Abstract
Inositol requiring enzyme 1 (IRE1) is a conserved sensor of the unfolded protein response (UPR) that has protein kinase and endoribonuclease (RNase) enzymatic activities and thereby initiates HAC1/XBP1 splicing. Previous studies demonstrated that human IRE1α (hIRE1α) does not cleave S. cerevisiae HAC1 mRNA. Using an in vitro cleavage assay, we show that adenine to cytosine nucleotide substitution at the +1 position in the 3’ splice site of HAC1 RNA is required for specific cleavage by hIRE1α. A similar restricted nucleotide specificity in the RNA substrate was observed for XBP1 splicing in vivo. Together these findings underscore the essential role of cytosine nucleotide at +1 in the 3’ splice for determining cleavage specificity of hIRE1α.
Keywords: IRE1, RNase, XBP1, HAC1
Introduction
Protein folding homeostasis in the endoplasmic reticulum (ER) is critical for cellular function. Extracellular and intracellular perturbations e.g., chemical treatments and/or physiological conditions result in the accumulation of unfolded/misfolded proteins in the ER [1]. The unfolded protein response (UPR) is an evolutionarily conserved signaling pathway in all eukaryotic cells designed to correct the challenged ER protein folding environment [2–4].
IRE1 (Inositol requiring enzyme 1) is a sensor that detects the accumulation of unfolded proteins in the ER lumen. It possesses dual catalytic functions, protein kinase and endoribonuclease (RNase) activities [5, 6]. Under conditions of ER stress, S. cerevisiae Ire1 (Ire1p) is activated by trans-autophosphorylation that elicits its RNase function to initiate unconventional splicing of HAC1 mRNA to remove a 252 nt intron [7, 8], causing a translational frame-shift to produce spliced form of HAC1 mRNA [9, 10]. Spliced HAC1 mRNA is translated into fully active Hac1p, a basic leucine zipper transcription factor, which induces transcription by binding to a 22-bp UPR element (UPRE) located in the promoter regions of genes encoding many functions in the early secretory pathway [10]. The activated Ire1p RNase domain recognizes and cleaves HAC1 mRNA precisely at a seven nucleotide stem-loop structure. Besides the stem-loop structure, nucleotide sequences within the loop also significantly contribute to the cleavage specificity of Ire1p. Previous experiments indicated that nucleotides at positions −3, −1, +3 and +5 relative to the cleavage sites at both the 5’ and 3’ splice site junctions of yeast HAC1 mRNA are required for cleavage by Ire1p. Mutation at these positions inhibited splicing during ER stress [11].
There are two forms of IRE1 in mammalian cells, IRE1α and IRE1β [4, 6], however IRE1α is only form that is expressed ubiquitously and triggers the mammalian UPR. Although a HAC1 mRNA homolog could not be identified in metazoan cells, the substrate of metazoan IRE1α was identified by several groups as X-box binding protein 1 (XBP1) mRNA [12, 13]. XBP1 shares common important features with HAC1 mRNA including a stem-loop structure at the exon-intron junctions and the conserved nucleotides of XBP1 mRNA at position −3, −1 and +3 [14]. There are differing reports on the ability of IRE1α to initiate splicing in HAC1 mRNA. Niwa et al. reported that the RNase activity of the truncated IRE1α cytoplasmic domain over-expressed in baculovirus efficiently cleaved HAC1 mRNA in vitro[15]. In contrast, full-length IRE1α expressed in mammalian cells efficiently cleaved HAC1 mRNA at the 5’ splice junction, but not at the 3’ splice site junction [6, 16]. The cause of the different substrate specificity at the 5’ and 3’ splice site junctions of HAC1 mRNA is not known. In order to identify the specificity of IRE1α RNase activity, we employed a well-characterized yeast model to investigate the function of human IRE1α (hIRE1α) in mediating HAC1 mRNA splicing, as well as in mediating fruit fly Drosophila melanogaster XBP1 (dmXBP1) mRNA splicing in mammalian cells. We found that hIRE1α can splice HAC1 mRNA in yeast only when the nucleotide at +1 position in the 3’ splice site junction is mutated from adenine to cytosine. In addition, cytosine at +1 in the 3’ splice site junction was required for hIRE1α to cleave dmXBP1 mRNA.
