was no significant difference between these 2 markers in predicting prognosis of CHF patients (P=0.2779).
Discussion
Heart failure (HF) is one of the most severe diseases, leading to death all over the world. Currently, there are 20 million people suffering from HF globally. A 2015 study demonstrated that the 5-year survival rate of HF patients in China is approximately 34%, which is much lower than that of many cancers, such as breast cancer, bladder cancer, prostate cancer, colon and rectum cancer, and cervical cancer, with 5-year survival rates of 73.1%, 67.3%, 53.8%, 47.2%, and 45.4%, respectively [15]. UCA1 is a critical lncRNA, first discovered in bladder cancer. It plays important roles in multiple biological processes. Accumulating evidence suggests that UCA1 is elevated in many cancers. Overexpression of UCA1 leads to cell proliferation, and depletion of UCA1 suppresses the growth and aggressiveness of tumor cells [16–19]. It is reported that UCA1 is abundantly expressed in the heart, suggesting UCA1 might play roles in maintaining heart functions [6]. A study in 2015 indicated that UCA1 in the plasma can be a good biomarker for acute myocardial infarction [12]. However, UCA1 has never been investigated in HF patients before.
In this study, UCA1 was found to be significantly elevated in the plasma from CHF patients but not in controls, indicating that UCA1 must play important roles in CHF. UCA1 is notably decreased in cardiomyocytes from rats with ischemia reperfusion injury due to the inducement of hypoxia and hydrogen peroxide. Moreover, UCA1 inhibits the expression of p27 and plays an anti-apoptosis role in cardiomyocytes [20]. Myocardial apoptosis is considered to be the most essential process taking place in HF [21]. Thus, we presumed that UCA1 might exert its role of anti-apoptosis in CHF. Correlation analysis illustrated that UCA1 was positively correlated with BNP and negatively associated with LVEF, suggesting that a high level of UCA1 is closely associated with poor heart function. Our study found that the diagnostic power of UCA1 for CHF was strong. Although it was not superior to the traditional HF marker BNP, high plasma UCA1 highly suggests a poor prognosis for CHF patients, which was similarly observed in patients with cancers in previous studies [22,23]. However, there was no significant difference in prediction of prognosis of CHF between UCA1 and BNP, although they had similar survival tendencies. The results demonstrated that UCA1 might be used as an ideal biomarker to predict the outcome of CHF.
Our study has some limitations. Firstly, our sample size was small and our conclusions need further validation in larger populations. Secondly, although UCA1 had a high sensitivity and specificity to diagnose CHF, many cancers, like bladder and prostate cancer, could also elevate UCA1 expression. Thus, when UCA1 was used as a diagnostic marker for CHF, other related diseases must be excluded. Additionally, qRT-PCR is the quickest and most convenient method to detect the concentration of UCA1, but this technology is time-consuming; therefore, there is a great need for new time-saving technologies, such as microarrays and probes for lncRNA detection. Lastly, the underlying mechanisms of UCA1 in CHF were not fully explored in our study. Since the functions of UCA1 in HF are still largely unknown, we plan to further investigate the roles of UCA1 related with CHF in cardiomyocytes and HF mouse models.
Conclusions
Our study is the first to demonstrate that plasma UCA1 is higher in CHF patients, and it might be an ideal indicator for CHF. Higher UCA1 in the plasma can predict poor prognosis for CHF. Furthermore, UCA1 might be a potential therapeutic target for treatment of CHF.
Footnotes
Conflict of interest
None.
