Abstract
Macaque species, specifically rhesus (Macaca mulatta), are the most common nonhuman primates (NHPs) used in biomedical research due to their suitability as a model of high priority diseases (e.g., HIV, obesity, cognitive aging), cost effective breeding and housing compared to most other NHPs, and close evolutionary relationship to humans. With this close evolutionary relationship, however, is a shared adaptation for a socially stimulating environment, without which both their welfare and suitability as a research model are compromised. While outdoor social group housing provides the best approximation of a social environment that matches the macaque behavioral biology in the wild, this is not always possible at all facilities, where animals may be housed indoors in small groups, in pairs, or alone. Further, animals may experience many housing changes in their lifetime depending on project needs, changes in social status, management needs, or health concerns. Here we review the evidence for the physiological and health effects of social housing changes and the potential impacts on research outcomes for studies using macaques, particularly rhesus. We situate our review in the context of increasing regulatory pressure for research facilities to both house NHPs socially and mitigate trauma from social aggression. To meet these regulatory requirements and further refine the macaque model for research, significant advances must be made in our understanding and management of rhesus macaque social housing, particularly pair-housing since it is the most common social housing configuration for macaques while on research projects. Because most NHPs are adapted for sociality, a social context is likely important for improving repeatability, reproducibility, and external validity of primate biomedical research.
Keywords: pairing, heterogeneous environment, enrichment, peer interaction
Introduction
Animals are in captivity for a wide variety of reasons and at research facilities their primary purpose is to serve as research subjects. Housing laboratory animals (defined as intended for research or teaching use, regardless of housing type [Association for Assessment and Accreditation of Laboratory Animal Care International, 2015a]) in simple and uniform conditions is done to limit environmental variability and increase the internal validity of studies, as well as facilitate sample collection, treatments, and husbandry procedures. Recently, it has been demonstrated that highly controlled and standardized laboratory environments for rodent models are generating reduced repeatability and reproducibility in research outcomes [Crabbe et al., 1999; Wurbel, 2000; Wahlsten et al., 2006; Paylor, 2009; Richter et al., 2009; Richter et al., 2010; Branchi et al., 2011; Richter et al., 2011; Schumann et al., 2014]. For example, rodent tests comparing results from four repeated experiments using subjects in highly controlled cages (same aged subjects and one enrichment item) versus subjects in less controlled cages (varying aged subjects and enrichment items) show that the highly controlled condition had low within experiment variation, but significant between experiment differences, resulting in low repeatability; the latter condition, however, had greater within experiment variation and low between experiment differences, resulting in greater repeatability in research results [Richter et al., 2009; Richter et al., 2010; Richter et al., 2011]. If an enriched context is this important for laboratory rodent research, one can reason that it is also likely important for nonhuman primates (NHPs), an outbred taxon whose hallmark adaptation is social complexity and intelligence [Fooden, 2000]. Socialization is widely regarded as the best form of enrichment for laboratory NHPs, and when on a study it is typically provided by pair-housing two animals in adjoined cages [Lutz and Novak, 2005a]. However, social housing exemptions with scientific justification are allowed that result in single-housing when an animal, or its pair-mate, is assigned to a project [Baker et al., 2007]. Such constraints may introduce an overly simplistic environment to which most primates are not well adapted and affect study subjects in ways that cannot always be addressed in analytical models. In this article, we review what is known, and what further research is needed, regarding the effects of macaque (Macaca) social housing changes on research outcomes.
Nonhuman primates are a critical resource for health research because of their close evolutionary relationship to humans [Phillips et al., 2014]. Macaques not only have a similar biology to humans, but also complex social and affective behavior repertoires [Fooden, 2000]. While their similarities to humans make them some of the best animal models for human health and disease, such social complexity also presents management challenges [Lutz and Novak, 2005b]. Social housing promotes well-being and its absence results in deleterious effects [Novak and Suomi, 1988]. It is also required by federal law and the agencies that accredit and inspect research facilities [NC3Rs, 2006; Commission Recommendation, 2007; Directive of the European Parliament, 2010; National Research Council, 2011; United States Department of Agriculture, 2013; Association for Assessment and Accreditation of Laboratory Animal Care International, 2015b; Office of Laboratory Animal Welfare, 2015]. Exceptions to social housing requirements occur, however, when scientifically justified and approved by an institutional oversight committee (Institutional Animal Care and Use Committee or IACUC in the US) for a project or when efforts to socially house an animal are not successful. Accumulating evidence suggests that not all types of social housing provide the same benefits, and further, that changes to animals' social housing can alter behavior and physiology in ways that indicate reduced welfare and quality as a research model [e.g., Gust et al., 1991; Clarke et al., 1996; DiVincenti and Wyatt, 2011; Gilbert and Baker, 2011; Xie et al., 2014; Capitanio and Cole, 2015]. Therefore, accomplishing social housing in a way that both benefits animal welfare and is compatible with research goals can be a challenge.
Social housing decisions for rhesus macaques (Macaca mulatta) represent a balance between welfare concerns and experimental needs or requirements. These decisions are also constrained by regulations, available space, climate, resources, caging, and available staff time to monitor and manage pairs. Because macaques are capable of inflicting severe wounding during fights, socialization must be carefully managed to prevent, as much as is possible, harm to the animals and costly treatment. Because socialization is the best form of enrichment for laboratory NHPs, it is given higher priority than other forms of enrichment [Lutz and Novak, 2005a]. While outdoor group housing is widely considered to be the gold standard for maintaining captive macaques, the reality is that in colder climates, urban areas, and for some studies, such outdoor housing may not be tenable. Animals in these situations are commonly housed indoors, typically in small groups or pairs [Baker, 2007]. Although male-female pairs are often easier, pairs are usually same sex to prevent uncontrolled breeding [Baker et al., 2007]. Pairs may be housed together over long durations of time, or social partners may be switched with some frequency based on health, temperament, pair compatibility, available resources, or IACUC approved research protocols [Baker et al., 2012a; Baker et al., 2014a; Baker et al., 2014b].
While reviews on pairing exist that detail the behavioral benefits, risks, and methods for socially housing macaques [e.g., Reinhardt and Reinhardt, 2000; DiVincenti and Wyatt, 2011; Truelove et al., 2015], the goal of this review is to synthesize a related literature. We review the evidence for behavioral and physiological differences between macaques that are housed in different social conditions, and the effects that social housing changes may have on these measures and on research outcomes. Our aim is to paint a picture of what is known, and what additional knowledge is needed, to advance best practices for both the welfare of laboratory macaques and the quality of the research conducted on them. We begin with an overview of the importance of macaques in laboratory research, followed by the importance of social life for normal macaque physiology. We then turn to recent regulatory changes affecting laboratory macaque social housing management and the challenges they present. We then review the literature on how changes to social housing (both social partner and physical environment) impact behavioral biology and in turn have the potential to alter research outcomes. This review is limited to the literature on macaques and is biased toward rhesus, the most common laboratory NHP and the species best represented in the relevant literature. It is worth noting that the current literature is quite limited. While there are studies on the impact of social housing changes, many questions remain unanswered. In the discussion we provide our assessment of what current literature tells us about providing for and managing macaque social housing and what further work is needed to better inform these efforts. This literature review adheres to the American Society of Primatologists Principles for the Ethical Treatment of Non-Human Primates.
What & Why: Social Housing for Laboratory Rhesus Macaques
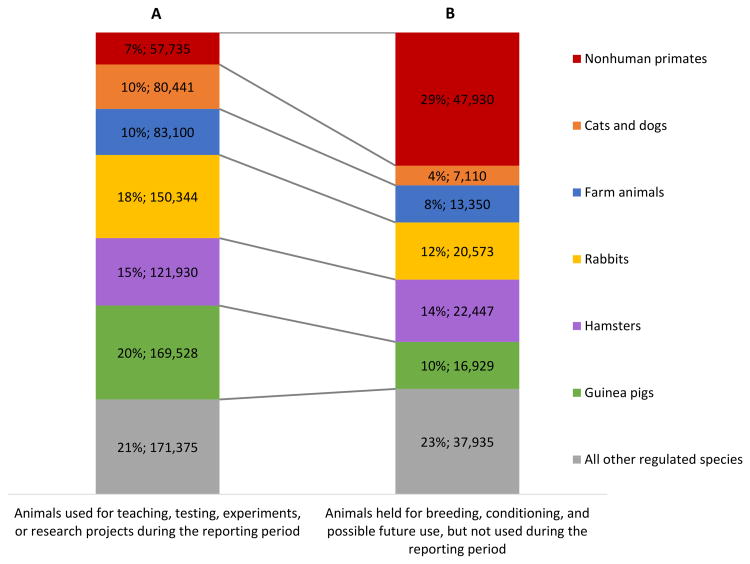
Nonhuman primates constitute the smallest percentage (7%) of regulated laboratory animal research subjects in the United States (Fig. 1A). However, among regulated laboratory animals held for breeding, conditioning, or future use, NHPs are a relatively large percentage (29%) (Fig. 1B) [data from: Animal Plant and Health Inspection Service, 2014]. This disparity is due to characteristics of primate development and sociality. Compared to many other animal species (e.g., rodents, drosophila), primates have a slow life history strategy characterized by a suite of evolved adaptations for slow maturation and reproduction with singleton births, prolonged parental investment, and long life spans [Harvey and Clutton-Brock, 1985; Stearns, 1992; Charnov and Berrigan, 1993; Lee, 1996]. This slow life history, in concert with the social needs of NHPs, requires complex management strategies to maintain breeding colonies with appropriate social partners. For this reason, NHPs cannot be bred and reared primarily in response to immediate research needs; they need time to grow and mature and at adulthood some may serve critical social roles (e.g., alpha male, alpha female, conflict policer, etc.) for the overall health and wellbeing of the social group that limit their use on projects. Thus relatively large (compared to the very small number of NHPs used) NHP breeding populations are needed to produce healthy, high quality, subjects and meet future research demands.
