To the Editor
A 20-year-old man was admitted to a tertiary referral trauma center for management of wound sepsis and femoral stump osteomyelitis in the setting of recent below-knee amputation following a high-speed motorbike accident. He received multiple antimicrobials (Figure 1A) for bacteremia and fever (>38.3°C) and subsequently developed a blistering rash involving more than 30% of body surface area associated with a positive Nikolsky sign (Figure 1B), consistent with toxic epidermal necrolysis (TEN). Antimicrobial therapy was changed and he received intravenous immunoglobulin therapy, surgical debridement, and pulse steroid therapy with full recovery.
FIGURE 1.
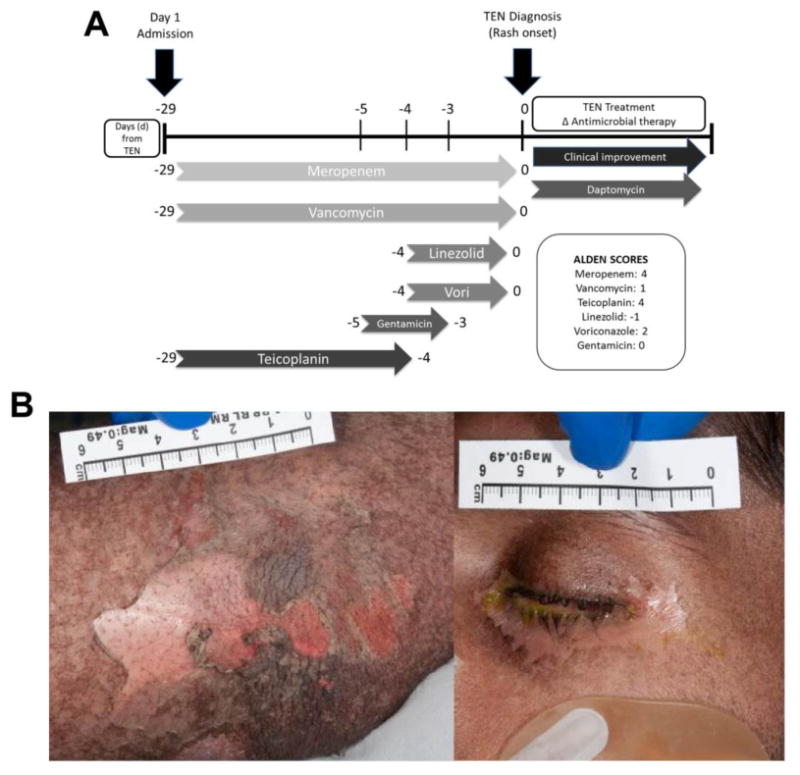
Implicated drug timeline and clinical images for a case of teicoplanin-induced TEN. A, Antbiotic timeline: Implicated antibiotics with corresponding ALDEN scores.9 B, Pictorial presentation: Clinical photography at day 4 of TEN demonstrating widespread skin (positive Nikolsky sign) and ocular involvement (conjunctivitis). ALDEN, Algorithm of drug causality of epidermal necrolysis; APC, allophycocyanin; Vori, voriconazole. ALDEN score definitions9: less than 0, very unlikely; 0 to 1, unlikely; 2 to 3, possible; 4 to 5, probable; 6 or more, very probable.
IFN-y release in response to overnight incubation with implicated antibiotics was measured by enzyme-linked immunospot assay (ELISpot) in triplicate as previously described1 on PBMCs from day 4 post-TEN onset (time point 1), with an unstimulated and positive control (anti-CD3 antibody; Mabtech, Victoria, Australia). PBMCs from time point 1 were stored at −80°C in liquid nitrogen before being used for IFN-γ release ELISpot 30 days after initial collection. One million PBMCs were incubated with 100 μg/mL of candidate drugs for 18 hours at 37°C. T-cell stimulation was assessed by measuring upregu-lation of the early activation marker CD137, a member of the TNF receptor family, on viable CD3+CD8+ T cells by flow cytometry. Four-digit HLA ABC DR DQ DP typing (Miseq, Illumina, San Diego, Calif) was performed in the American Society for Histocompatibility and Immunogenetics and National Association of Testing Authorities–accredited laboratories of the Institute for Immunology and Infectious Diseases.
