Abstract
Mandibular cortical bone measurement with x-ray imaging is known to be a potentially useful tool in the detection of dimensional changes caused by bisphosphonate. The primary purpose of this study was to assess the meaning and limitation of cortical bone measurement with computed tomography (CT) in patients with medication-related osteonecrosis of the jaw (MRONJ). The investigators obtained DentaScan images of the mandible from 15 patients with MRONJ, 15 patients with a history of antiresorptive agent administration without symptoms of MRONJ (non-MRONJ), and 15 control subjects. The cortical bone width measured on DentaScan images was compared between the three groups (ANOVA and Tukey’s test). Interobserver reliability between two observers was also assessed. The values of interclass correlation coefficient were 0.48 in the MRONJ group, 0.29 in the Non-MRONJ group, and 0.34 in control group. The cortical bone widths calculated both by observer 1 and observer 2 were thicker in patients with MRONJ than in the non-MRONJ group and controls. There were significant differences in cortical bone width among the MRONJ, non-MRONJ, and control groups in observer 1 (P < 0.001) and observer 2 (P < 0.001), specifically comparing the MRONJ group with the non-MRONJ group and the control group. Cortical bone width measurement is useful for the distinction between medication-related osteonecrosis of the jaw and normal bone, in spite of the low interobserver reliability.
Keywords: Bisphosphonates, Computed tomography, Denosumab, Mandible, Osteonecrosis
INTRODUCTION
Bisphosphonate-related osteonecrosis of the jaw was first reported in 2003, with the term recently changed to medication-related osteonecrosis of the jaw (MRONJ), because RANK ligand inhibitor (denosumab) used for the treatment of osteoporosis and cancer metastasis to bone is also known to cause osteonecrosis of jaw [5, 7, 12, 14, 15]. Bisphosphonates (BPs) inhibit osteogenic cells, osteoclasts, and human fibroblasts, and restrict vasculogenesis and angiogenesis, resulting in insufficient mucosal wound healing [10]. Furthermore, Bone turnover is suppressed by the administration of BPs and denosumab. Although there are numerous studies regarding the underlying mechanisms, the exact etiopathology of MRONJ has been still elusive.
MRONJ severely affects patients’ quality of life, causing pain, reduced oral hygiene, and eating difficulty. As a result, this condition has become an important therapeutic concern for oral and maxillofacial surgeons [17]. MRONJ risk factors have been reported previously and include that subjects treated with high-dose intravenous nitrogen-containing BPs or oral nitrogen-containing BPs for > 3 years have a higher risk of MRONJ than the general population [11]. Baseline osteomyelitis is also a strong risk factor for MRONJ [16].
The clinical assessment of MRONJ with radiological imaging contributes to informed consent and treatment planning. Although radiological imaging such as panoramic radiographs, computed tomography (CT), magnetic resonance imaging, and bone scintigraphy contribute to the clinical assessment of MRONJ patients, the relevance and efficacy of radiological imaging remain undetermined [1, 17]. To determine resection margin for surgical debridement, CT is adequate to detect osteolytic area in medullary cavity. Moreover, changes in cortices (e.g., cortical thickening) should be evaluated. Because cortical change which reflects the effect of medication or infection is one of important factors of the success of oral and maxillofacial surgery [13]. Mandibular cortical width measurement with x-ray imaging has been suggested as a screening tool for bone densitometry for osteoporosis investigation [19]. Some recent studies report that mandibular cortical bone measurement with cone-beam CT or panoramic radiographs is a potentially useful tool in the detection of bone dimensional changes caused by BPs [20, 21]. Another report established a simple method to detect MRONJ using CT, and revealed that there was a significant difference in cortical bone width between MRONJ patients and control subjects, however, did not show the value of interclass correlation coefficient, in spite of the evaluation by two observers [8].
The primary purpose of this study was to evaluate the meaning and limitation of cortical bone width measurement for diagnosis of MRONJ, by comparing the cortical bone width of MRONJ with control groups and assessing the interobserver reliability.
MATERIAL AND METHODS
Study design
This retrospective study was carried following approval from the Ethics Committee of Kobe University Hospital (No. 1708). All subjects gave written informed consent to use their CT images for the clinical study.