Fig 1.
Percent and count of total regulated animals used in 2014 for: A) animals used for teaching, testing, experimental, or research projects (left column), and; B) animals held for breeding, conditioning, or future use during the reporting period (right column). Data in each column is demarcated by USDA defined categories of regulated species used in research (excludes aquatic species, birds, rats, and mice). Data from the Annual Report of Animal Usage by Research Facilities, Fiscal Year 2013 [Animal Plant and Health Inspection Service 2014]. Figure footnote: It is important to note that the vast majority of animals used in research are “non-regulated” (e.g., rodents). Thus the percentage of nonhuman primates used in research is actually very small percentage of total number of animals used in research.
Macaques are the most commonly used laboratory NHP genus, comprising 34.2% of published NHP studies in the most recent survey; for comparison, vervets (19%) are the next most commonly used NHP taxon [Carlsson et al., 2004]. Among the macaques, the rhesus monkey is most common, comprising 53.9% of macaque species used in research [Carlsson et al., 2004]. Macaques, and rhesus in particular, are favored because of their hardiness in captive environments, relatively efficient breeding and housing requirements (compared to other NHPs), and their importance as a translational model for key discoveries in treating human diseases (e.g. HIV and AIDS, diet and obesity, etc.) [Carlsson et al., 2004]. Macaques are one of the most important translational models for human health, providing either the best relevant non-human model or the critical taxon needed for discovery about the safety and potential effectiveness of a treatment prior to human use.
Defining good versus poor welfare for laboratory NHPs, as well as measuring and assessing changes to it, generally follows standards established by a combination of regulation, enforcement, the scientific literature, and facility practices [Coleman et al., 2012]. In general, NHPs are considered to have good, even optimal, welfare when they have a healthy body condition and coat quality (weight to height ratio that accounts for distribution of fat and fur that uniformly covers skin), are free of injury or disease, exhibit species typical behavior, have a low occurrence of abnormal, anxious, depressive, and aggressive behaviors [Keeling and Wolf, 1975; Capitanio, 1986]. Changes to these metrics are typically used to monitor captive populations for declining welfare and identify and evaluate methods for improving it. While some conditions clearly constitute poor welfare if untreated (e.g., self-injurious behavior, serious illness), many others are in a gray area of change toward or away from optimal welfare [Coleman et al., 2012]. The welfare consequences associated with social housing changes are often assessed from any combination of the above measures. Generally, including multiple measures provides a robust assessment. Ideally, measures that reflect the biological status of the individual provide additional information about welfare—measures such as immune or autonomic function [e.g., Doyle et al., 2008]. But these measures are more difficult and expensive to measure and often do not figure prominently into welfare assessments. They may be among the most critical measures, however, for investigators that use NHPs for biomedical research. Given the literature review we present below, we believe that additional relevant research is needed to inform the welfare changes we expect given specific contexts.
Like most other primate species, rhesus macaque evolutionary history emphasizes adaptations for relatively large and complex brains and life in complex social groups, both of which are thus deeply intertwined in their behavior and biology [Crook and Gartlan, 1966; Hinde, 1976; van Schaik and van Hooff, 1983; Dunbar, 1988; Dunbar, 1998; Thierry et al., 2000; Thierry, 2004]. Outdoor and social group housing, either harem or multi-male/multi-female groups is the gold standard for housing macaques because it replicates, as closely as possible, the normal social environment of macaques [Bernstein, 1991]. This results in the healthiest and most normal macaques, both behaviorally and biologically, and thus optimal welfare [O'Neill et al., 1991; Westergaard et al., 2000; Capitanio et al., 2005; Fontenot et al., 2006; Karere et al., 2009; Vandeleest et al., 2011; Xie et al., 2014]. Social life is so critical that captive rhesus macaques in environments with no or limited social interaction frequently develop abnormal behavior and physiology [Harlow et al., 1965; Suomi et al., 1971; Anderson and Chamove, 1980; Capitanio, 1986; Eaton et al., 1994; Lutz et al., 2003; Novak, 2003; Lutz et al., 2007; Rommeck et al., 2011; Vandeleest et al., 2011; Gottlieb et al., 2013]. While milder abnormalities may manifest as abnormal repetitive behavior (e.g., pacing, flipping, etc.) or non-injurious self-directed behaviors (e.g., self-sucking, self-strumming, etc.), extreme forms can be self-abusive and include self-injury [e.g., Lutz et al., 2003; Novak, 2003; Lutz et al., 2007; Rommeck et al., 2009a; Gottlieb et al., 2013]. Monkeys that develop such behaviors have compromised welfare and those with the least socialization have the poorest welfare outcomes [Harlow and Harlow, 1962; Bayne et al., 1992; Mason and Latham, 2004; Rommeck et al., 2009b; Baker et al., 2012a]. In addition to welfare concerns, NHPs with such abnormal presentations are financially costly to maintain because their conditions require additional enrichment supplies, staff monitoring, and veterinary treatment that is rarely or minimally effective in the absence of increased socialization or outdoor housing [Bayne et al., 1995; Lutz et al., 2003; Novak, 2003; Lutz et al., 2004; Fontenot et al., 2006; Gottlieb et al., 2011]. Finally, underlying the externally observable abnormal behavior is often an abnormal physiology that limits their utility for research [Coe et al., 1989; Lubach et al., 1995; Garner, 2005; Capitanio, 2011; Rommeck et al., 2011; Prescott et al., 2012].
A large body of literature demonstrates that single-housing is stressful [reviewed in DiVincenti and Wyatt, 2011]. Accumulating evidence also suggests that suboptimal social housing, removal from the social group, and transitioning to other housing (even if it is social) is also associated with multiple physiological changes indicative of stress [e.g., Gust et al., 1994; Clarke et al., 1995; Capitanio et al., 2008; Baker et al., 2012b; Capitanio and Cole, 2015]. While the conditions that optimize social housing are of increasing interest [e.g., Capitanio et al., 2015; Truelove et al., 2015], little attention has been paid to the consequences of variation in social housing (including shifting animals between different social housing conditions) for physiological functioning despite the potential for such changes to dramatically impact research outcomes [Suomi et al., 1975; Cohen et al., 1992; Gordon et al., 1992; Gust et al., 1992; Gust et al., 1994; Capitanio et al., 1998a]. It is therefore important for both NHP welfare and the science for which they are used that they are provided with adequate social environments and that scientists understand the full implications of social housing changes for their research projects.
While it has long been recognized that social housing is important for laboratory NHP well-being [National Research Council, 1996], standards have recently changed with regards to expectations for providing social housing [National Research Council, 2011]. Social housing is considered so critical that the Office of Laboratory Animal Welfare [2015] in the United States has stated that there is now “universal agreement among oversight agencies that NHPs should be socially housed.” Social housing is now the default and single-housing is only allowed upon documented difficulty finding a suitable pair mate or via scientifically justified research exemption approved by the IACUC [Office of Laboratory Animal Welfare, 2015]—not because of lack of resources, for example. Similarly, regulations in the European Union also indicate that social housing should be the norm for social species of NHPs [Directive of the European Parliament, 2010]. Based on reports that NHPs in protected contact have similar levels of abnormal and anxious behaviors compared to single-housed animals and lower levels of allogrooming compared to full contact [e.g., Baker et al., 2012b], national guidelines consider protected contact no different than single-housing [National Research Council, 2011; Association of Primate Veterinarians Scientific Advisory Committee, n.d.]. For this reason, social housing that offers only protected contact, via a grate or mesh wall between animals, has come under increased scrutiny by regulatory agencies. It is worth noting, however, that Baker and colleagues [2014a] found that protected-contact housing is an improvement over single-housing even if it is not equivalent to full-contact housing. Thus, more research on alternatives that can meet research and management requirements is warranted.
