Abstract
We conducted an epidemiological study of intestinal parasitic infection in 572 schoolchildren aged 4 to 12 years old from six elementary schools in Sakon Nakhon Province, Thailand from June 2013 to August 2014. We collected fecal, blood, and urine samples to investigate parasitic infection and conducted a questionnaire survey. Soil samples were examined for egg contamination. Fecal examination, using the formalin-ether sedimentation method, revealed that 39% of schoolchildren were infected with eight genera and eight species of parasites; three nematodes, two trematodes, one cestode, and two protozoa. Prevalence rates across the six schools (schools A through F) were: A (13%), B (15%), C (53%), D (11%), E (20%), and F (43%). Schools C and F showed significantly higher prevalence rates than the other schools (p<0.05). In school C, Necator americanus was detected in 49% of schoolchildren tested, while in school F a high prevalence of Opisthorchis viverrini and Heterophyes heterophyes, at a rate of 23% and 21%, respectively, was detected. The questionnaire survey revealed that health, hygiene practices and awareness were poor in school C. However, school F showed high levels of cognizance and practices relating to the prevention of infection. The schoolchildren ate a staple diet of undercooked river fish and the results revealed a high rate of fish-borne parasites. Soil samples showed Toxocara sp. contamination in and around the campus. Toxocara antibodies were detected in over 6% of schoolchildren. The use of urine samples, as opposed to serum samples, was found to be effective for antibody testing.
Keywords: Fecal examination, soil contamination, urine ELISA, parasitic infection, zoonosis, Toxocara, Thailand
INTRODUCTION
Infection with intestinal parasites is most common in developing countries in tropical and sub-tropical areas, particularly in Sub-Saharan Africa, Asia, Latin America, and the Caribbean, where high prevalence rates have been recorded (de Silva et al, 2003). Currently, approximately 3.5 billion people worldwide suffer from this type of infection, with one report suggesting that 450 million children are infected with intestinal parasites but are asymptomatic. These extremely high rates of infection result in considerable social and economic losses (WHO, 2014). One of the factors common among all of the endemic regions is the warm and humid climate that promotes the development of infection (Tesana et al, 1987). In addition to this geographical factor, poverty, poor environmental conditions and other social factors such as a lack of public health measures, need to be considered (Raso et al, 2005; Ziegelbauer et al, 2012).
The rates of infection with intestinal parasites are high in Southeast Asian countries, where they present a major public health problem. The prevalence rates of Ascaris lumbricoides and Trichuris trichiura in Laos, Myanmar, and Vietnam are 36% and 21%, 29% and 18%, and 54% and 22%, respectively (UNICEF, 2003). In 1957 in Thailand, the Ministry of Health introduced a control program for parasitic infection and the prevalence rates dropped from 63% in 1957 to 23% in 2001, with a further decrease to 18% in 2009 indicating the effectiveness of this program (Anantaphruti et al, 2004; Wongsaroj et al, 2014). Although a number of studies have been carried out, the current rates of parasitic infection in Thailand are unclear as results vary among studies (Anantaphruti et al, 2002; Boonjarasinyo et al, 2013; Saksirisampant et al, 2006; Waikagul et al, 2002).
One reason for these differences seems to be the use of only one or two parameters, i.e. feces examination and/or a questionnaire survey. Few epidemiological studies have analyzed multiple parameters simultaneously.
In this study, we examined parasitic infection among schoolchildren in Sakon Nakhon Province, Thailand using the following parameters. Fecal and soil samples were used for egg detection, urine and blood samples were used for antibody examinations, and a questionnaire survey was used for understanding the lifestyle of local inhabitants. Using this five-parameter approach, we attempted to accurately determine and clarify the current situation regarding intestinal parasitic and zoonotic infections in this region of Thailand.