Patients
The inclusion criteria of this study were all of the patients who were referred to the Department of Oral and Maxillofacial Surgery at Kobe University Hospital from January 2007 to January 2015 for the treatment of MRONJ, and underwent DentaScan examinations before the treatment of MRONJ. DentaScan is a computer software program which provides CT imaging of the jaw in three planes of reference: axial, panoramic, and oblique sagittal, and its clinical usefulness has been reported [6, 22]. The patients who did not undergo DentaScan examinations were excluded in this retrospective study during the study period.
We included three study groups: MRONJ group, non-MRONJ group, and control group. The MRONJ group consisted of 15 patients who had a history of BP or denosumab administration and symptoms of MRONJ. The non-MRONJ group consisted of 15 patients who had a history of BP or denosumab administration and no symptoms of MRONJ, and who underwent DentaScan for diagnosis and treatment planning for other purposes (almost all of them were planning for dental implant insertion). The control group consisted of 15 patients who had no history of antiresorptive medications and no symptoms of MRONJ. They also had no systemic illness, no mandibular lesions, and had undergone DentaScan for other purposes (almost all of them were planning for dental implant insertion). The control group was similar to the study group in terms of age and sex distributions. Study subjects in the non-MRONJ and the control group were randomly selected before we evaluated the CT images. This study did not edentulous patient.
The following epidemiological data were retrospectively gathered from the medical charts: age, sex, primary disease, medication, the administration of duration and period of antiresorptive agents, MRONJ site and stage at the first visit.
MRONJ diagnostic criteria
The diagnosis of MRONJ at first visit was retrospectively made by reviewing medical charts according to the 2014 Guidelines of the American Association of Oral and Maxillofacial Surgeons as follows: current or previous treatment with antiresorptive or antiangiogenic agents, exposed bone or bone that could be probed through an intraoral or extraoral fistula(e) in the maxillofacial region that had persisted for more than 8 weeks, and no history of radiation therapy to the jaws or obvious metastatic disease to the jaws [15].
Computed tomography
CT examinations were performed in MRONJ patients at the time of diagnosis, using an Aquilion 64 (Toshiba Medical Systems, Tokyo, Japan) at the Hakubikai Imaging Support Center or Kobe University Hospital (tube voltage: 120kV; tube current: 100 mA; rotation time: 0.5 s/rotation; slice width: 0.5 mm; slice interval: 0.3mm; field of view: 25 cm; reconstruction kernel: FC30, scan pitch: 0.688). The scanned area extended from the floor of the orbit to the inferior border of the mandible. Multiplanar reconstructions using DentaScan software were done. CT images in the non-MRONJ and control group patients were obtained using the same settings as for the MRONJ patients.
Measurement and analysis methods
Hamada et al. previously established a simple evaluation method for cortical bone width calculation [8]. We modified their method as described in detail in Figure 1. All measurements were automatically done in enlarged view at the same magnification by using DICOM workstation (Yokogawa Medical Solutions Corporation, Tokyo, Japan), because the image size of DentaScan is very small. Cortical bone width was independently and blindly calculated by 2 observers [EI and MK].
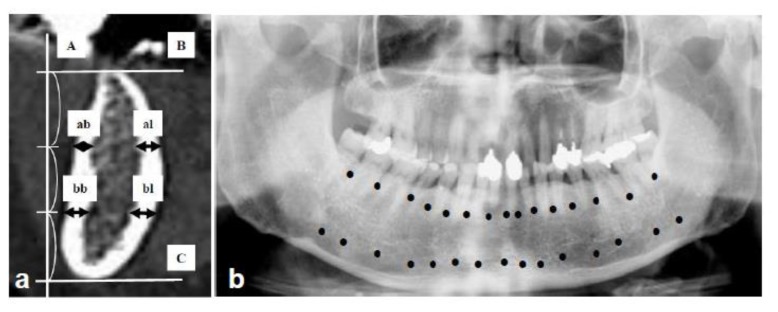
Figure 1.