Meeting the increased standards for socialization of laboratory macaques, particularly rhesus, is challenging for several reasons. Rhesus macaques are among the most despotic of the macaque species, managing their social systems with more frequent and more severe aggression than most other macaques [Thierry, 2004]. Aggression in rhesus groups can range from mild status interactions (including social signaling and minimal contact) to severe bites causing traumas requiring medical treatment, and in extreme cases, even death [Bernstein and Mason, 1963]. Some amount of trauma due to social aggression is normal and unavoidable for rhesus macaques, whether in the wild or in captivity. Further complicating this is the fact that the USDA has recently changed its policy on NHP canine tooth modifications for preventing social trauma—modifications breaching the pulp cavity can now only be done for therapeutic reasons and not to prevent injury [Animal Plant and Health Inspection Service, 2011: policy #3]. This has increased both the risk of injury and the severity of injuries due to social trauma [Hannibal et al., 2014]. Simultaneously, there is less tolerance for, and increased enforcement of, the amount of allowable injury or trauma resulting from social housing. The types of injuries allowed as part of the process of establishing and maintaining animals in social housing are also not clearly defined and largely left up to the interpretation of USDA inspectors. Finally, USDA representatives have stated at recent conferences that they will increasingly enforce full contact social housing for NHPs (Symposium on Social Housing of Laboratory Animals, 2014; American Association of Laboratory Animal Sciences, 2014). The combination of these competing requirements presents a new set of challenges for meeting the welfare needs of laboratory NHPs. While this is generating renewed discussion and investigation of methods to increase pairing rates and pairing success [e.g., Capitanio et al., 2015; Truelove et al., 2015], it will also likely be the impetus for additional changes in social housing as animals are increasingly removed from one social housing situation due to trauma and placed in a queue for introduction to new social housing. Additional investigation into the benefits and detriments, for both the animals' welfare and for research goals, is also warranted.
Changes in the Social Environment of Laboratory Macaques, Physiology, and Implications for Research Outcomes
The frequency of social housing changes and the types of social housing laboratory rhesus macaques experience with each change are established risk factors for developing abnormal behaviors [Gottlieb et al., 2013]. Such frequent changes in social relationships and housing could also affect research outcomes. Furthermore, given the variation in criteria for establishing and maintaining pairs across facilities [Baker et al., 2014b], it is possible, even probable, that there is variation in research outcomes from different facilities due to variation in social housing practices.
The extent to which animals experience reduced well-being during pairing depends on characteristics of the animals and the social environments that facilitate compatibility with potential pair-mates. These characteristics include stability and certainty of dominance relationships [Lynch, 1998], subject personality and temperament [Capitanio et al., 2008; Capitanio et al., 2015], occurrence of deleterious aggression [Crockett et al., 1994], type of housing [Schapiro et al., 2000], and previous social experience of the animals [Reinhardt et al., 1995]. A large body of literature has investigated the connection between anxious, depressive, and abnormal behaviors and social housing conditions for macaques with a focus on its importance for laboratory animal welfare [e.g., Suomi et al., 1973; Mineka and Suomi, 1978; Bayne et al., 1992; Eaton et al., 1994; Schapiro et al., 1996; Gilbert and Baker, 2011; Baker et al., 2012b; Gottlieb et al., 2013]. The literature examining the physiological consequences of changes to social housing is more limited and complicated due to the use of multiple outcome measures. Some studies have examined the impact of social housing changes on physiological measures of stress (i.e. cortisol levels) [Reinhardt et al., 1991; Laudenslager et al., 1995; Doyle et al., 2008; Baker et al., 2012a], with the assumption that high cortisol levels likely have implications for immunity and health. Only a handful of studies, however, have used alterations of the social environment to directly examine the impact on immunity [Gordon et al., 1992; Gust et al., 1992; Gust et al., 1993; Gust et al., 1994; Schapiro et al., 2000; Capitanio et al., 2008]. Since previous literature has addressed how social housing generally (that is, its presence or absence) alters welfare, we concentrate here on how changes to social housing can impact physiological measures relevant to biomedical research outcomes.
Social housing and physiology
What evidence exists on changes to social housing suggests that, generally, expansion of the social environment (e.g., single-housing to pair-housing) improves welfare while contraction of the social environment (e.g., group-housing to pair-housing) diminishes welfare. Welfare is typically measured in these contexts by assessing behaviors (e.g., abnormal, depressive, etc.) and less often by changes in biological measures (e.g., cortisol levels, immune response) indicative of a physiology that is altered from the normal macaque model [e.g., Schapiro, 2002; Doyle et al., 2008; Baker et al., 2012a; Baker et al., 2012b; Capitanio and Cole, 2015]. Furthermore, all changes to social housing conditions, whether they represent an expansion or contraction of the social environment, are likely to result in at least short-term alterations in physiological systems that can impact biomedical research outcomes [e.g., Cohen et al., 1992; Clarke et al., 1995; Capitanio et al., 1998a]. That is, even if pair-housing is ultimately better for animals than single-housing, the change to being paired could influence research outcomes if animals are not given enough time to acclimate to their new social environment. Despite the importance of changes in social environment for macaques and for research, little empirical attention has been paid to the topic of how changes impact welfare and research, and what the time course is for biobehavioral responses to stabilize to the changed conditions. The literature regarding physiological responses to changed social conditions primarily focuses on two different systems, the stress responsive hypothalamic-pituitary-adrenal (HPA) axis and the immune system. It is important to consider that in order to understand the potential impact of social changes on biomedical research outcomes, any single measurement is likely insufficient. Nevertheless there appear to be some consistencies in the existing literature—expanding the social environment when animals are compatible social partners tends to improve well-being, while contracting the social environment tends to reduce it.
Expanding the social environment and matching animals for compatibility improves well-being
Available behavioral and physiological evidence suggests that expansions of the social environment reduce negative, and promote positive, outcomes for macaques. However, behavioral and physiological measures of well-being do not always cohere. For example, Schapiro and colleagues evaluated both behavioral and physiological outcomes in rhesus macaques as their housing changed from outdoor social group, to indoor single-housing, then indoor pair-housing, and finally group housing again, over the course of two years [Schapiro et al., 1993; Schapiro et al., 1996]. While these changes did not influence serum cortisol levels, there were significant reductions in the rate of abnormal behaviors when subjects moved from single- to pair-housing and from pair- to group-housing [Schapiro et al., 1993; Schapiro et al., 1996]. Thus, as measured via behavioral observations, the most complex social housing (groups) appears to provide the greatest benefit to well-being. It is unclear, however, exactly how subjects were selected for pairing or group formations and whether animals were assessed for compatibility prior to introductions. Incompatibility may explain the lack of a change in cortisol values; alternatively, other factors in the environment, such as increased activity, which can also stimulate higher cortisol levels, may have overridden any changes in this physiological marker due to the expanding social environment. This illustrates the need for multiple behavioral and physiological measures to understand the impact of social housing changes.
Evidence suggests that when compatibility among social partners is considered for making housing decisions, it has both behavioral and physiological benefits. For example, Doyle and colleagues [2008] investigated behavioral and physiological responses of young adult male rhesus macaques moved from single- to pair-housing and preselected for compatibility based on weight disparity. Indicators of physiological processing (heart rate telemetry and fecal cortisol levels), as well as anxious (shake, scratch, yawn, etc.) and abnormal (motor stereotypies, hair pluck, feces paint, etc.) behaviors improved significantly after subjects were paired [Doyle et al. 2008]. Compared to baseline values, fecal cortisol concentrations and anxious and abnormal behaviors were significantly lower during pair introductions, with only modest changes occurring thereafter as pairs lived together for longer. Heart rate was highest and most variable over the course of a day during all phases of pair introduction, low at baseline and just after introduction, and finally lowest and least variable 20 weeks or more after pairing. The long lasting reduction in heart rate and cortisol suggest substantive and socially-induced changes in the sensitivity of the sympathetic nervous system and the regulation of the HPA axis for paired males.
In a similar study, both male and female macaques that were moved from single-housing into isosexual pair-housing showed evidence of improved welfare even though pairs varied in compatibility [Baker et al., 2012a]. In contrast to Doyle et al [2008], Baker et al [2012a] measured serum, instead of fecal, cortisol and had limited ability to match based on compatibility. Despite this, anxious and abnormal behaviors were reduced in the paired condition for both males and females and for both dominant and subordinate animals. While paired, animals exhibited affiliative behavior, some aggressive behavior, and an increase in locomotor behaviors. Behavioral improvements were found even in pairs that were tolerable, but not ideal, matches (those with low affiliation and more low-level aggression). However, there was no change in serum cortisol in the study. It may be the case that differences in cortisol findings between Doyle et al [2008] and Baker et al [2012a] are due to the differences cortisol measures used (blood versus fecal), in the pre-selection of well-matched pairs, or in the distinction between tolerable versus ideal pairs. It is possible that tolerated social partners provide enough social enrichment and stress reduction to affect behavior, but not physiological measures such as cortisol.