Materials and Methods
Bacterial strains, yeast strains and cell lines
Saccharomyces cerevisiae AWY14 (W303-1A, UPRE-CYC1-LacZ, UPRE-CYC1-LEU2) and S. cerevisiae AWY19 (Same as to AWY14 except Δire1::kanamycinr) were used for heterologous gene expression [17]. E. coli DH5α was used for propagation and construction of all plasmids. The S. cerevisiae Δire1/Δhac1 strain was constructed from AWY19. The HAC1 locus was replaced with a Zeocin resistance gene expression cassette (hac1::PTEF1-PEM7-Zeocinr-CYC1TT), flanked by the 5’ and 3’ UTRs of the HAC1 gene. To develop a trp1 deletion in the Δire1/Δhac1 strain required for the TRP1 auxotrophic marker gene of the pTB326 or pCM181 expression vectors, the TRP1 locus was disrupted with a hygromycin expression cassette (trp1::hph).
COS-1 cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% heat inactivated fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified 10% CO2-90% air atmosphere.
Plasmid construction
The hIRE1α expression plasmid for yeast was constructed by subcloning the hIRE1α fragment from pED-hIRE1α [6] into pYES2 (Invitrogen) yielding pYES-hIRE1α. The S. cerevisiae Ire1 expression plasmid (pYES-Ire1) was generated as described previously [17].
Wild-type HAC1 expression plasmid (pBluescript-HAC1) used for in vitro transcription template of unspliced HAC1 was obtained and constructed as described previously [6]. To construct mutated HAC1 variants for in vitro transcription, pBluescript-HAC1 was used as template for PCR-based site directed mutagenesis using Pfu DNA polymerase (Promega). The M1F and M1R were used to introduce a single nucleotide cytosine to adenine change at the +1 residue in HAC1 at the 3’ splice junction to generate pBluscript-mtHAC1. The mutation was confirmed by automated DNA sequencing.
To construct the HAC1 expression vector in yeast, the full-length HAC1 coding sequence was amplified from S. cerevisiae genomic DNA by PCR using HAC1F and HAC1R with Vent® DNA polymerase. The PCR product was subcloned into the multicopy plasmid pTB326 between the EcoRI and KpnI sites generating pTB-HAC1. To generate the mutated HAC1 recombinant plasmid, site-directed mutagenesis in pTB-HAC1 was performed using MHAC1F and MHAC1R primers with Phusion DNA polymerase (Thermo Scientific). In addition, the expression plasmid for both wild-type HAC1 and mutated HAC1 under regulation of its own promoter were generated. The HAC1 coding sequence was amplified using full-HAC1F and full-HAC1R primers and was subcloned into the low copy plasmid (pCM181) between the BamHI and ClaI sites yielding pCM181-HAC1. Site-directed mutagenesis was also performed using MHAC1F and MHAC1R generating pCM181-mtHAC1.
The mutant unspliced dmXBP1 expression plasmid was constructed by single nucleotide substitution at the +1 position in the 3’ splice site junction in pcDNA-dmXBP1u [18] by a PCR approach using MdmXBP1F and MdmXBP1R primers with Phusion DNA polymerase (Thermo Scientific).
HAC1 RNA in vitro cleavage
An in vitro cleavage assay was performed as described [8]. A 550 bp fragment of HAC1, covering the 252 bp intron, was transcribed in vitro in the presence of α-[32P]-UTP (Amersham Phamarcia) using T7 RNA polymerase (Ambion). The radiolabeled HAC1 RNA was purified by electrophoresis on a 6% urea polyacrylamide gel. The RNA was extracted and purified from the polyacrylamide gel slice using endoribonuclease buffer (20 mM HEPES, pH 7.3, 10 mM magnesium acetate, 50 mM potassium acetate, 1 mM dithiothreitol (DTT) and 2 mM ATP). The purified RNA was incubated with hIRE1α immunoprecipitated from transiently transfected COS-1 cells at 30°C for 30 and 60 min [6]. The reaction was stopped by phenol/chloroform extraction and then analyzed by electrophoresis on 10% polyacrylamide gels followed by autoradiography.
Transformation of the Δire1/ Δhac1 S. cerevisiae strain
Both HAC1 and IRE1 were expressed in the S. cerevisiae double mutant stain using exogenous chromosomal markers. One hundred nanograms of each expression plasmid for IRE1 (pYES-Ire1, pYES-hIRE1α) and HAC1 (pTB-HAC1, pTB-mtHAC1 or pCM181-HAC1, pCM181-mtHAC1) were co-transformed into S. cerevisiae by electroporation [19]. The positive clones were selected on uracil and tryptophan dropout (-URA, -TRP) medium and transformants were screened by colony PCRs using specific primers for each gene (Table 1).
Table 1.