Method to measure the cortical bone width in mandible using Dental CT, originally established by Hamada et al. [8]: (a) = Measurement sites in oblique sagittal images. A line was drawn vertical to the occlusal plane [A], and 2 lines were marked parallel to the occlusal plane which divided the space between the alveolar crest [B] and the lower edge of the mandible [C] into 3 equal parts. ↔: Cortical bone width was defined as [ab + al] on the alveolar level and [bb + bl] on the body level. ab: alveolar buccal, al: alveolar lingual, bb: body buccal, bl: body lingual. (b) = Measurement points in each patient. A total of 15 sites was between the teeth from the second molar of one side to the second molar of the opposite side. Measurements were made at 30 points [●] in the alveolar border and the mandibular body, resulting in 15 oblique sagittal images of DentaScan. [ab + al + bb + bl] ÷ 2 was calculated at 15 points. The average of 15 points in each case was defined as the value of cortical bone width and used for comparison.
Statistical analysis
Statistical analyses were performed using R software (R Development Core Team, 2011). Interclass corelation was used to evaluate the interobserver reliability. Each factor was expressed as mean and standard deviation (SD). ANOVA and a Turkey’s test to compare the 3 groups and unpaired t-test were performed. Data are presented as mean deviation. P < 0.05 was considered statistically significant.
RESULTS
Patients’ characteristics are summarized in Table I.
Table I.
Patients’ characteristics.
| MRONJ (N = 15) | Non-MRONJ (N = 15) | Control (N = 15) | P-value | |
|---|---|---|---|---|
| Average age (SD) | 73.7 ± 7.8 | 73.3 ± 10.4 | 72.8 ± 5.9 | NS |
| Gender (male/female) | 3/12 | 3/12 | 3/12 | |
| Primary disease | ||||
| Osteoporosis | 9 | 11 | ||
| Breast cancer | 3 | 1 | ||
| Lung cancer | 2 | 2 | ||
| Prostate cancer | 1 | - | ||
| Rheumatoid arthritis | - | 1 | ||
| Medication | ||||
| Bisphosphonate Oral | ||||
| Alendronate | 5 | 8 | ||
| Risedronate | 4 | 3 | ||
| Minodronate | - | 1 | ||
| Intravenous | ||||
| Zoledronate | 4 | 3 | ||
| Denosumab | 1 | - | ||
| Denosumab and zoledronate | 1 | - | ||
| Administration duration (months) | ||||
| Average (SD) | ||||
| Bisphosphonate Oral | 20.4 ± 14.2 | 35.7 ± 34.4 | NS | |
| Intravenous | 18 ± 11.3 | 31.0 ± 1.6 | NS | |
| Denosumab | 3 | - | ||
| Denosumab and zoledronate | 15 | - | ||
| Administration period (No. of patients) | ||||
| < 3 years | 11 | 12 | ||
| ≥ 3 years | 4 | 3 | ||
| MRONJ site (No. of patients) | ||||
| Anterior mandible | 5 | |||
| Posterior mandible | 10 | |||
| MRONJ stage (No. of patients) | ||||
| 0 | 2 | |||
| 1 | 3 | |||
| 2 | 6 | |||
| 3 | 4 | |||
MRONJ = medication-related osteonecrosis of the jaw. ANOVA to compare 3 groups and unpaired t-test to compare 2 groups were performed. Data are presented as mean and standard deviation. P < 0.05 was considered statistically significant.