Social housing status can also impact female reproductive outcomes, which are important for breeding colonies. Eaton et al [1994] experimentally manipulated the social housing of indoor breeding females to test differences in reproductive success. Females were randomly assigned to single- versus pair-housing, and then the paired females were randomly assigned a female pair-mate. After approximately three months in the experimental housing condition, females were briefly transferred to mate with a males and then transferred back to their experimental housing. There were no significant differences in ovulation or conception for single- versus pair-housed subjects. However, infant mortality was significantly higher for single-housed mothers and their surviving infants had poorer weight and growth trajectories [Eaton et al., 1994]. Their pair-housed subjects exhibited less abnormal behavior and spent 40% of their daytime hours and 80% of their nighttime hours in close physical contact with their pair-mate. Although reproductive and behavioral outcomes differed between housing conditions, no differences in immune measures (CBC and leucocyte proliferation) tested in this study.
While expansion of the social environment leads to positive psychosocial outcomes for captive NHPs, the initial introduction period can be stressful. During periods of social introductions social status and roles can be uncertain and until relationships are established, aggression, anxious behaviors, and biomarkers of stress (e.g., cortisol) can be high for a few hours or days, depending on the complexity of the social introduction [e.g., Clarke et al., 1995; Doyle et al., 2008]. The frequency of status signals is typically highest during the period immediately following introduction (about one week, as in [Eaton et al. 1994]) and decrease once dominance is established. Thus, macaques that are enrolled in a study in their paired condition should be well settled into their current pair to prevent unintended effects on study outcomes. The length of time required to be considered settled probably varies with the type of change (group to pair, weaning, single to pair, etc.), but currently the data is too limit to determine.
Contracting or destabilizing the social environment reduces well-being
Research on the consequences of contraction of the social environment (change from group- to pair- or single-housing, or from pair- to single-housing) suggests physiological alterations to hormonal and immune systems can last, at least for major social contractions, up to 3 months after the social relocation [Gordon et al., 1992; Gust et al., 1992; Gust et al., 1994; Capitanio et al., 1998a]. In juveniles, removal from a social group and relocation to either indoor single-housing [Gordon et al., 1992] or small group housing outdoors [Gust et al., 1992] resulted in alterations in glucocorticoid levels that lasted 2-8 weeks and to peripheral blood immune cell counts that lasted up to 11 weeks after the relocation. The impact of relocation on immunity in adults appears to be more variable. Research by Gust et al. [1994] examined the impact of single- versus pair-housing in adult female monkeys on cortisol and immune cell counts. Results indicate that the relocation from social group housing, regardless of the housing condition upon relocation (either single- or pair-housing), resulted in alterations in both cortisol levels and peripheral immune cell counts that persisted up to 96 hours later. Notably, while cortisol levels did not differ by housing condition (single versus pair), immune cell counts did—a greater decrease in lymphocytes was observed for females relocated to single-housing compared to pair-housing. Studies of adult males indicate that subjects relocated from large social groups to indoor single-housing can take 1-5 months to acclimate to this change based on cortisol levels and leukocyte numbers [Capitanio et al., 1998a]. Although these studies do not directly measure the impact of these hormone and immune related changes on biomedical research outcomes, a retrospective study of data from four primate research centers indicated housing relocations impacted survival after inoculation with SIV [Capitanio and Lerche, 1998]. A greater number of social separations in the 90 days prior to inoculation and in the 30 days after inoculation with SIV were associated with shorter survival. Finally, a review by Capitanio et al [2006] argued that three months is a reasonable expectation for macaques to acclimate following relocation from large, outdoor cages to individual cages indoors. It is unknown, however, how long the acclimation period might be for more modest changes (e.g., from pair- to single-housing for animals already adapted to indoor housing) or what the cumulative effect of repeated social changes is over the long run. Altogether these results indicate that physiological acclimation to social separation and relocation can take months and likely impacts biomedical research outcomes.
Contraction of the social environment affects different aspects of immunity in varying ways. Some measures remain consistent across social conditions, some measures change and then normalize over a relatively short period of time, and other measures change chronically. Benton et al [2013] found few changes in immune function (CD4+/CD8+ lymphocyte ratio, pro-inflammatory cytokines) and serum cortisol in sub-adult rhesus macaques moved from single, to pair, and then again to single-housing. However the study was of relatively short duration (<4 months) and subjects were randomly assigned to pair mates (not matched for compatibility). It is possible that a short period of random pairing does not create the enriched environment that social housing is intended to provide. When the study period is longer and subjects are housed with familiar social partners, there were differences in some measures of immunity (CD4+/CD8+ lymphocyte ratio, responses to four pathogens, responses to five mitogens, NK cell activity, and cytokine production) [Schapiro et al., 2000]. Subjects were removed from outdoor social groups to either pair- or single-housing, and changes in immunological values were assessed at baseline, 4, 8, and 12 month time points. Notably, some differences were identifiable even 12 months after relocation to new housing. All of the measured immune markers in Schapiro et al. [2000] tell a similar story—changes to social housing conditions result in changes to immunity that require time to resolve. Their results provide evidence that social housing changes likely alter an animal's immunity for at least several months. These results highlight the need for further research into how different social housing and changes to social housing impact a variety of aspects of immunity and therefore have consequences for research outcomes.
Taken together, these studies suggest that contraction of the social environment influences some measures of immunity and that the changes vary across social housing conditions (single versus paired versus group). Critically, not all measures show the same patterns and some measures fail to show differences across housing conditions at all [e.g., Lilly et al., 1999]. Some of these differences in immunity lasted up to 12 months suggesting either that animals were still responding to the new housing situation or that social housing type could alter immune system regulation either for a very long duration or possibly permanently. This is consistent with evidence showing that different socialization strategies (i.e., experimentally manipulated stable or unstable social conditions) can indeed alter regulation of the HPA axis and antibody responsiveness to immune challenge [Capitanio et al., 1998b]. A constantly changing or unstable social environment increases measures of stress with deleterious consequences for disease progression in male rhesus macaques. For example, there is a causal link from social stress, to lymphoid tissue and immune response changes, and proliferation of SIV [Sloan et al., 2007; Capitanio et al., 2008; Cole, 2008; Sloan et al., 2008a; Cole et al., 2009; Capitanio and Cole, 2015]. Adult male rhesus macaques exposed to daily changes in social partners exhibited increased innervation of their lymphoid tissues, a blunted HPA axis response to acute stress, and a causal link to weakened glucocorticoid regulations of leukocyte activity [Sloan et al., 2007; Sloan et al., 2008a; Sloan et al., 2008b; Cole et al., 2009]. The increased innervation of lymphoid tissues has also been associated with increased SIV replication [Sloan et al., 2006; Sloan et al., 2008b].
The literature reviewed in this paper demonstrates that different studies use different biomarkers to index stress and immunity on different scales and time periods of social change and this is creating heterogeneity in the literature. While measuring cortisol and activity of the HPA axis is one of the most prevalent ways to index stress, it is not the only stress response system. For example, the sympathetic adrenal medullary (SAM) system, a component of the autonomic nervous system, is also critically involved in how individuals respond to stress. The importance of understanding alterations in these other stress response systems is highlighted by the socially-induced plasticity seen in the patterns of lymph node innervation by the sympathetic nervous system [Sloan et al., 2007; Capitanio and Cole, 2015]. In addition, while cortisol is known to have potent impacts on immunity [Sapolsky et al., 2000], more detailed information is needed on how social housing conditions and changes not only alter stress responsive systems, but also exactly what immunological processes are impacted. Future research into how social housing changes influence well-being and research outcomes should investigate behavior and physiology using multiple measures concurrently to paint a more comprehensive picture of socially-induced changes that could affect research outcomes.
When looking normal is not enough
Behavioral observations are the most frequently used indicators to measure whether an animal has acclimated to a new social environment or stressor. While this is useful as a general assessment of the population, at the individual level it can be misleading due to variation in coping mechanisms and the expression of anxiety and abnormal behaviors [Mason and Latham, 2004]. The absence of behavioral indicators of distress may not mean an absence of physiological activation that can influence research results. Studies of laboratory chair restraint [Golub and Anderson, 1986; Ruys et al., 2004] found that animals exhibited anxiety-related behaviors with initial chair restraint, but relatively quickly animals appeared calm -- anxiety behaviors ceased with repeated exposure, and baseline cortisol levels returned to pre-restraint levels. Ruys et al. [2004] further demonstrated, however, that reduced cortisol was not due to reduced activation of the stress response, but rather to a blunting of the cortisol response (i.e., altered regulation of the HPA axis) during the repeated two-hour daily restraint sessions. Additional studies in squirrel monkeys have also shown that while distress vocalizations are reduced in infants after six 1-hr maternal separations (9-11 days apart), cortisol levels do not habituate and remain elevated across all six separations [Coe et al., 1983]. These findings show that dissociations between behavioral and physiological responses to repeated stress can occur. In future studies of laboratory macaque well-being related to socialization, it will be critical to measure multiple behavioral and biological markers to understand how they cope with social changes.