MATERIALS AND METHODS
Survey area and study period
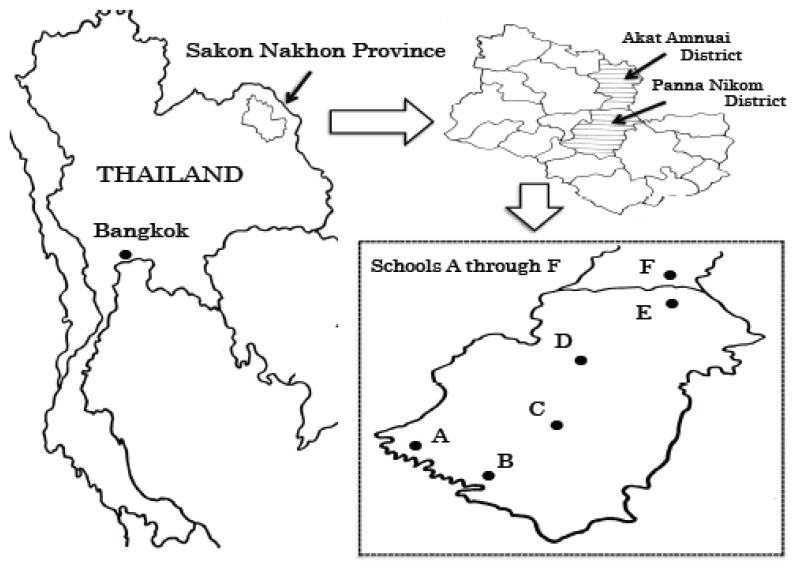
The study was conducted over a 15-month period from June 2013 to August 2014. A total of 572 elementary schoolchildren aged 4 to 12 years were selected from six elementary schools in Panna Nikhom and Akat Amnuai District, Sakon Nakhon Province, located in the northeast of the country approximately 500 km from the Thai capital Bangkok (hereafter referred to as schools A through F) (Fig. 1).
Fig. 1.
Map of Thailand showing Bangkok and Sakon Nakhon Province. Six elementary schools (A through F) in Panna Nikom and Akat Amnuai District were examined in this survey.
The residents in this region comprised four main ethnic groups. In general, children of “So” heritage attended school A and B, children of “Bru” heritage attended school C, children of “Phu Thai” heritage attended schools D and E, and children of “Thai Nyaw” heritage attended school F. The average annual temperature in the region is 31.7°C with an annual rainfall of 1,645 mm and an annual average relative humidity of 73% (Thai Meteorological Department, 2004; http://www.tmd.go.th/en/). The residents in this region mainly make a living from agriculture, livestock, and revenue generated from the sale of fish caught in the local rivers.
Fecal examination
Fecal samples of approximately 5 to 10 g were collected from a total of 417 schoolchildren. Fecal examination was conducted using the formalin-ether sedimentation method. The agar plate method was used to examine Strongyloides stercoralis and the filter paper culture method was used to identify the species of hookworm.
Soil collection and examination
To investigate egg contamination of the soil, a total of 117 samples (41 samples taken from around the school zone, 37 from around the places of residence, and 39 from inside the school) were collected. The collected soil samples were dried for 1 day at room temperature, after which, they were filtered using a 150-μm metal mesh and 2 g of sand particles were retained. Filtered soil samples were stored at 4°C and tested within 2 weeks using a centrifugal flotation method with a sucrose solution at a specific gravity of 1.20 (Uga et al, 1993). The soil samples were collected in the dry season (Mar. and Apr. 2013) and the rainy season (Sep. 2013 and Aug. 2014).
Urine and blood examination
Urine (361) and blood (286) samples were collected and subjected to an enzyme linked immunosorbent assay (ELISA) to determine Toxocara antibody titers. Approximately 5 ml of the collected urine was stored at room temperature in a solution containing 0.1% of sodium azide. The blood samples (maximum of 0.5 ml) were collected from the fingertips of schoolchildren using a lancet. The collected blood samples were absorbed into filter paper (Toyo Roshi, ADVANTEC, Tokyo, Japan), dried, and stored at room temperature. The blood-soaked filter paper was then cut into 5 mm squares and the serum was extracted in PBS-tween, left overnight, and the obtained extracts of serum was used. A 96-well microplate was sensitized at 25°C overnight with 500-fold diluted (5 μg/ml) excretory-secretory antigen from the 3rd-stage larvae of Toxocara canis. The second reaction was conducted at 37°C for 1 h using 4,000-fold diluted peroxidase-conjugated goat anti-human IgG (Tago, Camarillo, CA, USA). After triplicate washes, the plate was incubated with ABTS (KPL, Inc., Gaithersburg, MD, USA) for 1 h at 37°C. The absorbance (415 nm) was measured using a microplate ELISA reader and was expressed as units (Itoh et al, 2001).