Oligonucleotide primers used in this study
| Primer | Sequence (5’-3’) |
|---|---|
| HAC1F | GAC GAG AAT AAG GTC TGC |
| HAC1R | GGG TTC GCT TGC GGG AGC AC |
| full-HAC1F | GAC GAG AAT AAG GTC TGC |
| full-HAC1R | GG G TTC GCT TGC GGG AGC AC |
| MHAC1F | CTT GTA CTG TCC GCA GCG CAG TCA G |
| MHAC1R | CTG ACT GCG CTG CGG ACA GTA CAA |
| HAC1SF | GCC CAA GAG TAT GCC GAT TCC G |
| HAC1SR | CAA ACC TGA CTG CGC TTC TGG |
| 6319B | GGT TAC GGA TCC TCA GGA ACA GTA GTT T |
| 6320B | AAA CCT ACT CGA GGT AGA AAC GAG AAT AT |
| yActin F | GCC GGA TCC GCC GGT GAC GAC GCT CC |
| yActin R | TCG TCG AAT TCT TGT TTT GAG ATC |
| qRTKAR2 F | TGG GTG GTG GTA CTT TCG ATG TCT |
| qRTKAR2 R | AGC TAG GGC CTT GTT GTT GTC AGA |
| qRTPDI1 F | TCC CTC TAT TTG CCA TCC ACG ACA |
| qRTPDI1 R | CGC TCA ATT CGT CAA ACG CCT CTT |
| qRTActin F | CAC GTC GTT CCA ATT TAC GCT GGT |
| qRTActin R | TCG AAG TCC AAG GCG ACG TAA CAT |
| MdmXBP1 F | CTG ACC CTC TGA CGC AGG GTA TA |
| MdmXBP1 R | TGT ATA CCC TGC GTC AGA GGG TCA |
| dmXBP1F | CCG AAT TCA AGC AGC AAC AGC A |
| dmXBP1RR | TAG TCT AGA CAG AGG GCC ACA ATT TCC AG |
Heterologous protein expression in yeast and β-galactosidase assay
Yeast cells harboring the pTB (pCM181) and the pYES plasmids were grown in -URA, -TRP medium containing 1% (w/v) D-raffinose. IRE1 expression was induced by addition of D-galactose (2% (w/v) final). β-mercaptoethanol (β-ME) or DTT were applied to the final concentration of 15 mM or 5 mM, respectively, and cultures were incubated for another 6 hr or 2 hr. Cells were then collected cell for β-galactosidase activity measurement [20].
Transient DNA transfection and western blotting
Transient DNA transfection of the dmXBP1u expression plasmid into COS-1 cells was performed by Lipofectamine 2000 (Invitrogen) following the supplier’s instructions. At 48 hr after transfection, the cells were treated with 5 mM DTT for 2 hr. Cells were collected to measure dmXBP1 splicing.
For western blot analysis, cell extracts were harvested in RIPA lysis buffer [1% (v/v) NP-40, 0.5% sodium deoxycholate, 0.1%(w/v) SDS in phosphate buffer saline (PBS)] supplemented with protease inhibitor cocktail III (AG Scientific). The protein concentration was determined using DC protein assay (Bio-rad). COS-1 extracted proteins (~30 µg) were resolved on 4–15% polyacrylamide gradient gels (Bio-rad). The blot was probed with mouse polyclonal antibody against ORF1 of dmXBP1, and then antigen-antibody complexes were monitored with horseradish peroxidase conjugated with anti-mouse IgG (Sigma) and West Dura chemiluminescent substrate (Thermo Scientific).
RNA isolation, RT-PCR and qRT-PCR
Total RNAs from all strains of S. cerevisiae and COS-1 cells were extracted by TRIzole reagent (MRC). For RNA extraction from yeast cells, cells were pre-treated with lyticase before subjection to RNA extraction. First-strand cDNA synthesis was performed using 1 µg of DNaseI (Promega)-treated RNA by using Oligo(dT) primers and Impromp-II reverse transcriptase (Promega). To detect HAC1s mRNA level, HAC1SF and HAC1SR, specific primer pairs were used. The HAC1SF binding site is in exon1 of HAC1s whereas the HAC1SR binding site is at the junction of exon1 and exon2 in HAC1s mRNA. The Ire1 and hIRE1α expression levels were measured using 6319B and 6320B primer pairs, respectively. The yeast actin (internal control) was detected by yActinF and yActinR primers. All PCR amplifications were performed using Taq DNA polymerase (Promega). The expression of KAR2 and PDI1 transcript was quantitated by SYBR Green (KAPA SYBR®) qRT-PCR analysis. Yeast actin was used as an internal control. The dmXBP1 splicing was monitored by using dmXBP1F and dmXBP1R, intron flanking primers. The primers used for PCR and qRT-PCR are shown in Table 1.