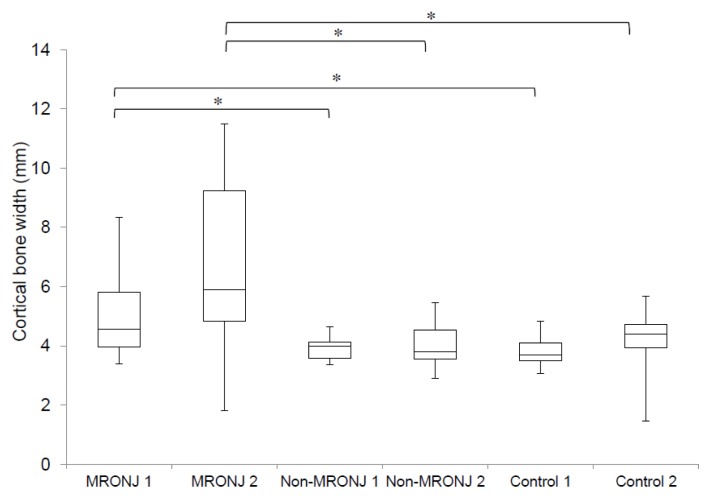
There was no significant differences between the values calculated by observer 1 and 2 (95 % CI −0.0099–0.79 in MRPNJ group, 95 % CI −0.024–0.69 in non-MRONJ group, and 95 % CI −0.11–0.7 in control group, respectively). The average cortical bone widths calculated by observer 1 were 4.98 (1.37) mm in patients with MRONJ, 3.91 (0.37) mm in the non-MRONJ group, and 3.81 (0.5) mm in controls. The average cortical bone widths calculated by observer 2 were 6.54 (2.92) mm in patients with MRONJ, 4.06 (0.74) mm in the non-MRONJ group, and 4.3 (1.03) mm in controls. There were significant differences in cortical bone width were found among the MRONJ, non-MRONJ, and control groups in observer 1 (P < 0.001) and observer 2 (P < 0.001), specifically comparing the MRONJ group with the non-MRONJ group (P = 0.004), and between the MRONJ group and the control group (P = 0.002) in observer 1, and comparing the MRONJ group with the non-MRONJ group (P = 0.002), and between the MRONJ group and the control group (P = 0.005) in observer 2. No significant difference was found between the non-MRONJ group and the control group both in observer 1 (P = 0.949) and 2 (P = 0.932) (Figure 2). The values of interclass correlation coefficient were 0.48 in the MRONJ group, 0.29 in the Non-MRONJ group, and 0.34 in control group.
Figure 2.
Comparison of cortical bone width among the 3 groups. Significant differences were found among the 3 groups. For individual group comparisons, significant differences were found between MRONJ and non-MRONJ, and between MRONJ and control. These results were consistent among the 2 observers. MRONJ 1, Non-MRONJ 1, and Control 1 were measured by observer 1. MRONJ 2, Non-MRONJ 2, and Control 2 were measured by observer 2. *Statistically significant at the level P < 0.05, Turkey’s test.
DISCUSSION
There are numerous studies about the imaging in diagnosis of MRONJ, however, in spite of its importance, there are few studies showing the actual value of the interclass correlation coefficient, as far as we know. Therefore, we assessed the interobserver reliability of cortical bone width measurement. This study indicates that cortical bone width measurement is useful to distinguish MRONJ from normal bone, because there was a significant difference in cortical bone width between MRONJ and non-MRONJ, and between MRONJ and control, and these results were consistent among the 2 observers. In contrast, we found the low interobserver reliability between 2 observers. Therefore, the determination of cutoff value of cortical bone width measured with DentaScan is difficult.
A previous study reported that cortical bone width did not reflect the progression of MRONJ staging [8]. It is known that osteosclerosis occurs in the early stages of MRONJ. Hutchison et al. reported that radiologic features of regional or diffuse osteosclerosis with extension beyond the involved site were observed even in stage 0 MRONJ [9]. In this study, the significant difference between Stage 0 – 1 and Stage 2 – 3 was found only in observer 1, but not observer 2 (data not shown). It is considered that the diagnosis of Stage 0 – 1 MRONJ by measuring cortical bone width is difficult. Moreover, we note that the significant differences between non-MRONJ and control were not found in this study. This result indicates that the increase of cortical bone width in this study was not due to pharmacological effect of antiresorptive agents.
