Abstract
Evidence regarding nursing support for delirium prevention is currently insufficient. An evaluation of changes in autonomic nervous activity over time after surgery would elucidate the features of autonomic nervous activity in patients with delirium. These results could provide a basis for effective nursing intervention and timing for preventing the onset of delirium. Here, we aimed to obtain basic data on effective nursing interventions for preventing the onset of postoperative delirium.
Heart rate variability was recorded during the morning and nighttime on the day before surgery until 3 days postoperatively in elderly patients who underwent orthopedic surgery to investigate the manner in which heart rate and autonomic nervous activity changed over time. Data were collected over 11 months from July 2013 to November 2014.
Surgical stress led to the maintenance of heart rate at a significantly higher value from the day of the surgery until postoperative day 3 compared to that before surgery. Moreover, the autonomic nervous activity remained unchanged during the morning, and it was significantly lower during the night from postoperative day 1 until postoperative day 3 than before the surgery.
These results suggest that there is a decrease in parasympathetic nervous activity during the nighttime postoperatively.
Keywords: Autonomic nervous activity, Delirium, Elderly patients, Heart rate variability, Orthopedic surgery
INTRODUCTION
Owing to the aging of the population and advances in medical technology, the number of elderly individuals undergoing highly invasive surgical procedures is increasing in Japan. (1) Postoperative delirium is a commonly observed complication after surgery in elderly patients. Fong et al. (2) indicated that postoperative delirium occurs in 15–53% of surgical patients aged >65 years, and in 70–87% of elderly patients admitted to an intensive care unit (ICU). Whitlock et al. (3) reported that delirium is associated with an increased duration of mechanical ventilation and ICU and hospital stay, as well as functional decline. Furthermore, delirium has been found to be related to an increase in the mortality rate and medical costs. Delirium can also be problematic in terms of nursing care, as the corresponding cognitive decline can lead to accidents such as falls and tumbles, or the removal of lines (i.e., intravenous lines, surgical drains, or urethral catheters). The postoperative environment and postoperative medication regimen are usually adjusted to prevent delirium and promote earlier withdrawal. However, current evidence regarding nursing support for delirium prevention is currently insufficient (4).
Circadian rhythm disorders (sleep disorders) are one of the main symptoms of delirium (5). Inouye et al. (6) adopted a six-item preventive intervention, including interventions for sleep deprivation and immobility, and they observed that the incidence of delirium was significantly lower in the intervention group than in the usual-care group. Moreover, Shigeta et al. (7) reported abnormal melatonin secretion in patients with delirium postoperatively. These studies indicated that changes in the life rhythm affect the onset and severity of delirium. Ono et al. (8) used bright light therapy and autonomic nervous activity as one of the indicators to verify its usefulness in patients after esophagectomy; they found that bright light therapy adjusted the sleep-wakefulness cycle, and they suggested that this therapy could also prevent delirium. On the basis of these findings, we hypothesized that the modulation of autonomic nervous activity is associated with delirium onset and severity.
The measurement of heart rate variability (HRV) is useful for assessing the function of the autonomic nervous system and the staging of clinical diseases (9). Akselrod et al. (10) reported that HRV can be used to noninvasively assess the autonomic nervous system, and power spectrum analysis of HRV offers a quantitative evaluation of the functioning of the sympathetic and parasympathetic nervous systems, which are involved in short-term cardiovascular control. When an individual experiences surgical stress, the sympathetic nerves are stimulated to maintain the body’s homeostatic balance. Due to such stimulation, the heart rate (HR) increases and peripheral vasoconstriction occurs, which consequently increases the blood pressure (11). Furthermore, aging has been found to be associated with a decrease in cardiac vagal activity (12) as well as a reduction in the amplitude of HRV (13,14). Therefore, we think that elderly individuals would be more likely to experience an imbalance in autonomic nervous activity because of surgical stress and to develop dysautonomia symptoms such as irritability and insomnia. Several studies have described the conditions underlying postoperative autonomic neuropathy. Zang et al. (15) reported that nighttime autonomic nervous activity on postoperative day 1 and day 2 in elderly patients was significantly lower than that preoperatively. Hansen et al. (16) also described a decrease in nighttime parasympathetic nervous activity after breast cancer surgery. However, these studies only investigated the condition during the nighttime, and to our knowledge, no study has conducted a continuous observation during the daytime as well.