Questionnaire survey and data analysis
We conducted a lifestyle survey of 352 schoolchildren (62% of the total participants). The questionnaire contained 12 items relating to daily habits and activities, such as hand washing and bowel movements. Statistical analysis of the data to compare two groups was performed using the χ2 test. When only a small dataset was available, the Fisher test was used. The significance level was set at p<0.05%.
Ethical considerations
This study was carried out with the approval of Kobe University Graduate School of Medicine, Japan (approval number 256) and the Ethics Committee of the Faculty of Science and Technology at Thammasat University in Thailand. Fully informed consent was also obtained from the principals of the participating schools. Permission was granted by the Ministry of Agriculture, Forestry and Fisheries, Kobe Plant Protection Station (25 shin-shoku No. 373), to bring the soil samples into Japan for analysis.
RESULTS
Fecal examination
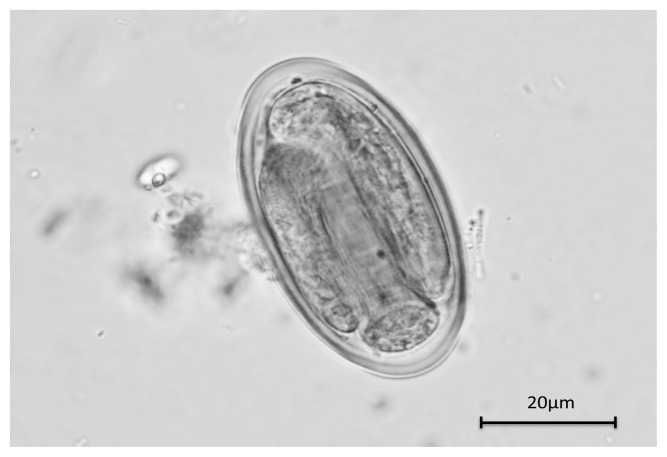
Of the 417 fecal samples examined, 163 (39%) were positive for at least one of the intestinal parasites. In total, eight genera and eight species (three nematodes, two trematodes, one cestode, and two protozoa) of parasites were detected (Table I). The most prevalent species were found to be Opisthorchis viverrini and Entamoeba sp. (each 10%), Necator americanus and Heterophyes heterophyes (each 9%). Of the 37 hookworm egg-positive samples, eight were cultured and identified and all were found to be N. americanus. It is worth noting that Gongylonema sp. eggs were detected in one sample (Fig. 2). The eggs displayed a clear, thick shell, of approximately 75×40 μm in size, with a “lid-like” shape at both ends. During observation, the eggs were found to be embryonated. An additional 98 specimens were examined for S. stercoralis using the agar plate method and all were found to be negative. During this study, A. lumbricoides eggs were not detected.
Table I.
Prevalence of intestinal parasites in schoolchildren of six elementary schools
| Species | No. of positive | Positive rate (%) |
|---|---|---|
| Necator americanus* | 37 | 9 |
| Trichuris trichiura | 1 | 0 |
| Gongylonema sp. | 1 | 0 |
| Opistorchis viverrini | 42 | 10 |
| Heterophyes heterophyes | 37 | 9 |
| Taenia sp. | 3 | 1 |
| Entamoeba sp. | 41 | 10 |
| Giardia intestinalis | 1 | 0 |
|
| ||
| Total | 163 | 39 |
The 417 fecal samples were examined.
Result of eight samples.
Fig. 2.
Egg of Gongylonema sp.
School prevalence rates and the questionnaire survey
The prevalence rates of infection with intestinal parasites specific to each of the six schools are shown in Table II.
Table II.