Although the radiological features of MRONJ were so diverse, osteosclerosis is one of notable findings of MRONJ [4]. Bedogni et al. proposed a staging system for MRONJ, in which non-specific CT findings associated with MRONJ were divided into early and late signs [3]. Early signs included cortical disruption and focal bone marrow sclerosis, and late ones diffuse osteosclerosis and osteosclerosis of adjacent bones (zygoma and hard palate) [3]. They proposed the following clinical and CT MRONJ staging system: Stage 1 is focal MRONJ, with increased bone density limited to the affected region; Stage 2 is diffuse MRONJ, with diffuse osteosclerosis; and Stage 3 is complicated MRONJ, with osteosclerosis of adjacent bones [3]. The loss of contrast definition between the endosteal cortex and the subjacent medullary bone (i.e., poor corticomedullary bone marrow differentiation) is also a notable CT finding with MRONJ [9, 16]. Osteolytic change is another notable CT finding in bone diseases such as osteomyelitis [18]. For example, in osteomyelitis caused by bacterial infection (i.e., non-diffuse sclerosing osteomyelitis), bone resorption occurs as a result of the inflammatory reaction, and bone formation (sclerotic changes) occurs around the site of bone resorption as a reactive change [18]. In contrast, osteolytic areas are scattered randomly in the sclerotic area in diffuse sclerosing osteomyelitis [18]. In MRONJ, bone alterations vary greatly in exposed and unexposed areas. A previous histological study revealed that the exposed areas show the features of an osteonecrotic process, whereas the unexposed areas resemble an osteomyelitic process [2]. Cortical bone width probably reflects complex alterations including not only osteosclerosis but also osteolysis. Therefore, it is difficult to achieve high interobserver reliability.
CONCLUSIONS
Cortical bone width measurement is useful for the distinction between medication-related osteonecrosis of the jaw and normal bone, in spite of the low interobserver reliability.
ACKNOWLEDGMENTS AND DISCLOSURE STATEMENTS
The authors report no conflicts of interest related to this study.
REFERENCES
- 1.Arce K, Assael LA, Weissman JL, Markiewicz MR. Imaging findings in bisphosphonate-related osteonecrosis of jaws. J Oral Maxillofac Surg. 2009;67(5 Suppl):75–84. doi: 10.1016/j.joms.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 2.Bedogni A, Blandamura S, Lokmic Z, Palumbo C, Ragazzo M, Ferrari F, Tregnaghi A, Pietrogrande F, Procopio O, Saia G, Ferretti M, Bedogni G, Chiarini L, Ferronato G, Ninfo V, Lo Russo L, Lo Muzio L, Nocini PF. Bisphosphonate-associated jawbone osteonecrosis: a correlation between imaging techniques and histopathology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:358–364. doi: 10.1016/j.tripleo.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 3.Bedogni A, Fusco V, Agrillo A, Campisi G. Learning from experience. Proposal of a refined definition and staging system for bisphosphonate-related osteonecrosis of the jaw (BRONJ) Oral Dis. 2012;18:621–623. doi: 10.1111/j.1601-0825.2012.01903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bianchi SD, Scoletta M, Cassione FB, Migliaretti G, Mozzati M. Computerized tomographic findings in bisphosphonate-associated osteonecrosis of the jaw in patients with cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:249–258. doi: 10.1016/j.tripleo.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 5.Brown JP, Roux C, Ho PR, Bolognese MA, Hall J, Bone HG, Bonnick S, van den Bergh JP, Ferreira I, Dakin P, Wagman RB, Recknor C. Denosumab significantly increases bone mineral density and reduces bone turnover compared with monthly oral ibandronate and risedronate in postmenopausal women who remained at higher risk for fracture despite previous suboptimal treatment with an oral bisphosphonate. Osteoporos Int. 2014;25:1953–1961. doi: 10.1007/s00198-014-2692-7. [DOI] [PubMed] [Google Scholar]