An evaluation of changes in autonomic nervous activity over time after surgery can help elucidate the features of autonomic nervous activity in patients with delirium. The results could also provide a basis for effective nursing intervention and timing of the intervention to prevent delirium onset. Here, we aimed to clarify changes in the HR and autonomic nervous activity over time in elderly patients who have undergone orthopedic surgery.
MATERIALS AND METHODS
Participants
We enrolled 49 subjects hospitalized in the orthopedic ward of hospital A; they were aged ≥65 years and were scheduled to undergo total knee arthroplasty, unicompartmental knee arthroplasty, total hip arthroplasty, or spinal surgery.
Patients taking β-blockers, patients with a cerebrovascular disease (in cases of autonomic neuropathy), and patients diagnosed as having diabetes were excluded from the present study, as these conditions would affect their autonomic nervous activity. Additionally, patients who found it difficult to follow instructions (e.g., those with dementia) were also excluded. Informed consent was obtained from all patients before study participation.
Setting
Data were collected over 11 months from July 2013 to November 2014. As postoperative delirium peaked between 1 and 3 days postoperatively, as reported by Whitlock et al. (3), we performed the survey 1 day before, on the day of, and 3 days postoperatively.
Data collection
Electrocardiography (ECG) recordings (FX-8222, CardiMax, Fukuda Denshi, Japan) were obtained for 200 heartbeats by using a lead II channel system. The average HR and R-R intervals were calculated from the ECG findings. Spectral analysis of HRV was performed by using the fast Fourier transform system (LabVIEW, National Instruments, Tokyo, Japan). The spectral bands used were 0.15–0.40 Hz (high-frequency component; HF) and 0.04–0.15 Hz (low-frequency component; LF). Considering the burden the measurements would place on the patients, we used an intermittent measurement method.
The investigators (nurses) visited patients twice per day at 10:00 and 20:00. Measurements were performed in the patient’s room while the patients were placed in the decubitus position, after approximately 15 minutes of bed rest. Subjects abstained from consuming beverages containing caffeine for at least 3 hours before the measurements were taken, and the measurements were performed at an interval of at least 2 hours after a meal was consumed. To ensure that the assessment distinguished cardiac vagal activity from sympathetic nervous activity, the respiratory rate was simultaneously confirmed to be at least 9/min (or a frequency of at least 0.15 Hz, which is the limiting transmittable frequency for sympathetic nerves). During the measurement, the air temperature and humidity were similar to those in the in-hospital environment; the sound and lighting conditions during each measurement were recorded.
As LF and HF data are not normally distributed, data were converted into the natural logarithm (ln) before analysis. Pomeranz et al. (17) found that the LF component represents the activity of the sympathetic and parasympathetic nervous system, whereas the HF component represents the activity of the parasympathetic nervous system alone. The maximum variation was determined by subtracting the minimum value (mean value) from the maximum value (mean value), which also indicates the fluctuation range.
Patients’ background
Delirium was defined using the Japanese version of the NEECHAM confusion scale (18). Nurses performed assessments for delirium during routine nursing care. In addition, sleep satisfaction and perceived pain were assessed by using the Visual Analog Scale by the investigators (nurses). Sleep satisfaction was measured at 10:00. Delirium and perceived pain were measured at 10:00 and 20:00.
Information on patients’ age, sex, anamnesis, diagnoses, and surgical procedures were collected from their medical records.
Data analysis
Repeated-measures analysis of variance (ANOVA; F-value) was used to investigate the manner in which HR and autonomic nervous activity changed over time postoperatively. The Mauchly sphericity test was also conducted to verify the equality of variance for the ANOVA; if a significant result was found, the Greenhouse-Geisser correction was applied. In addition, the Tukey test was used for post hoc analysis. IBM SPSS, version 21 for Windows (IBM Corp., Armonk, NY, USA) was used to perform statistical analysis, and the level of significance was set at 5%.