Prevalence rates of infection with intestinal parasites and species of recovered parasites in schools A through F
| School | No. of samples | No. of positive (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| examined | positive | % | N.A | T.T | G.sp | O.V | H.H | T.sp | E.sp | G.I | |
| A | 70 | 9 | 13 | 3 (4) | 1 (1) | 5 (7) | |||||
| B | 79 | 12 | 15 | 6 (8) | 1 (1) | 5 (6) | |||||
| C | 55 | 29 | 53* | 27 (49) | 3 (5) | 2 (4) | 7 (13) | ||||
| D | 28 | 3 | 11 | 2 (7) | 1 (4) | ||||||
| E | 20 | 4 | 20 | 1 (5) | 1 (5) | 1 (5) | 1 (5) | ||||
| F | 165 | 71 | 43** | 1 (0) | 38 (23) | 34 (21) | 22 (13) | 1 (0) | |||
N.A: Necator americanus, T.T: Trichuris trichiura, G.sp: Gongylonema sp., O.V: Opisthorchis viverrini, H.H: Heterophyes heterophyes, T.sp: Taenia sp., E.sp: Entamoeba sp., G.I: Giardia intestinalis
p<0.05 by χ2 test (C vs A, B, D, E, F)
p<0.05 by χ2 test (F vs A, B, C, D,E)
They ranged from 11 to 53%. Schools C (53%) and F (43%) had significantly higher prevalence rates than the other schools (p<0.05). Entamoeba sp. was detected in all six schools, however, O. viverrini and H. heterophyes were mainly found in schools C and F, while T. trichiura was detected only in school B. Necator americanus had a prevalence rate of 49% in school C. School F showed a high prevalence of the fish-borne parasites O. viverrini (23%) and H. heterophyes (21%). We analyzed the data from the questionnaire survey to better understand possible factors that may cause these high rates in schools C and F (Table III).
Table III.
Results of questionnaire in schools C and F, and correlation between school C/F and other schools
| Factor | School C | School F | ||
|---|---|---|---|---|
|
|
|
|||
| % of schoolchildren answered “yes” | % of schoolchildren answered “No” | % of schoolchildren answered “yes” | % of schoolchildren answered “No” | |
|
|
|
|
|
|
| Having a toilet | 83 | 84 | ||
| Washing hands before eating | 38 (a)* | 87 (a)** | ||
| Eating with hands | 66 (a)* | 64 (a)** | ||
| Drinking without tap water | 83 (b)* | 69 | ||
| Washing hands after defecating | 50 | 81(a)** | ||
| Ever pass stools outside | 97 (a)* | 71 | ||
| Wearing shoes outside | 7 (b)* | 97 | ||
| Keeping animals in the house | 97 | 95 | ||
p<0.05 Correlation between school C and other schools by χ2 test (a) or Fisher test (b).
p<0.05 Correlation between school F and other schools by χ2 test (a).
In answer to questions such as “How often do you eat by hand?”, “How often do you drink water from sources other than tap water?”, and “How often do you have bowel movements in an area not designated as a toilet area?”, the frequency of positive answers from school C was found to be significantly higher (p<0.05) than in other schools (66%, 83%, and 97%, respectively). These figures strongly suggest a low level of understanding and awareness regarding the prevention of parasitic infection. The data obtained from school F on questions such as “How often do you wash your hands before eating?”, “How often do you wash your hands after defecating?”, and “How often do you not eat using your hands?” showed a significantly higher (p<0.05) cognizant with regard to infection prevention and hygiene (81%, 87%, and 64%, respectively). The education of the Thai Nyaw, which includes home economics classes with the handling and cooking of river fish, and the lifestyle which is centered around river fishing, all increase the possibility for direct contact with infection sources potentially making them more susceptible to infection, which may partially explain the disparity in the results.
Egg contamination of soil
Fourteen (12%) of the 117 soil samples were positive for Toxocara sp. eggs, a known causative agent of larva migrans. Of these 14, six were from around the school zone (6/41; 15%), five out of 37 were from around places of residence (5/37; 14%), and three were from inside the school (3/39; 8%) (Table IV). The contamination rate with Toxocara sp. eggs was higher in the rainy season (15%) than in the dry season (8%), but this difference was not statistically significant (p>0.05). Throughout the soil survey, no eggs other than Toxocara sp. eggs were detected.
Table IV.