What Constitutes Social Housing “Success”?
Our review above highlights the inherent complexity and heterogeneity in measuring stress and well-being. Given this complexity, defining what constitutes social housing “success” is challenging. At the extremes, what constitutes successful and unsuccessful social housing by current standards established by facilities' practices, animal care recommendations, and regulatory guidelines at a given point in time is clear. At one extreme, pairs with reciprocal pro-social relationships characterized by frequent grooming, joint social signaling to humans or other monkeys, and clear dominance relationships without the presence of anxiety are considered successful. At the other extreme, pairs with repeated or severe injurious aggression are not considered successful. For most laboratory macaques, pairing outcomes are somewhere between those two extremes. There are a myriad of factors that influence the success of pairs and the long-term stability of their relationships including temperament, sex, reproductive state, age and body size, and social history [Capitanio et al., 2015; Truelove et al., 2015]. It is also important to recognize that success at one time point may not ensure success at a later time point. Compatible pairs can become incompatible over time. Many factors can influence the stability and indelibility of pairs, including changes associated with maturation into adulthood, changes in rank for individuals in a pair, change to the membership of the housing room (e.g., new monkeys that are housed across from the pair). The definition of “success” varies between research facilities, even among those that house the same species, and animals of the same age and weight in similar or identical caging [Baker et al., 2014b]. Some facilities consider a pair successful only if grooming or other pro-social behaviors are observed. Other facilities consider a pair successful if they do not hurt or constantly aggress each other. Still other facilities may consider any level of social wounding indicative of an unsuccessful pair, even though some amount of aggression, even minor bite and scratch wounds, is normal for rhesus macaques [Baker et al., 2014b]. There is no consensus on which of the above criteria result in optimal welfare largely because there is limited research available to inform them. We simply do not know, for example, if pairs that do not fight, but also do not affiliate, have better welfare, and provide a better biomedical model, together than apart. Given these complexities, the importance of future research on this topic is clear.
Discussion
The existing evidence suggests that changes to social housing matter not only for macaque well-being but also for physiology that can impact research outcomes. Consider a hypothetical study on cortisol reactivity to an acute experimental stressor with animals that have recently been relocated from large outdoor group enclosures to indoor housing (either paired or single). Changes in physiology and immunology are not just possible, but probable from just the housing change itself. These changes could last 3-5 months [Capitanio et al., 1998a; Capitanio et al., 1998b] or possibly a year or more [Schapiro et al., 2000]. Depending on the relevant measures for any given study, and based on our interpretation of some physiological and immunological parameters reported in Schapiro et al. [2000], indoor single or pair-housed macaques may have a different baseline after acclimation than when in outdoor social groups. As a result, reactivity to intended acute experimental stressors would likely be altered. Further, variance in outcome measures may occur as a result of acclimation over time to the new housing, with study results early in the experiment differing from those seen later in the experiment. Such patterns can be further complicated because an absence of behavioral indicators of stress does not necessarily indicate a return of physiological parameters to baseline [Ruys, et al., 2004].
While historically limitations on socialization have been viewed as necessary for most research, the evidence reviewed here suggests that past and even current practices may decrease validity by introducing an environment that animals are not well adapted to cope with and may result in outcomes that are less relevant to humans living in a social context. Investigators with limited knowledge of macaque behavioral biology might assume that single-housed subjects represent fewer problems for their research than pair-housed subjects; such subjects would not, however, represent a normal macaque and this has largely unknown repercussions for the translational value of findings. A social context is the first requirement for creating an adequately complex environment for a NHP research model. In many cases, limited social housing may impede research discoveries by introducing environmental contexts that have low repeatability, reproducibility, and external validity. While reproducibility and external validity are arguably a crisis for scientific research [e.g., Garner, 2014; Open Science Collaboration, 2015], when investigated, the causes are usually contextual [discussed in: Garner, 2014; Barrett, 2015], and thus highlight the importance of environmental contexts for biomedical research. Recent work demonstrates that social housing is possible in research settings that were previously believed to require non-social housing. For example, it used to be the case that animals with neural implants and head posts were not socially housed because there was a fear that social interaction could lead to injury or infection [Roberts and Platt, 2005]. Modern neuroscience laboratories, however, now readily pair such animals. At our facility, animals with implants even spend times in large outdoor cages with their pair-mates. Future advances in NHP biomedical research should endeavor to find more opportunities to incorporate socialization into research design where it has previously been eliminated. As much as is possible, investigators should conduct research on subjects living in a social setting approximating their normal social structure, with indoor pair-housing being next considered, and single-housing being considered as rarely as possible. While social housing is the default, many investigators request and receive exemptions. We are proposing that investigators and IACUCs reconsider what is possible for research in a social setting and to increase efforts to work toward incorporating a social context into the study design before considering eliminating it as an extraneous variable. Garner [2014] and Festing [Festing, 2014] provide examples of established, but underutilized, research methods (such as randomized block design) to account for increased environmental variability.
One of the challenges to evaluating the impact of social changes on macaque behavioral biology is that reports in the scientific literature are often not sufficient for thorough evaluation. In light of evidence about the impact of social housing changes (reviewed above) and variation in social housing status [Capitanio et al., 2015; Truelove et al., 2015] on animal well-being, it is therefore possible that some reported scientific effects may be the result of animals settling into new housing. A review by Carlsson et al. [2004] on the use of NHPs in research found that rarely are all critical life history details reported for laboratory NHPs. These details include rearing history, social housing status, previous study enrollments, or other aspects of subjects' environments that would allow readers to evaluate the extent to which study results are influenced by potential stressors in, or recent changes to, subjects' environments. We suggest that, moving forward, authors publishing studies using laboratory NHPs provide, at a minimum, rearing history, social housing status, and recent (within 1 year) housing changes (We note some journals now require reporting such information as part of the “Animal Research: Reporting In Vivo Experiments” [ARRIVE] guidelines [Kilkenny et al., 2010]). At the very least, it is to the benefit of scientific advancement if scientists who regularly employ limited social housing in research designs reconsider the impact that social housing status and changes to it have on their subjects, the variation that recent changes can stimulate, and the external validity of research produced from NHPs in limited social environments. Consideration of these details in research design will ultimately serve not only to improve animal welfare but also translational and comparative science.
Acknowledgments
The authors would like to thank Kate Baker and Amanda Dettmer-Erard for inviting us to make a contribution to this special issue. We would also like to thank Amy Nathman, Allison Barnard, and K. Adams [AWIC] for assistance with library materials and anonymous reviewers for valuable feedback on the manuscript.