Ethical considerations
The present study was approved by the ethics committee of the Kobe University Graduate School of Health Sciences (approval number: 227). The protocol followed the guidelines of the Declaration of Helsinki. Permission was obtained from attending physicians regarding patients’ suitability for study participation. After providing the subjects with verbal and written explanations of the study objectives and research methods, written consent was obtained. The survey was conducted in coordination with the nurses to avoid interfering with rehabilitation, examination, and other aspects of patient therapy.
RESULTS
Patients’ demographic and clinical characteristics
Table I presents data on patients’ demographic and clinical characteristics. Forty-nine patients provided informed consent and were permitted by their primary physician to participate in this study. Of 49 patients, we excluded 4 with diabetes and 13 with missing values. Thus, 32 patients were included in the final analysis. Patients’ mean age was 74.4 ± 5.7 years (9 men and 23 women), and their average body mass index was 24.8 ± 3.0 kg/m2. Of all the subjects, 3 had delirium. All patients were treated with routine pain medication.
Table I.
Patients’ demographic and clinical characteristics
| Total (n = 32) | ||
|---|---|---|
| Age (years) | Mean | 74.4 |
| SD | 5.7 | |
| Sex (male/female) | 9/23 | |
| Body mass index (kg/m2) | Mean | 24.8 |
| SD | 3 | |
| Body temperature (°C) | Mean | 36.3 |
| SD | 0.6 | |
| bSBP (mmHg) | Mean | 117.7 |
| SD | 13.5 | |
| bDBP (mmHg) | Mean | 62.6 |
| SD | 6.8 | |
| Hb (g/dL) | Mean | 12.4 |
| SD | 1.3 | |
| WBC (×102/μL) | Mean | 54.5 |
| SD | 11.4 | |
| CRP (mg/dL) | Mean | 0.129 |
| SD | 0.142 | |
| Surgical procedure | ||
| TKA and UKA | 11 | |
| THA | 10 | |
| Spinal surgery | 11 | |
| Duration of anesthesia (min) | Mean | 224.2 |
| SD | 82.8 | |
| Drainage volume (mL) | Mean | 314.4 |
| SD | 285.3 | |
| Delirium | ||
| Yes | 3 | |
| No | 29 | |
Notes: TKA=total knee arthroplasty,
UKA=unicompartmental knee arthroplasty,
THA=total hip arthroplasty,
bSBP=brachial systolic blood pressure,
bDBP=brachial diastolic blood pressure,
Hb=hemoglobin, WBC=white blood cell,
CRP=C-reactive protein
Changes in HR and autonomic nervous activity over time in elderly patients after orthopedic surgery
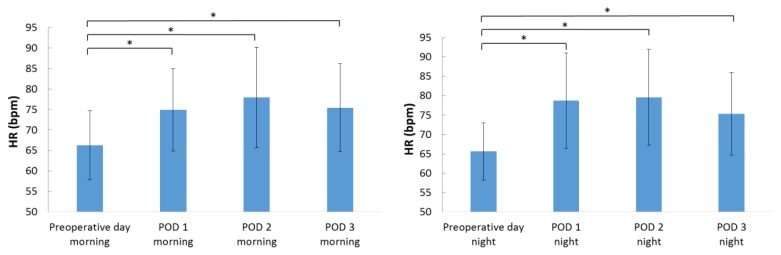
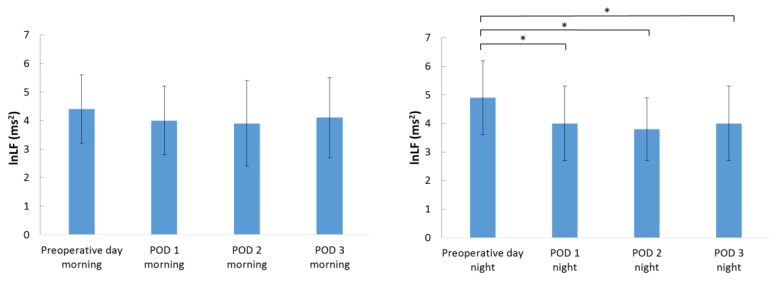
Table II indicates changes in the HR and autonomic nervous activity over time in elderly patients who had undergone orthopedic surgery. The HR increased from the night before the surgery (65.6 ± 7.4 beats/min) to the night on the day of surgery (73.0 ± 10.8 beats/min); the HR continued to gradually increase thereafter, and it peaked on the night of postoperative day 2 (79.6 ± 12.4 beats/min) and then gradually decreased. The change in HR from before the surgery to 3 days postoperatively was significant (F = 25.592, p < 0.001), and the HR was significantly higher from the night of the day of surgery until the night of postoperative day 3 than before the surgery (p < 0.001). The lnLF increased and peaked from the morning before the surgery (4.4 ± 1.2 ms2) to the night before the surgery (4.9 ± 1.3 ms2), after which it remained constant at a lower value than that before the surgery. The change in lnLF from before the surgery to 3 days after the surgery was significant (F = 4.151, p < 0.001), and the lnLF values were significantly lower from the morning of postoperative day 1 until the night of postoperative day 3 than on the night before the surgery (p = 0.001–0.028). The lnHF also increased and peaked from the morning before the surgery (4.5 ± 1.3 ms2) to the night before the surgery (4.9 ± 1.4 ms2), and then it remained constant at a lower value than that before the surgery; however, the difference in the lnHF values during this period was not significant (F = 1.401, p = 0.197).