Recovery of Toxocara sp. eggs from soil samples in different sites and seasonal changes of contamination rate with Toxicara sp. eggs
| Site | Total samples | No. of Toxocara sp. eggs | % | No. of Toxocara sp. eggs in season | % | |
|---|---|---|---|---|---|---|
| Outside the school | Surrounding the school zone | 41 | 6 | 15 | 1/14 (DS*) | 7 |
| 5/27 (RS**) | 19 | |||||
|
| ||||||
| Surrounding the residence | 37 | 5 | 14 | 1/10 (DS) | 10 | |
| 4/27 (RS) | 15 | |||||
|
| ||||||
| Inside the school | 39 | 3 | 8 | 2/26 (DS) | 8 | |
| 1/13 (RS) | 8 | |||||
|
| ||||||
| Total | 117 | 14 | 12 | 4/50 (DS) | 8 | |
| 10/67 (RS) | 15 | |||||
DS: dry season (Mar. and Apr. 2013)
RS: rainy season (Sep. 2013 and Aug. 2014)
Antibody distribution
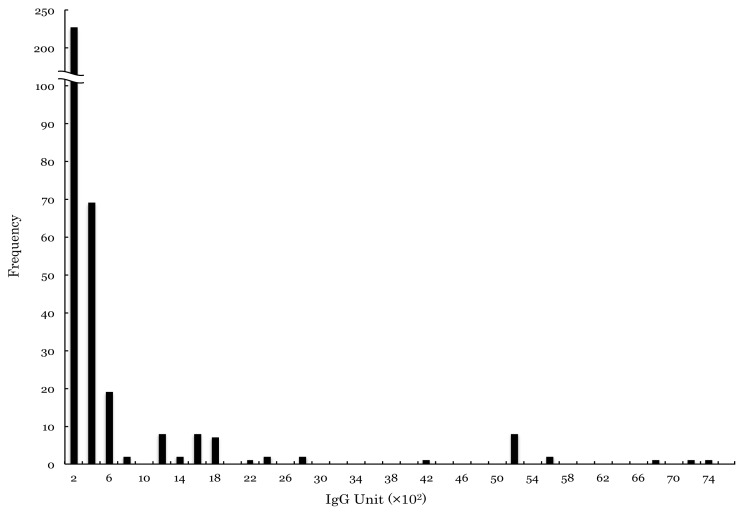
To investigate the distribution of antibodies against Toxocara sp., urine samples from 361 schoolchildren were examined (Fig. 3). Despite the relatively small sample size, two distinct groups were detected. Assuming a maximum titer of 3,000 U for the negative group, with a cut-off titer around 3,200 to 3,400 U, 6% (23/361) of samples were considered to be positive (peak at 5,200 U), and 94% (338/361) of samples were considered to be negative (peak at 200 U) (Fig. 3).
Fig. 3.
Frequency distribution of ELISA values in urine samples.
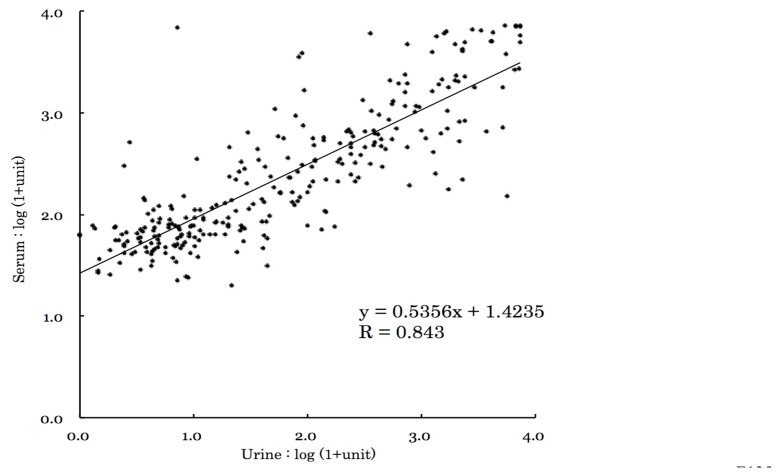
Finally, we measured and compared the ELISA values of the serum and the urine antibody titer using the Pearson Correlation Coefficient test and the ELISA values of both were well correlated (R = 0.843, P <0.05) (Fig. 4).
Fig. 4.
Correlation of ELISA values between serum and urine samples.