References
- Anderson JR, Chamove AS. Self-aggression and social aggression in laboratory-reared macaques. Journal of Abnormal Psychology. 1980;89:539–550. doi: 10.1037//0021-843x.89.4.539. [DOI] [PubMed] [Google Scholar]
- Animal Plant and Health Inspection Service. Animal Care and Policy Manual. United States Department of Agriculture; 2011. http://www.aphis.usda.gov/animal_welfare/downloads/Animal%20Care%20Policy%20Manual.pdf: [Google Scholar]
- Animal Plant and Health Inspection Service. Annual report of animal usage by research facilities, fiscal year 2014. United States Department of Agriculture; 2014. Jul, http://www.aphis.usda.gov/animal_welfare/downloads/7023/Animals%20Used%20In%20Research%202014.pdf. 2015. [Google Scholar]
- Association for Assessment and Accreditation of Laboratory Animal Care International. [Accessed: 2015];Position Statement: Laboratory Animal. 2015a https://www.aaalac.org/accreditation/positionstatements.cfm#labanimals:
- Association for Assessment and Accreditation of Laboratory Animal Care International. [Accessed: 2015];Position Statements: Social. 2015b http://www.aaalac.org/accreditation/positionstatements.cfm#social:
- Association of Primate Veterinarians Scientific Advisory Committee. n.d. Socialization guidelines for nonhuman primates in biomedical research. [Accessed: 2015]; http://www.primatevets.org/Content/files/Public/education/APV%20Social%20Housing%20Guidelines%20final.pdf:
- Baker K. Enrichment and primate centers: Closing the gap between research and practice. Journal of Applied Animal Welfare Science. 2007;10:49–54. doi: 10.1080/10888700701277618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, Schoof V, Maloney M. Benefits of pair housing are consistent across a diverse population of rhesus macaques. Applied Animal Behaviour Science. 2012a;137:148–156. doi: 10.1016/j.applanim.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, Schoof VAM. Comparing options for pair housing rhesus macaques using behavioral welfare measures. American Journal of Primatology. 2014a;76:30–42. doi: 10.1002/ajp.22190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Coleman K, Bloomsmith MA, McCowan B, Truelove MA. Pairing rhesus macaques (Macaca mulatta): Methodology and outcomes at four national primate research centers. American Society of Primatologists; Decatur, GA: 2014b. p. 104. [Google Scholar]
- Baker KC, Crockett CM, Lee GH, Oettinger BC, Schoof V, Thom JP. Pair housing for female longtailed and rhesus macaques in the laboratory: Behavior in protected contact versus full contact. Journal of Applied Animal Welfare Science. 2012b;15:126–143. doi: 10.1080/10888705.2012.658330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker KC, Weed JL, Crockett CM, Bloomsmith MA. Survey of environmental enhancement programs for laboratory primates. American Journal of Primatology. 2007;69:377–394. doi: 10.1002/ajp.20347. [DOI] [PubMed] [Google Scholar]
- Barrett LF. Psychology Is Not in Crisis. The New York Times; 2015. p. 23. p Section A; Column 0; Editorial Desk; Op-Ed Contributor. [Google Scholar]
- Bayne K, Dexter S, Suomi S. A preliminary survey of the incidence of abnormal behavior in rhesus monkeys (Macaca mulatta) relative to housing condition. Lab Animal. 1992;21:38–46. [Google Scholar]
- Bayne K, Haines M, Dexter S, Woodman D, Evans C. Nonhuman primate wounding prevalence: A retrospective analysis. Lab Animal. 1995;24(4):40–44. [Google Scholar]
- Benton CG, West MW, Hall SM, Marko ST, Johnson JC. Effect of short-term pair housing of juvenile rhesus macaques (Macaca mulatta) on immunologic parameters. Journal of the American Association for Laboratory Animal Science. 2013;52:240–246. [PMC free article] [PubMed] [Google Scholar]
- Bernstein IS. Social housing of monkeys and apes: group formations. Laboratory Animal Science. 1991;41:329–333. [PubMed] [Google Scholar]
- Bernstein IS, Mason WA. Group formation by rhesus monkeys. Animal Behaviour. 1963;11:28–31. [Google Scholar]
- Branchi I, D'Andrea I, Santarelli S, Bonsignore LT, Alleva E. The richness of social stimuli shapes developmental trajectories: Are laboratory mouse pups impoverished? Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2011;35:1452–1460. doi: 10.1016/j.pnpbp.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Capitanio JP. Behavioral pathology. In: Mitchell G, Erwin J, editors. Comparative 680 primate biology, vol 2, part A: Behavior, conservation, and ecology. New York: Alan R. Liss; 1986. pp. 411–454. [Google Scholar]
- Capitanio JP. Nonhuman Primate Personality and Immunity: Mechanisms of Health and Disease. 2011:233–255. [Google Scholar]
- Capitanio JP, Abel K, Mendoza SP, Blozis SA, McChesney MB, Cole SW, Mason WA. Personality and serotonin transporter genotype interact with social context to affect immunity and viral set-point in simian immunodeficiency virus disease. Brain Behavior and Immunity. 2008;22:676–689. doi: 10.1016/j.bbi.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Blozis SA, Snarr J, Steward A, McCowan B. Do “birds of a feather flock together” or do “opposites attract”? Behavioral responses and temperament predict success in pairings of rhesus monkeys in a laboratory setting. American Journal of Primatology. 2015 doi: 10.1002/ajp.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Cole SW. Social instability and immunity in rhesus monkeys: The role of the sympathetic nervous system. Philosophical Transactions of the Royal Society 694 B-Biological Sciences. 2015;370 doi: 10.1098/rstb.2014.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Kyes RC, Fairbanks LA. Considerations in the selection and conditioning of Old World monkeys for laboratory research: Animals from domestic sources. Institute for Laboratory Animal Research Journal. 2006;47:294–306. doi: 10.1093/ilar.47.4.294. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Lerche NW. Social separation, housing relocation, and survival in simian AIDS: A retrospective analysis. Psychosomatic Medicine. 1998;60:235–244. doi: 10.1097/00006842-199805000-00001. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW. Individual differences in peripheral blood immunological and hormonal measures in adult male rhesus macaques (Macaca mulatta): Evidence for temporal and situational consistency. American Journal of Primatology. 1998a;44:29–41. doi: 10.1002/(SICI)1098-2345(1998)44:1<29::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Lerche NW, Mason WA. Social stress results in altered glucocorticoid regulation and shorter survival in simian acquired immune deficiency syndrome. Proceedings of the National Academy of Sciences. 1998b;95:4714–4719. doi: 10.1073/pnas.95.8.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Developmental Psychobiology. 2005;46:318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Carlsson HE, Schapiro SJ, Farah I, Hau J. Use of primates in research: A global overview. American Journal of Primatology. 2004;63:225–237. doi: 10.1002/ajp.20054. [DOI] [PubMed] [Google Scholar]
- Charnov EL, Berrigan D. Why do female primates have such long lifespans and so few babies? Or life in the slow lane. Evolutionary Anthropology: Issues, News, and Reviews. 1993;1:191–194. [Google Scholar]
- Clarke AS, Czekala NM, Lindburg DG. Behavioral and adrenocortical responses of male cynomolgus and lion-tailed macaques to social stimulation and group formation. Primates. 1995;36:41–56. [Google Scholar]
- Clarke MR, Harrison RM, Didier ES. Behavioral, immunological, and hormonal responses associated with social change in rhesus monkeys (Macaca mulatta) American Journal of Primatology. 1996;39:223–233. doi: 10.1002/(SICI)1098-2345(1996)39:4<223::AID-AJP3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Coe CL, Glass JC, Wiener SG, Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8:401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Ershler WB, Klopp RG. Influence of early rearing on lymphocyte proliferation responses in juvenile rhesus monkeys. Brain Behavior and Immunity. 1989;3:47–60. doi: 10.1016/0889-1591(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kaplan JR, Cunnick JE, Manuck SB, Rabin BS. Chronic social stress, affiliation, and cellular immune-response in nonhuman primates. Psychological Science. 1992;3:301–304. [Google Scholar]
- Cole SW. Social regulation of leukocyte homeostasis: The role of glucocorticoid sensitivity. Brain, Behavior, and Immunity. 2008;22:1049–1055. doi: 10.1016/j.bbi.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Mendoza SP, Capitanio JP. Social stress desensitizes lymphocytes to regulation by endogenous glucocorticoids: Insights from in vivo cell trafficking dynamics in rhesus macaques. Psychosomatic Medicine. 2009;71:591–597. doi: 10.1097/PSY.0b013e3181aa95a9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman K, Bloomsmith MA, Crockett CM, Weed JL, Schapiro SJ. Behavioral Management, Enrichment, and Psychological Well-being of Laboratory Nonhuman Primates. In: Abee CR, Mansfield K, Tardif S, Morris T, editors. Nonhuman Primates in Biomedical Research, Vol 1: Biology and Management. San Diego: Elsevier Academic Press Inc; 2012. pp. 149–176. [Google Scholar]