Table II.
Changes in heart rate and autonomic nervous activity from before surgery to postoperative day 3 in elderly patients who had undergone orthopedic surgery
| Preoperative day | Surgery day | POD1 | POD2 | POD3 | Max variation | F value | p value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Morning | Night | Night | Morning | Night | Morning | Night | Morning | Night | ||||
| HR (bpm) | 66.3 ± 8.4 | 65.6 ± 7.4 | 73.0 ± 10.8 | 74.9 ± 10.1 | 78.7 ± 12.3 | 77.9 ± 12.3 | 79.6 ± 12.4 | 75.4 ± 10.7 | 75.3 ± 10.6 | 14 | 25.592 | 0 |
| lnLF (ms2) | 4.4 ± 1.2 | 4.9 ± 1.3 | 4.3 ± 1.4 | 4.0 ± 1.2 | 4.0 ± 1.3 | 3.9 ± 1.5 | 3.8 ± 1.1 | 4.1 ± 1.4 | 4.0 ± 1.3 | 1.1 | 4.151 | 0 |
| lnHF (ms2) | 4.5 ± 1.3 | 4.8 ± 1.4 | 4.6 ± 1.4 | 4.3 ± 1.4 | 4.3 ± 1.8 | 4.3 ± 1.8 | 4.1 ± 1.7 | 4.4 ± 1.5 | 4.2 ± 1.4 | 0.7 | 1.401 | 0.197 |
Data are presented as a mean ± standard deviation. HR, heart rate; lnLF, natural log of low frequency values; lnHF, natural log of high frequency values; POD, postoperative day; Max variation: maximum value (mean) – minimum value (mean).
Changes in the HR and autonomic nervous activity during the morning and nighttime
Figure 1 indicates changes in the HR during the morning and nighttime. The HR was significantly higher after the surgery than before the surgery during the morning and nighttime (p < 0.001). The lnLF values in the morning were not significantly different before surgery and after surgery. However, the lnLF values during the nighttime were significantly lower on postoperative day 1 (p = 0.003), postoperative day 2 (p < 0.001), and postoperative day 3 (p = 0.002) than before the surgery (Figure 2). The lnHF values during the morning and nighttime did not significantly change before the surgery and after the surgery.
Figure 1.
Changes in the HR during the morning and nighttime.
Data are presented as a mean ± standard deviation. POD: postoperative day; HR: heart rate.
*p < 0.01 according to the Tukey post hoc test.
Figure 2.
Changes in the lnLF values during the morning and nighttime.
Data are presented as a mean ± standard deviation. POD: postoperative day; lnLF, natural log of low frequency.
*p < 0.01 according to the Tukey post hoc test.
DISCUSSION
Our results indicated the following 2 points regarding changes in the HR and autonomic nervous activity in these patients. (1) The HR was significantly higher from the night of the day of surgery until postoperative day 3 than before surgery. (2) Autonomic nervous activity in the morning remained unchanged from the period before surgery compared to the period after surgery. However, the autonomic nervous activity during the nighttime was significantly lower from postoperative day 1 to postoperative day 3 than before the surgery.