DISCUSSION
As a result of fecal examination of 572 schoolchildren from six elementary schools in northeast Thailand, 39% of schoolchildren tested positive for intestinal parasites. Of the parasites detected, eight genera and eight species were identified. The prevalence rates varied between schools, with schools C and F showing significantly higher prevalence rates. Necator americanus was the dominant species in school C with a significantly higher rate of prevalence compared with the other schools. It has been suggested that one of the main factors affecting these results may be the lifestyle of the “Bru” population living in this region. The poverty in which they live may partially explain the endemic of N. americanus among this population. Hookworm infection causes physical growth disorder, mental retardation, and iron deficiency anemia, etc. in schoolchildren, and there is a report that the future potential income for these people has been reduced by 43% due to hookworm infection (Bleakey, 2007). This continued poverty compounds the potential for further hookworm infection leading to a vicious cycle among the people in this region. By contrast, School F comprised mainly schoolchildren from “Thai Nyaw” heritage who reside close to the river and eat a staple diet of uncooked river fish. Both in home economics classes and in the family home, river fish is often cooked and eaten. Any increase in potential contact with fresh water fish needs to be carefully considered. The eating habits of the parents are reported to increase the risk of O. viverrini infection in children (Tomokawa et al., 2008). In addition, it is estimated that the number of people infected in Thailand with O. viverrini, which is known to induce liver cirrhosis and cancer of the bile duct system (Haswell-Elikins et al., 1994), is about seven million (Sithithaworn et al., 2003), suggested that public health measures for not only schoolchildren but also their families and communities are necessary for prevention of this illness.
In this study, we detected one Gongylonema sp. egg among our samples. Gongylonema sp. is distributed worldwide, however, human infection is rare. There have, to date, been fewer than 60 cases reported worldwide (Pesson et al., 2013). The intermediate hosts of this parasite are the common beetle, dung beetles, and cockroach. In our study, we observed local people cooking and eating dung beetles collected from around the home. Additionally in an interview with one of the schoolchildren we detected Gongylonema sp. egg from feces, we learned that there was a history of refractory stomatitis with mobility. Pasuralertsakul et al. (2008) reported nine Thai patients who had been found to be infected with Gongylonema sp. due to the detection of eggs in their feces. However, they concluded that these were not examples of actual human infection with Gongylonema sp. They suspected that the eggs detected in the feces were due to the consumption of infected chicken and the subsequent excretion of eggs. In our study, we did find one patient who had been directly infected with Gongylonema sp. Unlike participants in previous studies, as mentioned earlier our participant had a history of refractory stomatitis and was living in an area where the dung beetle was present and often eaten on a regular basis. Given the unique lifestyle circumstances, participants’ history and information collected from interviews, this seems to be the first reported case of human Gongylonema sp. infection in Thailand (Doi et al., 2014).
In our study, we did not detect the presence of any A. lumbricoides eggs. One of the reasons for this may be attributed to the control programs carried out by the Ministry of Public Health in Thailand (Anantaphruti et al., 2004; Wongsaroj et al., 2014). However, it remains unclear why this widely spread soil-transmitted nematode was not detected. Similar results were reported by Ngrenngarmlert et al. (2003) and Wongsaroj et al. (2014) in other studies in northeast Thailand.
Fourteen (12%) out of the 117 soil samples from Sakon Nakhon Province were positive for Toxocara sp. eggs. Eggs were detected from samples taken inside elementary school campuses, indicating that fecal contamination from dogs and cats is widespread. Soil contamination with Toxocara eggs was also reported in Songkhla Province (positivity rate of 19%; Uga et al., 1997) and Bangkok (6%; Wiwanitkit et al., 2004). Future health measures should include controlling the breeding of domestic animals to prevent the spread of zoonotic parasites, particularly larva migrans.
In this study, more than 6% of schoolchildren tested were considered to be positive for Toxocara antibodies. Uga et al. (1990) reported similar results in schoolchildren in Japan (6%), and similar results have also been reported in Malaysia (6%; Romano et al., 2010) and Korea (9%; Kim et al., 2014). Taken together, these findings indicate that the sero-prevalence rates of Toxocara range from 6 to 10% across the Asian region. The reasons for the similarities in rates across these countries remain unclear and future research could consider clarifying these factors. Uga et al. (1989) and Itoh et al. (2001) conducted a urine ELISA using schistosomiasis and filariasis patients, respectively. The use of urine instead of blood for sero-epidemiology is advantageous because it is less invasive and raises fewer ethical concerns. In this study, we performed ELISA using urine and serum and the antibody titers were compared. The results showed a strong correlation suggesting that urine can be used effectively for Toxocara studies in this type of research. The use of urine has the additional benefit of being easy to collect as it does not require a skilled practitioner for sample collection, facilitating the design and plausibility of studies with large sample sizes.