Immunoprecipitation
hIRE1α protein expression and collection was performed as previously described [18]. Briefly, cell lysates from transfected COS-1 cells were incubated with protein G sepharose (Amersham Pharmacia) conjugated with anti-IRE1α antibody overnight at 4 °C. The IRE1α-antibody protein complex was retrieved by centrifugation at 2,000 rpm for 5 min at 4 °C and washed with PBS containing 1%, 0.5% and 0.05% (v/v) Triton X-100.
Results
hIRE1α cannot restore UPR signaling in Ire1-null S. cerevisiae
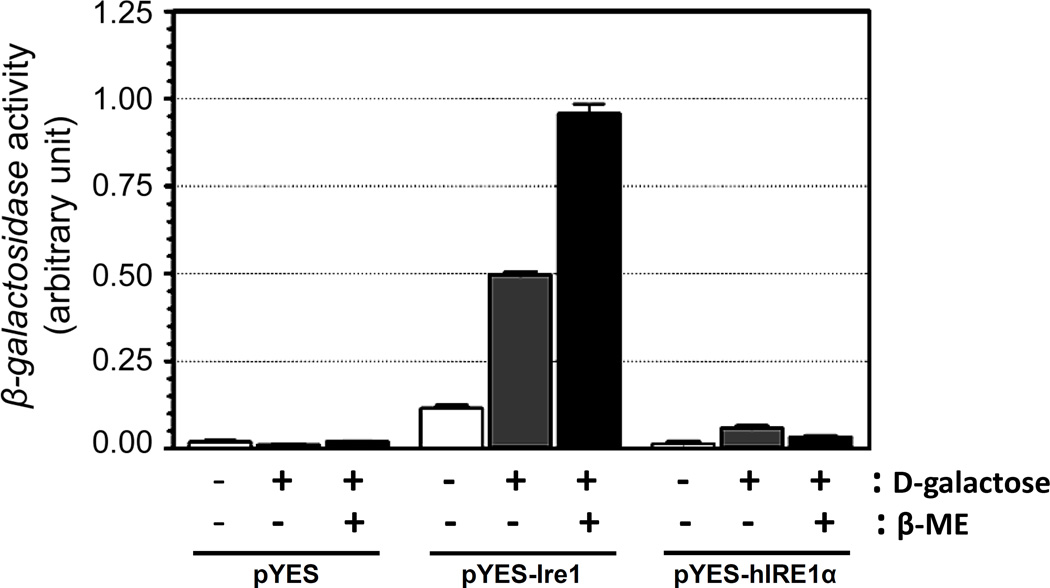
To investigate the ability of hIRE1α to signal the UPR in yeast, hIRE1α and Ire1 expression plasmids were transformed into the ire1-null strain of S. cerevisiae AWY19 in which the endogenous IRE1 gene was disrupted. Yeast cells expressing Ire1 showed significant increase in the basal level of β-galactosidase activity even in D-raffinose containing medium compared to the basal level of empty vector alone (Figure 1). Upon switching the carbon source to D-galactose the expression of the UPR reporter increased significantly (>5 fold). Addition of β-mercaptoethanol (β-ME) to the medium, to induce ER stress, further enhanced reporter gene activity by 10-fold compared to its basal activity, indicating that expression of Ire1p fully complements the UPR in a yeast strain devoid of the endogenous IRE1 locus. In contrast, the expression of hIRE1α in S. cerevisiae AWY19 failed to activate the UPR reporter gene as indicated by the low level of β-galactosidase activity in both the absence and presence β-ME, similar to results obtained from the strain carrying an empty plasmid. The failure of hIRE1α to restore the UPR in this system suggests that significant differences exist in the catalytic properties between Ire1p and hIRE1α.
Figure 1. The UPR is not induced by hIRE1α expression in Δire1 yeast.
The AWY19 (Δire1) yeast strains containing the IRE1 expression plasmids (pYES-Ire1 or pYES-hIRE1α) were cultured in -URA medium containing D-glucose (−) or D-galactose (+) as a carbon source. The induction of UPRE- LacZ reporter activity was measured in the absence (−) or presence (+) β-ME. The results are presented as mean±SEM (n=3).