- Commission Recommendation. Commission recommendation of 18 June 2007 on guidelines for the accommodation and care of animals used for experimental and other scientific purposes. Official Journal of the European Union 3072007 L187/1-L197/88 2007 [Google Scholar]
- Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: Interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Bowers CL, Bowden DM, Sackett GP. Sex differences in compatibility of pair-housed adult longtailed macaques. American Journal of Primatology. 1994;32:73–94. doi: 10.1002/ajp.1350320202. [DOI] [PubMed] [Google Scholar]
- Crook JH, Gartlan JS. Evolution of primate societies. Nature. 1966;210:1200–1203. doi: 10.1038/2101200a0. [DOI] [PubMed] [Google Scholar]
- Directive of the European Parliament. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of the European Union 20102010 L276/33-L276/79 [Google Scholar]
- DiVincenti L, Wyatt JD. Pair Housing of Macaques in Research Facilities: A Science-Based Review of Benefits and Risks. Journal of the American Association for Laboratory Animal Science. 2011;50:856–863. [PMC free article] [PubMed] [Google Scholar]
- Doyle LA, Baker KC, Cox LD. Physiological and behavioral effects of social introduction on adult male rhesus macaques. American Journal of Primatology. 2008;70:542–550. doi: 10.1002/ajp.20526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar RIM. Primate social systems. Ithaca, New York: Cornell University Press; 1988. [Google Scholar]
- Dunbar RIM. The social brain hypothesis. Evolutionary Anthropology. 1998;6:178–190. [Google Scholar]
- Eaton GG, Kelley ST, Axthelm MK, Iliff-Sizemore SA, Shiigi SM. Psychological well-being in paired adult female rhesus (Macaca mulatta) American Journal of Primatology. 1994;33:89–99. doi: 10.1002/ajp.1350330204. [DOI] [PubMed] [Google Scholar]
- Festing MFW. Randomized Block Experimental Designs Can Increase the Power and Reproducibility of Laboratory Animal Experiments. ILAR Journal. 2014;55:472–476. doi: 10.1093/ilar/ilu045. [DOI] [PubMed] [Google Scholar]
- Fontenot MB, Wilkes MN, Lynch CS. Effects of outdoor housing on self-injurious and stereotypic behavior in adult male rhesus macaques (Macaca mulatta) Journal of the American Association for Laboratory Animal Science. 2006;45:35–43. [PubMed] [Google Scholar]
- Fooden J. Systematic review of the rhesus macaque, Macaca mulatta (Zimmermann, 1780) Fieldiana Zoology. 2000;96:1–180. [Google Scholar]
- Garner JP. Stereotypies and other abnormal repetitive behaviors: Potential impact on validity, reliability, and replicability of scientific outcomes. Institute for Laboratory Animal Research Journal. 2005;46:106–117. doi: 10.1093/ilar.46.2.106. [DOI] [PubMed] [Google Scholar]
- Garner JP. The significance of meaning: Why do over 90% of behavioral neuroscience results fail to translate to humans, and what can we do to fix it? Institute for Laboratory Animal Research Journal. 2014;55:438–456. doi: 10.1093/ilar/ilu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MH, Baker KC. Social buffering in adult male rhesus macaques (Macaca mulatta): Effects of stressful events in single vs. pair housing. Journal of Medical Primatology. 2011;40:71–78. doi: 10.1111/j.1600-0684.2010.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golub MS, Anderson JH. Adaptation of pregnant rhesus monkeys to short-term chair restraint. Laboratory Animal Science. 1986;36:507–511. [PubMed] [Google Scholar]
- Gordon TP, Gust DA, Wilson ME, Ahmedansari A, Brodie AR, McClure HM. Social separation and reunion affects immune-system in juvenile rhesus monkeys. Physiology & Behavior. 1992;51:467–472. doi: 10.1016/0031-9384(92)90166-y. [DOI] [PubMed] [Google Scholar]
- Gottlieb DH, Capitanio JP, McCowan B. Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): Animal's history, current environment, and personality. American Journal of Primatology. 2013;75:995–1008. doi: 10.1002/ajp.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DH, Ghirardo S, Minier DE, Sharpe N, Tatum L, McCowan B. Efficacy of 3 Types of Foraging Enrichment for Rhesus Macaques (Macaca mulatta) Journal of the American Association for Laboratory Animal Science. 2011;50:888–894. [PMC free article] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Brodie AR, McClure HM. Effect of a preferred companion in modulating stress in adult female rhesus monkeys. Physiology & Behavior. 1994;55:681–684. doi: 10.1016/0031-9384(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Hambright MK. Response to removal from and return to a social group in adult male rhesus monkeys. Physiology & Behavior. 1993;53:599–602. doi: 10.1016/0031-9384(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Ahmed-Ansari A, Brodie AR, McClure HM. Formation of a new social group of unfamiliar female rhesus monkeys affects the immune and pituitary adrenocortical systems. Brain, Behavior, and Immunity. 1991;5:296–307. doi: 10.1016/0889-1591(91)90024-5. [DOI] [PubMed] [Google Scholar]
- Gust DA, Gordon TP, Wilson ME, Brodie AR, Ahmedansari A, McClure HM. Removal from natal social group to peer housing affects cortisol levels and absolute numbers of t-cell subsets in juvenile rhesus monkeys. Brain Behavior and Immunity. 1992;6:189–199. doi: 10.1016/0889-1591(92)90018-j. [DOI] [PubMed] [Google Scholar]
- Hannibal DL, Beisner B, Cital S, Maness A, Kashyap N, McCowan B, Sammak R. Effect of Adult Male Canine Tooth Modification on Group Welfare in Rhesus Macaques. American Association for Laboratory Animal Science National Meeting; San Antonio, TX. 2014. [Google Scholar]
- Harlow HF, Dodsworth RO, Harlow MK. Total social isolation in monkeys. Proceedings of the National Academy of Sciences. 1965;54:90–97. doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Harlow MK. Social deprivation in monkeys. Scientific American. 1962;207:136–146. doi: 10.1038/scientificamerican1162-136. [DOI] [PubMed] [Google Scholar]
- Harvey PH, Clutton-Brock TH. Life history variation in primates. Evolution. 1985;39:559–581. doi: 10.1111/j.1558-5646.1985.tb00395.x. [DOI] [PubMed] [Google Scholar]
- Hinde RA. Interactions, relationships and social structure. Man. 1976;11:1–17. [Google Scholar]
- Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “Adverse” Environment? Interactions of Rearing Experiences and MAOA Genotype in Rhesus Monkeys. Biological Psychiatry. 2009;65:770–777. doi: 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling ME, Wolf RH. Medical management of the rhesus monkey In: Bourne GH, editor The rhesus monkey: Management, reproduction, and pathology. New York, NY: Academic Press; 1975. pp. 11–96. [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biology. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenslager ML, Boccia ML, Berger CL, Gennaroruggles MM, McFerran B, Reite ML. Total cortisol, free cortisol, and growth-hormone associated with brief social separation experiences in young macaques. Developmental Psychobiology. 1995;28:199–211. doi: 10.1002/dev.420280402. [DOI] [PubMed] [Google Scholar]
- Lee PC. The meanings of weaning: growth, lactation, and life history. Evolutionary Anthropology. 1996;5:87–96. [Google Scholar]
- Lilly AA, Mehlman PT, Higley JD. Trait-like immunological and hematological measures in female rhesus across varied environmental conditions. American Journal of Primatology. 1999;48:197–223. doi: 10.1002/(SICI)1098-2345(1999)48:3<197::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Lubach GR, Coe CL, Ershler WB. Effects of early rearing environment on immune-responses of infant rhesus monkeys. Brain Behavior and Immunity. 1995;9:31–46. doi: 10.1006/brbi.1995.1004. [DOI] [PubMed] [Google Scholar]
- Lutz C, Tiefenbacher S, Meyer J, Novak M. Extinction deficits in male rhesus macaques with a history of self-injurious behavior. American Journal of Primatology. 2004;63:41–48. doi: 10.1002/ajp.20037. [DOI] [PubMed] [Google Scholar]
- Lutz C, Well A, Novak M. Stereotypic and self-injurious behavior in rhesus macaques: A survey and retrospective analysis of environment and early experience. American Journal of Primatology. 2003;60:1–15. doi: 10.1002/ajp.10075. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Davis EB, Ruggiero AM, Suomi SJ. Early predictors of self-biting in socially-housed rhesus macaques (Macaca mulatta) American Journal of Primatology. 2007;69:584–590. doi: 10.1002/ajp.20370. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Novak MA. Environmental enrichment for nonhuman primates: theory and application. Institute for Laboratory Animal Research Journal. 2005a;46:178–191. doi: 10.1093/ilar.46.2.178. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Novak MA. Primate natural history and social behavior: implications for laboratory housing. In: Wolfe-Coote S, editor. The Laboratory Primate. San Diego: Elsevier Academic Press; 2005b. pp. 133–142. [Google Scholar]
- Lynch R. Successful pair-housing of male macaques. Laboratory Primate Newsletter. 1998;37:4–5. [Google Scholar]
- Mason GJ, Latham NR. Can't stop, won't stop: is stereotypy a reliable animal welfare indicator? Animal Welfare. 2004;13:S57–S69. [Google Scholar]