In summary, our findings indicated that the parasite epidemic in this region of Thailand is affected by a number of factors, for example, poor hygiene in school C, eating habits in school F, and uncontrolled breeding of domestic cats and dogs as exemplified by the egg contamination of soil. The most prevalent and widely distributed parasitic disease was toxocariasis with 6% of the schoolchildren being infected. The results from this study also confirm the benefits of adopting a multi-parameter approach to research offering a more comprehensive and accurate dataset with a view to enabling future prevention and control programs to be designed in a more appropriate and effective manner.
ACKNOWLEDGEMENTS
We greatly appreciated the support received from the principals and staff members of Ba Na Lao school, Ban Huai Bun Na Than school, Ban Hin Taek school, Ban Ba Hua Moei school, Ban Kut Nam school, and Ban Ba Yao school. We would like to extend our thanks to Mr. Daisaku Yanagihara and Mr. Kohei Naoi for their assistance during fieldwork. We also wish to thank Mr. Ahmed Youssef for his help on laboratory works.
REFERENCES
- 1.Anantaphruti MT, Miapanich W, Muennoo C, Pubampen S, Sanguankiat S. Hookworm infections of schoolchildren in southern Thailand. Southeast Asian J Trop Med Public Health. 2002;33:468–473. [PubMed] [Google Scholar]
- 2.Anantaphruti MT, Waikagul J, Maipanich W, Nuamtanong S, Pubampen S. Soil-transmitted helminthiases and health behaviors among schoolchildren and community members in a west-central border area of Thailand. Southeast Asian J Trop Med Public Health. 2004;35:260–266. [PubMed] [Google Scholar]
- 3.Bleakley H. Disease and development: evidence from hookworm eradication in the American South. Q J Econ. 2007;122:73–117. doi: 10.1162/qjec.121.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonjaraspinyo S, Boonmars T, Kaewasamut B, et al. A cross-sectional study on intestinal parasitic infections in rural communities, northeast Thailand. Korean J Parasitol. 2013;51:727–734. doi: 10.3347/kjp.2013.51.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–551. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Doi R, Youssef AI, Uga S. A suspected case of Gongylonema sp. infection in Thai school children: Interview and review of the literature. J Clin Parasitol. 2014;25:13–15. [Google Scholar]
- 7.Haswell-Elikins MR, Mairiang E, Mairiang P, et al. Cross-section study of Opisthorchis viverrini infection and cholangiocarcinoma in communities within a high-risk area in northeast Thailand. Int J Cancer. 1994;59:505–509. doi: 10.1002/ijc.2910590412. [DOI] [PubMed] [Google Scholar]
- 8.Itoh M, Weerasooriya MV, Qui XG, et al. Sensitive and specific enzyme-linked immunosorbent assay for the diagnosis of Wuchereria Bncrofti infection in urine samples. Am J Trop Med Hyg. 2001;65:362–365. doi: 10.4269/ajtmh.2001.65.362. [DOI] [PubMed] [Google Scholar]
- 9.Kim HS, Jin Y, Choi MH, et al. Significance of serum antibody test for toxocariasis healthy healthcare examinees with eosinophilia in Seoul and Gyeongsangnam-do, Korea. J Korean Med Sci. 2014;29:1618–1625. doi: 10.3346/jkms.2014.29.12.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ngrenngarmlert W, Chaturawit C, Sripommasa S, et al. Epidemiology of soil-transmitted helminth infection in Koodprao village, Sakon-Nakhon Province, Thailand. Bull Chiang Mai Assoc Med Sci. 2003;36:36–43. [Google Scholar]
- 11.Pasuralertsakul S, Yaicharoen R, Sripochang S. Spurious human infection with Gongylonema: nine cases reported from Thailand. Ann Trop Med Parasitol. 2008;102:455–457. doi: 10.1179/136485908X300869. [DOI] [PubMed] [Google Scholar]