The nucleotide at +1 position in the 3’ stem loop of HAC1 is critical for in vitro cleavage by hIRE1α
Apart from the consensus nucleotides at −3, −1, and +3 relative to cleavage site in both HAC1 and XBP1 mRNA, the cytosine (C) at +1 in the 3’ splice site junction of XBP1 from various organisms is conserved whereas the adenine (A) is present in yeast HAC1 (Figure 2A). To test whether this nucleotide is important for hIRE1α catalytic specificity, in vitro cleavage of HAC1 RNA was performed. The in vitro transcript HAC1 was incubated with immunoprecipitated hIRE1α from transiently transfected COS-1 cells. Denaturing gel electrophoresis was used to measure the cleavage of HAC1 RNA by hIRE1α (Figure 2B). The wild-type HAC1 transcript was partially cleaved by hIRE1α. The cleavage could be observed within 30 min generating only two fragments of approximately 224 nt and 326 nt corresponding to the sizes of the 5’ exon and intron plus 3’ exon, respectively. There was no RNA fragment represented as the 3’ exon (74 nt) in this reaction. Prolonged incubation did not alter the pattern or the efficiency of cleavage. In contrast, incubation with a mock-transfected sample did not demonstrate any RNA cleavage, confirming that HAC1 RNA was cleaved due to hIRE1α activity restricted at the 5’ splice site as previously reported [6]. Incubation of the mutant HAC1 RNA with hIRE1α also generated the fragments corresponding to the 5’exon and intron-plus 3’ exon, as well as two additional bands corresponding to sizes of the intron (252 nt) and 3’ exon of HAC1 RNA similar to the in vitro cleavage pattern of HAC1 RNA mediated by Ire1p [6]. Increasing the time of hIRE1α incubation decreased the intron-plus 3’ exon RNA level in contrast to the 3’ exon that was increased. This indicates that the 3’ splice site junction on mutant HAC1 RNA was correctly cleaved by hIRE1α. Thus, we conclude that C at this position in the 3’ stem loop structure of HAC1 RNA is crucial specifically for hIRE1α cleavage activity (Figure S1A) as same as C (−3), G (−1), and G (+3) in the RNA substrate [21].
Figure 2. hIRE1α cleavage activity is nucleotide sequence specific.
(A) Nucleotide sequence and predicted secondary structure at the exon/intron junctions of yeast HAC1 and human XBP1 mRNA. The black arrows indicate the RNA cleavage site by IRE1 at the 5’ and 3’ splice site junctions. The nucleotide sequence at the +1 position in the 3’cleavage sites of HAC1 and XBP1 are indicated with boxes. (B) An in vitro cleavage reaction using in vitro transcribed 32P-labeled HAC1 and immunoprecipitated hIREα. After incubation for the indicated times, the labeled RNA was analyzed by electrophoresis on 10% polyacrylamide denaturing gels. The mt represents mutant HAC1 that carries the single point mutation of adenine at +1 on 3’ splice junction. The right panel represents the predicted cleavage sites in HAC1. The numbers in brackets indicate the expected sizes of labeled RNA.
hIRE1α can restore UPR function in S. cerevisiae if the HAC1 3’splice site junction is mutated to cytosine
The ability of hIRE1α to cleave wild-type and mutant HAC1 mRNA to signal the UPR in yeast was studied by transforming the corresponding recombinant plasmids into Δire1/Δhac1 S. cerevisiae. This double-deleted strain lacks two essential components of the UPR and was unable to activate the UPR reporter in response to ER stress. The presence of a plasmid encoding only one UPR component (Hac1p or Ire1p) slightly increased the basal level of UPR reporter activity when compared to the empty vector alone (Figure 3A). UPR reporter activity was further increased upon stress induction. UPR induction measured by β-galactosidase activity was markedly increased in clones harboring wild-type or mutant HAC1 with the Ire1 expression plasmid (20-fold over basal). UPR activity in these clones responded to an ER stress inducer, ~ 25-fold induction upon ER stress generated by β-ME. Unlike Ire1 co-expression, cells expressing hIRE1α with wild-type HAC1 were not able to induce reporter expression. In response to ER stress, β-galactosidase activity by this clone was the same as the clone expressing HAC1. In contrast, the clone with mutated HAC1 and hIRE1α exhibited the similar UPR induction as wild-type or mutant HAC1 with Ire1 (30-fold induction over basal). These data indicate that the yeast UPR can be complemented by hIRE1α if the yeast expresses the mutant HAC1. Notably, cells expressing mutant HAC1 and hIRE1α showed a similar growth rate to wild-type or mutant HAC1 with Ire1 under non-stress and ER stress conditions induced by addition of DTT (β-ME) (Figure S2).
Figure 3. Specific point mutation in HAC1 mRNA complements the ability of hIRE1α to signal the UPR.