- Mineka S, Suomi SJ. Social separation in monkeys. Psychological Bulletin. 1978;85:1376–1400. [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academies Press; 1996. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- NC3Rs. Primate accomodations, care and use. London: 2006. [Google Scholar]
- Novak MA. Self-injurious behavior in rhesus monkeys: New insights into its etiology, physiology, and treatment. American Journal of Primatology. 2003;59:3–19. doi: 10.1002/ajp.10063. [DOI] [PubMed] [Google Scholar]
- Novak MA, Suomi SJ. Psychological well-being of primates in captivity. American Psychologist. 1988;43:765–773. doi: 10.1037//0003-066x.43.10.765. [DOI] [PubMed] [Google Scholar]
- O'Neill PL, Novak MA, Suomi SJ. Normalizing laboratory-reared rhesus macaque (Macaca mulatta) behavior with exposure to complex outdoor enclosures. Zoo Biology. 1991;10:237–245. [Google Scholar]
- Office of Laboratory Animal Welfare. National Institutes of Health Website; 2015. http://grants.nih.gov/grants/olaw/faqs.htm#1655. [Google Scholar]
- Open Science Collaboration Estimating the reproducibility of psychological science. Science. 2015;349:943–951. doi: 10.1126/science.aac4716. [DOI] [PubMed] [Google Scholar]
- Paylor R. Questioning standardization in science. Nature Methods. 2009;6:253–254. doi: 10.1038/nmeth0409-253. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, 't Hart BA, Hopkins WD, Hu SL, Miller LA, Nader MA, et al. Why primate models matter. American Journal of Primatology. 2014;76:801–827. doi: 10.1002/ajp.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott MJ, Nixon ME, Farningham DAH, Naiken S, Griffiths MA. Laboratory macaques: When to wean? Applied Animal Behaviour Science. 2012;137:194–207. [Google Scholar]
- Reinhardt V, Cowley D, Eisele S. Serum cortisol concentrations of single-housed and isosexually pair-housed adult rhesus macaques. Journal of Experimental Animal Science. 1991;34:73–76. [PubMed] [Google Scholar]
- Reinhardt V, Liss C, Stevens C. Social housing of previously single-caged macaques - what are the options and the risks. Animal Welfare. 1995;4:307–328. [Google Scholar]
- Reinhardt V, Reinhardt A. Social enhancement for adult nonhuman primates in research laboratories: A review. Lab Animal. 2000;29:34–41. [Google Scholar]
- Richter SH, Garner JP, Auer C, Kunert J, Wurbel H. Systematic variation improves reproducibility of animal experiments. Nature Methods. 2010;7:167–168. doi: 10.1038/nmeth0310-167. [DOI] [PubMed] [Google Scholar]
- Richter SH, Garner JP, Wurbel H. Environmental standardization: cure or cause of poor reproducibility in animal experiments? Nature Methods. 2009;6:257–261. doi: 10.1038/nmeth.1312. [DOI] [PubMed] [Google Scholar]
- Richter SH, Garner JP, Zipser B, Lewejohann L, Sachser N, Touma C, Schindler B, Chourbaji S, Brandwein C, Gass P, et al. Effect of population heterogenization on the reproducibility of mouse behavior: A multi-laboratory study. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SJ, Platt ML. Effects of isosexual pair-housing on biomedical implants and study participation in male macaques. Contemporary Topics in Laboratory Animal Science. 2005;44:13–18. [PubMed] [Google Scholar]
- Rommeck I, Anderson K, Heagerty A, Cameron A, McCowan B. Risk factors and remediation of self-injurious and self-abuse behavior in rhesus macaques. Journal of Applied Animal Welfare Science. 2009a;12:61–72. doi: 10.1080/10888700802536798. [DOI] [PubMed] [Google Scholar]
- Rommeck I, Capitanio J, Strand SC, McCowan B. Early social experience affects behavioral and physiological responsiveness to stressful conditions in infant rhesus macaques (Macaca mulatta) American Journal of Primatology. 2011;73:692–701. doi: 10.1002/ajp.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommeck I, Gottlieb DH, Strand SC, McCowan B. The effects of four nursery rearing strategies on infant behavioral development in rhesus macaques (Macaca mulatta) Journal of the American Association for Laboratory Animal Science. 2009b;48:395–401. [PMC free article] [PubMed] [Google Scholar]
- Ruys JD, Mendoza SP, Capitanio JP, Mason WA. Behavioral and physiological adaptation to repeated chair restraint in rhesus macaques. Physiology & Behavior. 2004;82:205–213. doi: 10.1016/j.physbeh.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ. Effects of social manipulations and environmental enrichment on behavior and cell-mediated immune responses in rhesus macaques. Pharmacology Biochemistry and Behavior. 2002;73:271–278. doi: 10.1016/s0091-3057(02)00779-7. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Kessel AL, Shively CA. Effects of enrichment and housing on cortisol response in juvenile rhesus monkeys. Applied Animal Behaviour Science. 1993;37:251–263. [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Porter LM, Suarez SA. Enrichment effects on rhesus monkeys successively housed singly, in pairs, and in groups. Applied Animal Behaviour Science. 1996;48:159–171. [Google Scholar]
- Schapiro SJ, Nehete PN, Perlman JE, Sastry KJ. A comparison of cell-mediated immune responses in rhesus macaques housed singly, in pairs, or in groups. Applied Animal Behaviour Science. 2000;68:67–84. doi: 10.1016/s0168-1591(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Schumann K, Guenther A, Jewgenow K, Trillmich F. Animal housing and welfare: Effects of housing conditions on body weight and cortisol in a medium-sized rodent (Cavia aperea) Journal of Applied Animal Welfare Science. 2014;17:111–124. doi: 10.1080/10888705.2014.884407. [DOI] [PubMed] [Google Scholar]
- Sloan EK, Capitanio JP, Cole SW. Stress-induced remodeling of lymphoid innervation. Brain Behavior and Immunity. 2008a;22:15–21. doi: 10.1016/j.bbi.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: Mechanisms and implications for viral pathogenesis. Journal of Neuroscience. 2007;27:8857–8865. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Nguyen CT, Cox BF, Tarara RP, Capitanio JP, Cole SW. SIV infection decreases sympathetic innervation of primate lymph nodes: The role of neurotrophins. Brain Behavior and Immunity. 2008b;22:185–194. doi: 10.1016/j.bbi.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan EK, Tarara RP, Capitanio JP, Cole SW. Enhanced replication of simian immunodeficiency virus adjacent to catecholaminergic varicosities in primate lymph nodes. Journal of virology. 2006;80:4326–4335. doi: 10.1128/JVI.80.9.4326-4335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns SC. The evolution of life histories. New York: Oxford University Press; 1992. [Google Scholar]
- Suomi SJ, Collins ML, Harlow HF. Effects of permanent separation from mother on infant monkeys. Developmental Psychology. 1973;9:376–384. [Google Scholar]
- Suomi SJ, Eisele CD, Grady SA, Harlow HF. Depressive behavior in adult monkeys following separation from family environment. Journal of Abnormal Psychology. 1975;84:576–578. doi: 10.1037/h0077066. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Harlow HF, Kimball SD. Behavioral effects of prolonged partial social isolation in rhesus monkey. Psychological Reports. 1971;29:1171–1177. doi: 10.2466/pr0.1971.29.3f.1171. [DOI] [PubMed] [Google Scholar]
- Thierry B, Iwaniuk AN, Pellis SM. The influence of phylogeny on the social behaviour of macaques (Primates: Cercopithecidae, genus Macaca) Ethology. 2000;106:713–728. [Google Scholar]
- Thierry BR. Social epigenesis. In: Thierry BR, Singh M, Kaumanns W, editors. Macaque societies: A model for the study of social organization. New York: Cambridge Univ Press; 2004. pp. 267–290. [Google Scholar]
- Truelove MA, Martin AL, Perlman JE, Wood JS, Bloomsmith MA. Pair housing of macaques: A review of partner selection, introduction techniques, monitoring for compatibility, and methods for long-term maintenance of pairs. American Journal of Primatology. 2015 doi: 10.1002/ajp.22485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture. Animal Welfare Act and Animal Welfare Regulations. National Agricultural Library; 2013. p. 143. http://awic.nal.usda.gov/government-and-professional-resources/federal-laws/animal-welfare-act: [Google Scholar]
- van Schaik CP, van Hooff JARAM. On the ultimate causes of primate social systems. Behaviour. 1983;85:91–117. [Google Scholar]
- Vandeleest J, McCowan B, Capitanio J. Early rearing interacts with temperament and housing to influence the risk for motor stereotypy in rhesus monkeys (Macaca mulatta) Applied Animal Behaviour Science. 2011;132:81–89. doi: 10.1016/j.applanim.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard GC, Izard MK, Drake JH. Reproductive performance of rhesus macaques (Macaca mulatta) in two outdoor housing conditions. American Journal of Primatology. 2000;50:87–93. doi: 10.1002/(SICI)1098-2345(200001)50:1<87::AID-AJP8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Wurbel H. Behaviour and the standardization fallacy. Nature Genetics. 2000;26:263–263. doi: 10.1038/81541. [DOI] [PubMed] [Google Scholar]
- Xie L, Zhou QM, Liu SG, Xu F, Shively CA, Wu QY, Gong W, Ji YJ, Fang L, Li LL, et al. Effect of Living Conditions on Biochemical and Hematological Parameters of the Cynomolgus Monkey. American Journal of Primatology. 2014;76:1011–1024. doi: 10.1002/ajp.22285. [DOI] [PubMed] [Google Scholar]