- 12.Pesson B, Hersant C, Biehler JF, et al. First case of human gongylonemosis in France. Parasite. 2013;20:1–4. doi: 10.1051/parasite/2013007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raso G, Utzinger J, Silue KD, et al. Disparities in parasitic infections, perceived ill health and access to health care among poorer and less poor schoolchildren of rural Cote d’Ivoire. Trop Med Int Health. 2005;10:42–57. doi: 10.1111/j.1365-3156.2004.01352.x. [DOI] [PubMed] [Google Scholar]
- 14.Romano N, Nor Azah MO, Rahmah N, Lim YAL, Rohela M. Seroprevalence of toxocariasis among Orang Asli (Indigenous people) in Malaysia using two immunoassays. Trop Biomed. 2010;27:585–594. [PubMed] [Google Scholar]
- 15.Saksirisampant W, Prownebon J, Kulkumthorn M, Yenthakam S, Janpla S, Nuchprayoon N. The prevalence of intestinal parasitic infections among school children in the central region of Thailand. J Med Assoc Thai. 2006;89:1928–1933. [PubMed] [Google Scholar]
- 16.Sithithaworn P, Haswell-Elikins M. Epidemiology of Opisthorchis viverrini. Acta Trop. 2003;88:187–194. doi: 10.1016/j.actatropica.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Tesana S, Sithithavorn P, Prasongwatana J, Kaewkes S, Pipitgool V. Geographic distribution of soil-transmitted helminthes in northeastern part of Thailand. J Trop Med Parasitol. 1987;10:57–62. [Google Scholar]
- 18.Tomokawa S, Kobayashi T, Kaneda E, Moji K, Nisaygnang B, Boupha B. Surveying factors related to children’s Opisthorchis viverrini infection to primary school children in rural central-southern Laos-Influence of parent’s habit of eating raw fish on their children’s one-Minzoku Eisei. 2008;74:13–21. [Google Scholar]
- 19.Uga S, Gatika SM, Kimura E, Muhoho DN, Waiyaki G. Enzyme-linked immunosorbent assay as a diagnostic method for schistosomiasis haematobium. Standardization and application in the field. J Trop Med Hyg. 1989;92:407–411. [PubMed] [Google Scholar]
- 20.Uga S, Matsumura T, Fujisawa K, Okubo K, Kataoka N, Kondo K. Incidence of seropositivity to human Toxocariasis in Hyougo Prefecture, Japan, and its possible role in ophthalmic disease. Jpn J Parasitol. 1990;39:500–502. [Google Scholar]
- 21.Uga S, Nagnaen W, Chongsuvivatwong V. Contamination of soil with parasite eggs and oocysts in sourthern Thailand. Southeast Asian J Trop Med Public Health. 1997;28:14–17. [PubMed] [Google Scholar]
- 22.Uga S. Prevalence of Toxocara eggs and number of fecal deposits from dogs and cats in sandpits of public parks in Japan. J Helminthol. 1993;67:78–82. doi: 10.1017/s0022149x0001289x. [DOI] [PubMed] [Google Scholar]
- 23.Unicef. Human helminth infections in Greater Mekong Region. 2003. http://www.tm.nagasaki-u.ac.jp/schoolhealth/kenkyuhan.eng/Mapping_human_helminth_UNICEF.pdf.
- 24.Waikagul J, Krudsood S, Radomyos P, et al. A cross-sectional study of intestinal parasitic infections among schoolchildren in Nan province, northern Thailand. Southeast Asian J Trop Med Public Health. 2002;33:218–223. [PubMed] [Google Scholar]
- 25.WHO. World Health Report 2000-Conquering Suffering Enriching Humanity. Geneva: World Health Organization; 2014. [Google Scholar]
- 26.Wiwanitkit V, Waenlor W. The frequency rate of Toxocara species contamination in soil samples from public yards in a urban area “Payathai”, Bangkok, Thailand. Rev inst Med trop S Paulo. 2004;46:113–114. doi: 10.1590/s0036-46652004000200011. [DOI] [PubMed] [Google Scholar]
- 27.Wongsaroj T, Nithikathkul C, Rojkitikul W, Nakai W, Royal L, Rammasut P. National survey of helminthiasis in Thailand. Asian Biomed. 2014;8:779–783. [Google Scholar]
- 28.Ziegelbauer K, Speich B, Mausezahl D, Bos R, Keiser J, Utzinger J. Effect of sanitation on soil-transmitted helminth infection: sysytemic review and meta-analysis. PLoS Med. 2012;9:1–17. doi: 10.1371/journal.pmed.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]