IFN-γ ELISpot assay 4 days following clinical recognition of TEN onset showed positive responses only to the glycopeptide antibiotic teicoplanin (Figure 2A). In addition, at this same time point, only teicoplanin induced upregulation of the activation marker CD137 (0.47% of total CD8+ T cells) (Figure 2B). Teicoplanin-specific responses were absent 5 and 8.5 months post-TEN by both flow cytometry and IFN-γ ELISpot using the same methodology (data not shown). In healthy donors (controls), teicoplanin did not induce the upre-gulation of CD137+ CD8+ T cells by flow cytometry and failed to elict IFN-γ release on ELISpot when incubated with up to 100 μg/mL of teicoplanin (data not shown).
FIGURE 2.
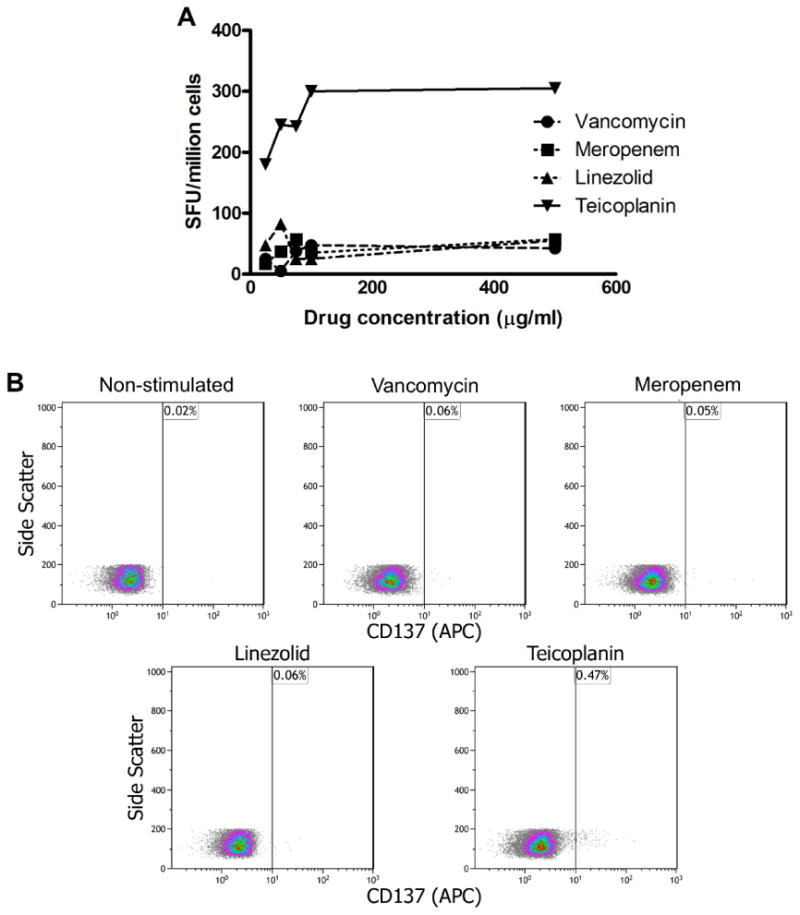
IFN-γ ELIspot and flow cytometry results for a case of teicoplanin-induced TEN. A, IFN-γ ELIspot day 4 of TEN: Responses (spot-forming units [SFUs]/mL) were determined by subtracting the mean of the negative control wells from the mean of triplicate stimulated wells, with a positive result defined as more than 50 SFUs/million cells. Teicoplanin was the only antibiotic to stimulate strong consistent dose-dependent IFN-γ responses. B, Drug-induced T-cell activation (flow cytometry): Teicoplanin was the only antibiotic to induce reproducible CD137 expression on CD8+ Tcells at day 4 of illness. APC, Allophycocyanin.