Δire/Δhac1 yeast harboring IRE1 and HAC1 recombinant plasmids were cultured in D-galactose containing medium for indicated times followed by addition of β-ME or DTT. (A) The UPRE-LacZ reporter activity after cells were cultured in -URA, -TRP synthetic medium containing D-galactose (2%) for 12 hr and then exposed to β-ME for 6 hr. (B) RT-PCR of spliced HAC1 (HAC1s) transcript from cells at 12 hr after IRE1 induction and 5mM DTT treatment (2 hr) using specific primers. Actin was used as an internal control. (C) qRT-PCR of KAR2 and PDI1 from yeast cells cultured in D-galactose containing medium for 16 hr and DTT treatment for 2 hr. The mRNA levels of both KAR2 and PDI1 were measured by qRT-PCR using specific primers and normalized to actin levels. * represents significant difference (p<0.05) of UPR responsive genes by one- way ANOVA analysis.
To confirm that co-expression of hIRE1α and mutant HAC1 restored the UPR, the amount of unspliced HAC1 (HAC1u) and spliced HAC1 (HAC1s) transcript was quantified by RT-PCR. Initially, we used a primer pair that flanks the intron nucleotides in order to amplify both HAC1u and HAC1s but the HAC1s was weakly detected. We speculated that HAC1s was not efficiently amplified by using this primer pair because HAC1s expression is much lower than HAC1u. Therefore, we designed primers that specifically bind to the HAC1s transcript to prevent the competitive amplification of HAC1u in the PCR reaction. After growth of yeast cells in selective medium supplemented with D-galactose, the 250 bp of HAC1s was exclusively observed in response to ER stress after allowing hIRE1α expression with the mutant HAC1 (Figure 3B).
In addition, expression of KAR2, one of the endogenous UPR target genes that is highly induced upon ER stress was measured by qRT-PCR. KAR2 mRNA expression was induced up to 2-fold in yeast cells expressing hIRE1α with the mutant HAC1 compared to wild-type HAC1 which displayed a similar KAR2 mRNA level as the negative control group. Similarly, PDI1 expression was also increased in this clone (Figure 3C). Collectively, these results indicate that co-expression hIRE1α and mutant HAC1 activate the UPR in yeast cells.
Specific C to A mutation at +1 in the 3’ splice junction of Drosophila melanogaster XBP1 mRNA abolishes hIRE1α cleavage in mammalian cells
It was demonstrated that hIRE1α can splice dmXBP1 similar to human XBP1 mRNA [18]. Therefore, to test whether the cytosine at +1 in the 3’ splice site junction is critical for the hIRE1α cleavage, the cytosine (C) at +1 in the 3’splice junction of pcDNA-dmXBP1u was mutated to adenine (A), guanine (G) or thymine (T). In untreated cells, only a 240 nt of dmXBP1u transcript was observed (Figure 4A). Addition of DTT resulted in excision of a 23 nt intron, 217 nt of dmXBP1s was detected simultaneously with dmXBP1u in wild-type dmXBP1u transfected cells. In contrast, hIRE1α failed to splice mutant dmXBP1u to generate a 217 nt fragment. We next determined the expression pattern of exogenous dmXBP1 by western blot analysis using polyclonal antibody against amino acid sequence of ORF1 (Figure 4B). A single protein of dmXBP1u (~37 kDa) was detected in untreated cells, where the dmXBP1s (~50 kDa) appeared together with dmXBP1u in DTT treated cells that express wild-type dmXBP1u. The dmXBP1s protein was not detected in COS-1 transfected with the mutant dmXBP1u expression plasmids. Taken together, we conclude that cytosine at +1 in the 3’ splice site junction is crucial for hIRE1α cleavage.
Figure 4. hIRE1α cannot mediate dmXBP1 splicing if the nucleotide at +1 in the 3’ splice site junction is mutated.
COS-1 cells were transiently transfected with wild-type (WT) and mutant dmXBP1u recombinant plasmids in which the cytosine (C) at +1 position in the 3’ splice site junction was mutated to be either adenine (A), guanine (G), or thymine (T). S represents spliced dmXBP1 recombinant plasmid transfection. XBP1 splicing was analyzed after transfected cells were either untreated (−) or treated with 5mM DTT treatment for 2 hr (+). (A) RT-PCR to measure dmXBP1s using primers flanking the splice site. (B) Western blot analysis of dmXBP1 protein from transfected cells using ORF1 polyclonal antibody. The level of vinculin was used as an internal control.
Discussion
The UPR is a vital eukaryotic intracellular signaling pathway for coping with stress from unfolded protein accumulation within the ER. In metazoans, the UPR is signaled through three ER transmembrane protein sensors (ATF6, IRE1, and PERK) but only IRE1 is conserved in yeast [3]. By using the Δire1/Δhac1 yeast strain as an analysis platform, we first elucidated that hIRE1α can be expressed in yeast and it is capable of complementing Ire1p function.