- 6.Chandak S, Shetty CM. Comparative study of dentascan and radiography for radiological evaluation of impacted teeth. J Clin Diagn Res. 2014;8:RC01–5. doi: 10.7860/JCDR/2014/7997.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dore RK, Cohen SB, Lane NE, Palmer W, Shergy W, Zhou L, Wang H, Tsuji W, Newmark R Denosumab RA Study Group. Effects of denosumab on bone mineral density and bone turnover in patients with rheumatoid arthritis receiving concurrent glucocorticoids or bisphosphonates. Ann Rheum Dis. 2010;69:872–875. doi: 10.1136/ard.2009.112920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamada H, Matsuo A, Koizumi T, Satomi T, Chikazu D. A simple evaluation method for early detection of bisphosphonate-related osteonecrosis of the mandible using computed tomography. J Craniomaxillofac Surg. 2014;42:924–929. doi: 10.1016/j.jcms.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Hutchinson M, O’Ryan F, Chavez V, Lathon PV, Sanchez G, Hatcher DC, Indresano AT, Lo JC. Radiographic findings in bisphosphonate-treated patients with stage 0 disease in the absence of bone exposure. J Oral Maxillofac Surg. 2010;68:2232–2240. doi: 10.1016/j.joms.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Imai Y, Hasegawa T, Takeda D, Kusumoto J, Akashi M, Ri S, Furudoi S, Komori T. Evaluation and comparison of CT values in bisphosphonate-related osteonecrosis of the jaw. J Oral Maxillofac Surg Med Pathol. 2016;28:19–25. [Google Scholar]
- 11.Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E American Society for Bone and Mineral Research. American Society for Bone and Mineral Research: Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2007;22:1479–1491. doi: 10.1359/jbmr.0707onj. [DOI] [PubMed] [Google Scholar]
- 12.Marx RE. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1157. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 13.Miyamoto I, Ishikawa A, Morimoto Y, Takahashi T. Potential risk of asymptomatic osteomyelitis around mandibular third molar tooth for aged people: a computed tomography and histopathologic study. PLoS One. 2013;8:e73897. doi: 10.1371/journal.pone.0073897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ristow O, Gerngroß C, Schwaiger M, Hohlweg-Majert B, Kehl V, Jansen H, Hahnefeld L, Koerdt S, Otto S, Pautke C. Effect of antiresorptive drugs on bony turnover in the jaw: denosumab compared with bisphosphonates. Br J Oral Maxillofac Surg. 2014;52:308–313. doi: 10.1016/j.bjoms.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 15.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial: American Association of Oral and Maxillofacial Surgeons position Paper on Medication-Related Osteonecrosis of the Jaws- 2014 Update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 16.Saia G, Blandamura S, Bettini G, Tronchet A, Totola A, Bedogni G, Ferronato G, Nocini PF, Bedogni A. Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction. J Oral Maxillofac Surg. 2010;68:797–804. doi: 10.1016/j.joms.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Stockmann P, Hinkmann FM, Lell MM, Fenner M, Vairaktaris E, Neukam FW, Nkenke E. Panoramic radiograph, computed tomography or magnetic resonance imaging. Which imaging technique should be preferred in bisphosphonate-associated osteonecrosis of the jaw? A prospective clinical study. Clin Oral Investig. 2010;14:311–317. doi: 10.1007/s00784-009-0293-1. [DOI] [PubMed] [Google Scholar]
- 18.Suei Y, Taguchi A, Tanimoto K. Radiographic evaluation of possible etiology of diffuse sclerosing osteomyelitis of the mandible. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:571–757. doi: 10.1016/s1079-2104(97)90275-4. [DOI] [PubMed] [Google Scholar]
- 19.Taguchi A, Suei Y, Sanada M, Ohtsuka M, Nakamoto T, Sumida H, Ohama K, Tanimoto K. Validation of dental panoramic radiography measures for identifying postmenopausal women with spinal osteoporosis. AJR Am J Roentgenol. 2004;183:1755–1760. doi: 10.2214/ajr.183.6.01831755. [DOI] [PubMed] [Google Scholar]
- 20.Torres SR, Chen CS, Leroux BG, Lee PP, Hollender LG, Lloid M, Drew SP, Schubert MM. Mandibular inferior cortical bone thickness on panoramic radiographs in patients using bisphosphonates. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:584–592. doi: 10.1016/j.oooo.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torres SR, Chen CS, Leroux BG, Lee PP, Hollender LG, Santos EC, Drew SP, Hung KC, Schubert MM. Mandibular cortical bone evaluation on cone beam computed tomography images of patients with bisphosphonate-related osteonecrosis of the jaw. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:695–703. doi: 10.1016/j.oooo.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Yanagisawa K, Friedman CD, Vining EM, Abrahams JJ. DentaScan imaging of the mandible and maxilla. Head Neck. 1993;15:1–7. doi: 10.1002/hed.2880150102. [DOI] [PubMed] [Google Scholar]