Patch testing was performed 6 months post-TEN onset using 0.005% vancomycin, 0.05% vancomycin, 4% teicoplanin, and 5% meropenem in white petroleum jelly. Negative responses to all drugs and controls (white petroleum jelly alone) were apparent after patch removal at 48 hours and 72 hours (no skin reaction at testing site). HLA class I/II typing showed the patient to carry HLA-A*01:01 and 02:02, HLA-B*37:01 and 41:01, HLA-C*10:01 and 17:01, HLA-DPB1*10:01, HLA-DQA1*01:01 and 02:01, HLA-DQB1*02:02 and 05:01, and HLA-DRB1*07:01 and 10:01.
We highlight a unique case of teicoplanin-induced TEN, without demonstrable ex vivo cross-reactivity with the glyco-peptide vancomycin. For this adverse drug reaction, multiple antibiotics with high likelihood of drug causality of epidermal necrolysis (Algorithm of drug causality of epidermal necrolysis [ALDEN]) scores were implicated. Indeed, based on ALDEN score and the usual latency period of Steven-Johnson syndrome (SJS)/TEN of 4 to 28 days, meropenem may have been initially considered the most likely causative drug. Teicoplanin was discontinued before noted onset of blisters; however, this drug has a long half-life of 72 hours and would have been present in therapeutic concentrations at the time of TEN onset. Given the complexity of this patient's treatment, which involved multiple potential implicated antibiotics and negative patch testing, which has been previously noted to have poor sensitivity in SJS/TEN phenotypes, ex vivo IFN-γ ELISpot and flow cytometry were useful tools to identify the implicated causal antibiotic. IFN-γ ELISpot studies have demonstrated cytokine responses to drugs, up to 20 years after initial stimulation.2-4 Our results however highlight the potential waning of response with time. Using flow cytometry to demonstrate selective upregulation of CD137 on CD8+ T cells in the presence of teicoplanin suggests that live cell approaches are modalities to identify and characterize drug-specific activated T cells in SJS/TEN.
IFN-γ ELISpot has previously been used to assess hypersen-sitivity reactions to a limited number of antiinfectives.4–7 ELI-Spot has also been used to help confirm T-cell–mediated hypersensitivity against cephalosporins, piperacillin, ticarcillin, meropenem, amikacin, and aztreonam. The noted high specificity of this test would be useful to the clinician in the case of a positive result. Further research is required to evaluate this testing and factors potentially impeding sensitivity.
Using a combination of clinical causality assessment and ex vivo data teicoplanin was implicated as the cause of SJS/TEN in this patient. As far as we are aware, this is the first reported combined use of IFN-γ ELISpot and flow cytometry to assign antimicrobial causality to TEN. Interestingly, and not readily explainable in our patient given the CD8+ T-cell dependency of SJS/TEN and expected long-lasting memory T-cell response, a diminished drug-specific ex vivo response was noted over time, highlighting the potential importance of performing assays during the acute or early recovery phase of drug reactions. This diminishing response in the peripheral blood requires further explanation although it is intriguing to hypothesize that in the later recovery phase in at least some cases of SJS/TEN drug-specific skin, resident CD8+ T cells are housed at the dermal-epidermal junction and drug-specific effector memory CD8+ T cells may be rare or absent in the peripheral blood. Prolonged expression of CD8+CD137+ T cells in the periphery in the absence of drug reexposure may be related in part to chronic low-level viral stmulation that maintains the response.5,8 The absence of a positive patch test is likely to be related to the known poor sensitivity of this testing modality in SJS/TEN phenotypes,6 rather than the absence of resident effector CD8+ T cells.
Although confirmatory intravenous challenge with vancomy-cin was not performed, the patient tolerated oral beta-lactam antibiotics (amoxicillin, flucloxacillin). The use of cellular ex vivo techniques such as IFN-γ ELISpot and flow cytometry in the early recovery phase of antibiotic-associated delayed (T-cell–mediated) reactions have the potential to contribute to clinical drug causality assessments in severe cutaneous adverse reactions, as highlighted by Porebski et al7 who demonstrated the utility of granzyme-B ELISpot and flow cytometry for primarily non–antibiotic-associated SJS or TEN. TEN is a severe disease for which rechallenge is associated with significant risk, and currently available in vivo diagnostics such as skin testing show poor sensitivity. Therefore, as our case illustrates, ex vivo diagnostics such as flow cytometry and IFN-γ ELISpot are potentially useful combined adjunctive tools that deserve further validation in larger controlled studies.