Despite using the same expression platform, mutant HAC1 splicing mediated by hIRE1α was much less effective than Ire1p to induce the UPR. This was not due to inappropriate targeting of hIRE1α to the ER because the signal peptide sequence at the N-terminus of hIRE1α efficiently targets Ire1p to the ER in yeast [22]. We propose that hIRE1α may not be fully active to cleave mutant HAC1 as they may not efficiently to form oligomers in yeast cell. Secondly, the reduced UPR induction may due to an incompatibility between yeast tRNA ligase and hIRE1α in mediating mutant HAC1 splicing. In metazoans, the XBP1 mRNA ligation is mediated by tRNA ligase complex having RtcB as a catalytic subunit [23, 24]. Insight specific into the molecular mechanism of RNA ligation, yeast tRNA ligase utilizes 5’-3’ RNA ligation in HAC1 splicing that is different from that demonstrated in XBP1 splicing (3’–5’ RNA ligation) [25, 26]. Although HAC1 and XBP1 mRNA share some features important for unconventional splicing, including a stem loop structure at the exon/intron junction and conserved nucleotides at −3(C), −1(G) and +3(G) within the loops at both the 5’ and 3’ splice site junctions [14], the mechanism by which IRE1α could not cleave the 3’splice site in HAC1 was unknown [6, 15, 16, 27]. We found that hIRE1α was unable to cleave wild-type HAC1. However, replacement of the adenine at the +1 position at the 3’ splice junction in HAC1 mRNA with a cytosine restored cleavage by hIRE1α whereas adenine (A) to guanine (G) or thymine (T) substitution at this position did not (Figure S1A). Not only was a cytosine required at the +1 position of the 3’ splice junction, the cytosine at +1 position of the 5’ splice junction was also required for HAC1 mRNA cleavage by hIRE1α (Figure S1B). Consistent with this observation, mutation of the +1 cytosine residue at the 3’ and 5’ splice site junction in D. melanogaster XBP1 mRNA to other nucleotides completely abolished cleaving by hIRE1α (Figure 4A and B, figure S3A and B). The crystal structure of IRE1 catalytic domain revealed that the helix loop element (HLE) within the RNase domain of each monomer forms the cavity that spatially serves as a recognition site for the HAC1/XBP1 stem-loop structure. Precisely, the CNGCNG motif is docked within this cavity of the dimer/oligomer [28]. It was proposed that the RNA binding cavity created in the IRE1α RNase domain is more stable by the close packing of the HLEs than that in Ire1p [28]. In this case, the cytosine at +1 of the 3’ splice junction on HAC1/XBP1 mRNA substrate may be required for IRE1α-RNA complex formation.
Besides XBP1 splicing, the IRE1α RNase activity also triggers regulated IRE1-dependent decay (RIDD) to degrade some of ER-localized mRNAs under chronic ER stress [29, 30]. Interestingly, all recently reported RIDD mRNA substrates such as CD59, RUVBL1, and INS exhibit the same unique cleavage site between G and C within the highly conserved recognition CNGCNG motif within XBP1 mRNA [31, 32]. Notably, some RIDD substrates are associated with diseases, including cancer (PPP2R1A, PEPD, and GEMIN5 mRNA) and inflammatory disease (CD59, PRKCD, and INS1 mRNA) [33]. Therefore, our basic knowledge of hIRE1α cleavage specificity may lead to new strategies for treating diseases associated with altered IRE1α activity [34].
In conclusion, our findings provide direct evidence that hIRE1α can signal the UPR in yeast. However, hIRE1α splicing of HAC1 mRNA requires a cytosine at position +1 in the 3’ splice site junction.
Supplementary Material
Acknowledgments
We thank Dr. Sutipa Tanapongpipat (National Center for Genetic Engineering and Biotechnology, National Science and Technology Development Agency) for critical review of this manuscript. WT received a student fellowship by the Royal Golden Jubilee, Ph.D. program (Grant No. PHD/0332/2551) under the Thailand Research Fund and Mahidol University. Portions of this work were supported by NIH/NCI Grants R37DK042394, R01DK103183, R24DK110973 and R01CA128814 (R.J.K.), Sanford Burnham Prebys NCI Cancer Center Grant P30 CA030199.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Author contributions
JP designed and performed experiments, analyzed data and wrote the manuscript.
PS performed experiments.
RJK designed experiments, analyzed data and edited the manuscript.