Source of support: This work was supported by the National High Technology Research and Development Program (863) (bj-2004-732), Beijing, P.R. China
References
- 1.Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics – 2008 update: A report from the american heart association statistics committee and stroke statistics subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 3.Stewart S, Jenkins A, Buchan S, et al. The current cost of heart failure to the national health service in the uk. Eur J Heart Fail. 2002;4:361–71. doi: 10.1016/s1388-9842(01)00198-2. [DOI] [PubMed] [Google Scholar]
- 4.Bertinchant JP. [Brain natriuretic peptide (bnp) and n-terminal-pro bnp in chronic haemodialysed renal failure]. Arch Mal Coeur Vaiss. 2004;97:881–88. [in French] [PubMed] [Google Scholar]
- 5.Henriksen JH, Gotze JP, Fuglsang S, et al. Increased circulating pro-brain natriuretic peptide (probnp) and brain natriuretic peptide (bnp) in patients with cirrhosis: Relation to cardiovascular dysfunction and severity of disease. Gut. 2003;52:1511–17. doi: 10.1136/gut.52.10.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F, Li X, Xie X, et al. Uca1, a non-protein-coding rna up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett. 2008;582:1919–27. doi: 10.1016/j.febslet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Wang XS, Zhang Z, Wang HC, et al. Rapid identification of uca1 as a very sensitive and specific unique marker for human bladder carcinoma. Clin Cancer Res. 2006;12:4851–58. doi: 10.1158/1078-0432.CCR-06-0134. [DOI] [PubMed] [Google Scholar]
- 8.Na XY, Liu ZY, Ren PP, et al. Long non-coding rna uca1 contributes to the progression of prostate cancer and regulates proliferation through klf4-krt6/13 signaling pathway. Int J Clin Exp Med. 2015;8:12609–16. [PMC free article] [PubMed] [Google Scholar]
- 9.Fang Q, Chen X, Zhi X. Long non-coding rna (lncrna) urothelial carcinoma associated 1 (uca1) increases multi-drug resistance of gastric cancer via downregulating mir-27b. Med Sci Monit. 2016;22:3506–13. doi: 10.12659/MSM.900688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nie W, Ge HJ, Yang XQ, et al. Lncrna-uca1 exerts oncogenic functions in non-small cell lung cancer by targeting mir-193a-3p. Cancer Lett. 2016;371:99–106. doi: 10.1016/j.canlet.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 11.Kamel MM, Matboli M, Sallam M, et al. Investigation of long noncoding rnas expression profile as potential serum biomarkers in patients with hepatocellular carcinoma. Transl Res. 2016;168:134–45. doi: 10.1016/j.trsl.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Yan Y, Zhang B, Liu N, et al. Circulating long noncoding rna uca1 as a novel biomarker of acute myocardial infarction. Biomed Res Int. 2016;2016:8079372. doi: 10.1155/2016/8079372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yancy CW, Jessup M, Bozkurt B, et al. 2013 accf/aha guideline for the management of heart failure: A report of the american college of cardiology foundation/american heart association task force on practice guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 14.Ponikowski P, Voors AA, Anker SD, et al. 2016 esc guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the european society of cardiology (esc). Developed with the special contribution of the heart failure association (hfa) of the esc. Eur J Heart Fail. 2016;18:891–975. doi: 10.1002/ejhf.592. [DOI] [PubMed] [Google Scholar]
- 15.Zeng H, Zheng R, Guo Y, et al. Cancer survival in china, 2003–2005: A population-based study. Int J Cancer. 2015;136:1921–30. doi: 10.1002/ijc.29227. [DOI] [PubMed] [Google Scholar]
- 16.Jiao C, Song Z, Chen J, et al. Lncrna-uca1 enhances cell proliferation through functioning as a cerna of sox4 in esophageal cancer. Oncol Rep. 2016;36:2960–66. doi: 10.3892/or.2016.5121. [DOI] [PubMed] [Google Scholar]
- 17.Fotouhi Ghiam A, Taeb S, Huang X, et al. Long non-coding rna urothelial carcinoma associated 1 (uca1) mediates radiation response in prostate cancer. Oncotarget. 2017;8(3):4668–89. doi: 10.18632/oncotarget.13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuo YL, Li XM, Luo J. Long noncoding rna uca1 modulates breast cancer cell growth and apoptosis through decreasing tumor suppressive mir-143. Eur Rev Med Pharmacol Sci. 2015;19:3403–11. [PubMed] [Google Scholar]
- 19.Lee JJ, Kim M, Kim HP. Epigenetic regulation of long noncoding rna uca1 by satb1 in breast cancer. BMB Rep. 2016;49:578–83. doi: 10.5483/BMBRep.2016.49.10.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Zhou D, Li G, et al. Long non coding rna-uca1 contributes to cardiomyocyte apoptosis by suppression of p27 expression. Cell Physiol Biochem. 2015;35:1986–98. doi: 10.1159/000374006. [DOI] [PubMed] [Google Scholar]
- 21.van Empel VP, Bertrand AT, Hofstra L, Crijns HJ, Doevendans PA, De Windt LJ. Myocyte apoptosis in heart failure. Cardiovasc Res. 2005;67:21–29. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 22.He A, Hu R, Chen Z, et al. Role of long noncoding rna uca1 as a common molecular marker for lymph node metastasis and prognosis in various cancers: A meta-analysis. Oncotarget. 2017;8(1):1937–43. doi: 10.18632/oncotarget.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Cao X, Zhang L, et al. Uca1 overexpression predicts clinical outcome of patients with ovarian cancer receiving adjuvant chemotherapy. Cancer Chemother Pharmacol. 2016;77:629–34. doi: 10.1007/s00280-016-2963-4. [DOI] [PubMed] [Google Scholar]