Clinical Implications.
There are major challenges defining drug causality in cases of severe cutaneous adverse reactions (SCAR) involving multiple antibiotics. We describe a case of toxic epidermal necrolysis (TEN) occurring in the setting of multiple antibiotics and the use of IFN-γ enzyme-linked immunospot assay and flow cytometry to clarify causality.
Acknowledgments
We thank the multiple treating teams involved in the patient care and the patient for donation of blood for these experiments.
This work was supported in part by the National Institutes of Health (NIH; grant nos. 1P50GM115305-01 and 1R01AI103348-01 to E.P.), NIH-funded Tennessee Center for AIDS Research (grant no. P30 AI110527), and the NHMRC. J.A.T. was supported by a National Health and Medical Research Council (NHMRC) postgraduate scholarship and Austin Medical Research Foundation (AMRF) grant.
R. Pavlos has received research support from the NHMRC and the Australian Centre for HIV and Hepatitis Virology Research. E. Phillips has received research support from NHMRC Australia, the National Institutes of Health, and ACH2 Australia; has received personal fees from UptoDate; has received honoraria as a consultant from Biocryst; is codirector of the company holding a patent for HLA-B*57:01 testing for abacavir HSR; and has received a nondirect consultancy fee from Aicuris.
Footnotes
Conflicts of interest: The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Keane NM, Roberts SG, Almeida CA, Krishnan T, Chopra A, Demaine E, et al. High-avidity, high-IFNγ-producing CD8 T-cell responses following immune selection during HIV-1 infection. Immunol Cell Biol. 2012;90:224–34. doi: 10.1038/icb.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beeler A, Engler O, Gerber BO, Pichler WJ. Long-lasting reactivity and high frequency of drug-specific T cells after severe systemic drug hypersensitivity reactions. J Allergy Clin Immunol. 2006;117:455–62. doi: 10.1016/j.jaci.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 3.Rozieres A, Hennino A, Rodet K, Gutowski MC, Gunera-Saad N, Berard F, et al. Detection and quantification of drug-specific T cells in penicillin allergy. Allergy. 2009;64:534–42. doi: 10.1111/j.1398-9995.2008.01674.x. [DOI] [PubMed] [Google Scholar]
- 4.Pavlos R, Mallal S, Ostrov D, Buus S, Metushi I, Peters B, et al. T cell-mediated hypersensitivity reactions to drugs. Annu Rev Med. 2015;66:439–54. doi: 10.1146/annurev-med-050913-022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu J, Peng T, Johnston C, Phasouk K, Kask AS, Klock A, et al. Immune surveillance by CD8αα+ skin-resident T cells in human herpes virus infection. Nature. 2013;497:494–7. doi: 10.1038/nature12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbaud A, Collet E, Milpied B, Assier H, Staumont D, Avenel-Audran M, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555–62. doi: 10.1111/bjd.12125. [DOI] [PubMed] [Google Scholar]
- 7.Porebski G, Pecaric-Petkovic T, Groux-Keller M, Bosak M, Kawabata TT, Pichler WJ. In vitro drug causality assessment in Stevens-Johnson syndrome— alternatives for lymphocyte transformation test. Clin Exp Allergy. 2013;43:1027–37. doi: 10.1111/cea.12145. [DOI] [PubMed] [Google Scholar]
- 8.Pavlos R, Mallal S, Ostrov D, Pompeu Y, Phillips E. Fever, rash, and systemic symptoms: understanding the role of virus and HLA in severe cutaneous drug allergy. J Allergy Clin Immunol Pract. 2014;2:21–33. doi: 10.1016/j.jaip.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sassolas B, Haddad C, Mockenhaupt M, Dunant A, Liss Y, Bork K, et al. ALDEN, an algorithm for assessment of drug causality in Stevens-Johnson Syndrome and toxic epidermal necrolysis: comparison with case-control analysis. Clin Pharmacol Therapeut. 2010;88:60–8. doi: 10.1038/clpt.2009.252. [DOI] [PubMed] [Google Scholar]