With regard to the aforementioned first point, patients usually experience an increase in HR postoperatively, as this ensures better perfusion of vital organs such as the brain, heart, and skeletal muscles (11). Hence, the increase in HR postoperatively in the present study is reasonable, and it may represent a biological response to surgical stress. However, further examination is needed to elucidate the relationship between postoperative pain or the side effects of medication and HR.
The significantly lower postoperative lnLF values suggest that after surgery, patients experienced a functional decline in autonomic nervous activity. Zhang et al. (15) measured autonomic nervous activity on the day before the surgery, postoperative day 1, and postoperative day 2 from 11:00 pm to 7:00 am on the following day to clarify differences in autonomic nervous activity among elderly patients after surgery according to the presence of diabetes. The authors found that the lnLF values on postoperative day 1 were significantly lower than before the surgery, regardless of the presence of diabetes. Thus, our study was consistent with their result and extended to that autonomic nervous activity declines until postoperative day 3 than before surgery. As aforementioned, Pomeranz et al. (17) reported that the LF component reflects sympathetic and parasympathetic modulation, whereas the HF component only represents parasympathetic modulation. In the present study, the significant decrease in lnLF values during the nighttime after the surgery could be attributed to a decrease in parasympathetic nervous activity, based on the following two points. First, the HR was significantly higher postoperatively in the present study. Second, nighttime parasympathetic nervous activity is significantly reduced postoperatively compared to preoperatively (15,16). However, in the current study, no significant decrease in parasympathetic nervous activity was observed. We think that the main effect of respiration on parasympathetic nervous activity may contribute to this finding (12), in other words parasympathetic nervous activity is often influenced by respiration, therefore, using controlled breathing might be more suitable for evaluation of parasympathetic nervous activity. However, it is impractical to regulate the respiration of patients postoperatively, as the difficulty of precisely measuring parasympathetic nervous activity remains a main limitation of the present study.
In our study, the autonomic nervous activity decreased during the night time, whereas there was no change in the morning. The mechanism of this result remains unclear, however a possible explanation is as follows. In the morning, sympathetic nervous activity dominates, resulting in the decrease of parasympathetic nervous activity. This dominance of sympathetic nervous activity might contribute to the no change in autonomic nervous activity in the morning. Further investigation is needed.
The present study also has five limitations. First, we only included subjects who underwent orthopedic surgery with minimal impact on autonomic nervous activity. Hence, only a limited number of subjects could be enrolled in the present study. Second, we did not consider the differences of surgical invasiveness about patients’ surgical procedures; TKA, UKA, THA, and spinal surgery. Third, although anemia from surgery affects postoperative heart rate, we have no data regarding blood transfusion. Fourth, considering the burden on the patients, we used an intermittent measurement method; however, as a result, we were unable to obtain continuous data. Fifth, we did not consider the effect of medications taken during the perioperative period.
In future studies, we recommend the continuous measurement of autonomic nervous activity to clarify the changes in this parameter, along with evaluating these changes based on patients’ sleep satisfaction and perceived pain, and the presence or absence of delirium or its severity.
Conclusions
Our findings indicate that surgical stress leads to the maintenance of the HR at a significantly higher value than that before surgery, from the day of the surgery until postoperative day 3. We also found that the autonomic nervous activity remained unchanged in the morning compared to before surgery, but it was significantly lower during the nighttime than before surgery, from postoperative day 1 until postoperative day 3. These results suggest that there is a decrease in parasympathetic nervous activity during the nighttime postoperatively.
ACKNOWLEDGEMENTS
We are deeply grateful to the subjects and their families, nurses and staff, and all persons who greatly contributed to this study. This work was supported by a grants-in-aid of the “YAMAJI FUMIKO NURSING RESEARCH FUND”. None of the authors has any conflicts of interest or any financial ties to disclose.
REFERENCES
- 1.Saisho S, Suto I, Eda I, Suemitsu K, Ohtsuka A. Surgical care and outcomes after abdominal surgery in the elderly patients over 90 years old. Journal of Japan Surgical Association. 2005;66:1540–1547. [Google Scholar]
- 2.Fong TG, Tulebaev SR, Inouye SK. Delirium in the elderly adults: diagnosis, prevention and treatment. Nature Reviews Neurology. 2009;5:210–220. doi: 10.1038/nrneurol.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitlock EL, Vannucci A, Avidan MS. Postoperative delirium. Minerva Anestesiologica. 2011;77:448–456. [PMC free article] [PubMed] [Google Scholar]
- 4.Sugawara M. A review of nursing intervention for delirium in elderly patients. Japan Academy of Gerontological Nursing. 2011;16:94–103. [Google Scholar]
- 5.Meagher DJ, Moran M, Raju B, Gibbons D, Donnelly S, Saunders J, Trzepacz PT. Phenomenology of delirium. Assessment of 100 adult cases using standardised measures. British Journal of Psychiatry. 2007;190:135–141. doi: 10.1192/bjp.bp.106.023911. [DOI] [PubMed] [Google Scholar]
- 6.Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM., Jr A multicomponent intervention to prevent delirium in hospitalized older patients. New England Journal of Medicine. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 7.Shigeta H, Yasui A, Nimura Y, Machida N, Kageyama M, Miura M, Menjo M, Ikeda K. Postoperative delirium and melatonin levels in elderly patients. The American Journal of Surgery. 2001;182:449–454. doi: 10.1016/s0002-9610(01)00761-9. [DOI] [PubMed] [Google Scholar]
- 8.Ono H, Taguchi T, Kido Y, Fujino Y, Doki Y. The usefulness of bright light therapy for patients after oesophagectomy. Intensive and Critical Care Nursing. 2011;27:158–166. doi: 10.1016/j.iccn.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Ushiyama T, Mizushige K, Wakabayashi H, Nakatsu T, Ishimura K, Tsuboi Y, Suzuki Y. Analysis of heart rate variability as index of noncardiac surgical stress. Heart and Vessels. 2008;23:53–59. doi: 10.1007/s00380-007-0997-6. [DOI] [PubMed] [Google Scholar]
- 10.Akselrod S, Gordon G, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat- to-beat cardiovascular control. Science. 1981;213(4504):220–22. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 11.Copel LC. Homeostasis, stress, and adaptation. In: Smeltzer SC, Bare BG, editors. Brunner and Suddarth’s Textbook of Medical-Surgical Nursing. 9th ed. Lippincott Williams & Wilkins; Philadelphia, USA: 2000. pp. 71–88. [Google Scholar]
- 12.Hayano J. The autonomic function analysis by heart rate variability. In: Inoue H, editor. Autonomic Function and Cardiovascular Disease. Igaku-Shoin P; Tokyo, Japan: 2001. pp. 71–109. [Google Scholar]
- 13.Hrushesky WJM, Fader D, Schmitt O, Gilbertsen V. The respiratory sinus arrhythmia: a measure of cardiac age. Science. 1984;224:1001–1004. doi: 10.1126/science.6372092. [DOI] [PubMed] [Google Scholar]
- 14.Shannon DC, Carley DW, Benson H. Aging of modulation of heart rate. American Journal of Physiology. 1987;253:H874–H877. doi: 10.1152/ajpheart.1987.253.4.H874. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Tu W, Dai J, Lv Q, Yang X. Dysfunction of pre- and post-operative cardiac autonomic nervous system in elderly patients with diabetes mellitus. Archives of Gerontology and Geriatrics. 2011;53:334–337. doi: 10.1016/j.archger.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Hansen MV, Rosenberg J, Gögenur I. Lack of circadian variation and reduction of heart rate variability in women with breast cancer undergoing lumpectomy: a descriptive study. Breast Cancer Research & Treatment. 2013;140:317–322. doi: 10.1007/s10549-013-2631-x. [DOI] [PubMed] [Google Scholar]
- 17.Pomeranz B, Macaulay RJ, Caudill MA, Kutz I, Adam D, Gordon D, Benson H. Assessment of autonomic function in humans by heart rate spectral analysis. American Journal of Physiology. 1985;248:H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 18.Watanuki S, Sakai I, Takeuchi T, Suwa H, Taruya T, Ichinose K. Development and pilot testing of the Japanese version of the NEECHAM Confusion Scale: a tool to assess delirium/acute confusion. Advances in Nursing art and Science. 2001;12:46–63. [Google Scholar]