WT designed experiments, analyzed data and edited the manuscript.
References
- 1.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 2.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J. Clin. Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol Cell. Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 4.Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. EMBO J. 1998;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 6.Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Deve. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kawahara T, Yanagi H, Yura T, Mori K. Unconventional splicing of HAC1/ERN4 mRNA required for the unfolded protein response - Sequence-specific and non-sequential cleavage of the splice sites. J. Biol. Chem. 1998;273:1802–1807. doi: 10.1074/jbc.273.3.1802. [DOI] [PubMed] [Google Scholar]
- 8.Sidrauski C, Walter P. The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell. 1997;90:1031–1039. doi: 10.1016/s0092-8674(00)80369-4. [DOI] [PubMed] [Google Scholar]
- 9.Kawahara T, Yanagi H, Yura T, Mori K. Endoplasmic Reticulum Stress-induced mRNA Splicing Permits Synthesis of Transcription Factor Hac1p/Ern4p That Activates the Unfolded Protein Response. Mol. Biol. Cell. 1997;8:1845–1862. doi: 10.1091/mbc.8.10.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori K, Ogawa N, Kawahara T, Yanagi H, Yura T. mRNA splicing-mediated C-terminal replacement of transcription factor Hac1p is required for efficient activation of the unfolded protein response. Proc. Natl. Acad. Sci. U S A. 2000;97:4660–4665. doi: 10.1073/pnas.050010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez TN, Sidrauski C, Dörfler S, Walter P. Mechanism of non-spliceosomal mRNA splicing in the unfolded protein response pathway. EMBO J. 1999;18:3119–3132. doi: 10.1093/emboj/18.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, Yoshida H, Morimoto R, Kurnit DM, Mori K, Kaufman RJ. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell. 2001;107:893–903. doi: 10.1016/s0092-8674(01)00612-2. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA Is Induced by ATF6 and Spliced by IRE1 in Response to ER Stress to Produce a Highly Active Transcription Factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 14.Hooks KB, Griffiths-Jones S. Conserved RNA structures in the non-canonical Hac1/Xbp1 intron. RNA Biol. 2011;8:552–556. doi: 10.4161/rna.8.4.15396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niwa M, Sidrauski C, Kaufman RJ, Walter P. A Role for Presenilin-1 in Nuclear Accumulation of Ire1 Fragments and Induction of the Mammalian Unfolded Protein Response. Cell. 1999;99:691–702. doi: 10.1016/s0092-8674(00)81667-0. [DOI] [PubMed] [Google Scholar]
- 16.Bowring CE, Llewellyn DH. Differences in HAC1 mRNA Processing and Translation between Yeast and Mammalian Cells Indicate Divergence of the Eukaryotic ER Stress Response. Biochem. Biophys. Res. Commun. 2001;287:789–800. doi: 10.1006/bbrc.2001.5633. [DOI] [PubMed] [Google Scholar]
- 17.Poothong J, Sopha P, Kaufman RJ, Tirasophon W. Domain compatibility in Ire1 kinase is critical for the unfolded protein response. FEBS Lett. 2010;584:3203–3208. doi: 10.1016/j.febslet.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plongthongkum N, Kullawong N, Panyim S, Tirasophon W. Ire1 regulated XBP1 mRNA splicing is essential for the unfolded protein response (UPR) in Drosophila melanogaster. Biochem. Biophys. Res. Commun. 2007;354:789–794. doi: 10.1016/j.bbrc.2007.01.056. [DOI] [PubMed] [Google Scholar]
- 19.Wu S, Letchworth GJ. High efficiency transformation by electroporation of Pichia pastoris pretreated with lithium acetate and dithiothreitol. Biotechniques. 2004;36:152–154. doi: 10.2144/04361DD02. [DOI] [PubMed] [Google Scholar]
- 20.Fu Y, Xiao W. Study of Transcriptional Regulation Using a Reporter Gene Assay. In: Xiao W, editor. Yeast Protocol. Totowa, NJ: Humana Press; 2006. pp. 257–264. [DOI] [PubMed] [Google Scholar]
- 21.Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Deve. 2002;16:452–466. doi: 10.1101/gad.964702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu CY, Schroder M, Kaufman RJ. Ligand-independent dimerization activates the stress response kinases IRE1 and PERK in the lumen of the endoplasmic reticulum. J. Biol. Chem. 2000;275:24881–24885. doi: 10.1074/jbc.M004454200. [DOI] [PubMed] [Google Scholar]
- 23.Jurkin J, Henkel T, Nielsen AF, Minnich M, Popow J, Kaufmann T, Heindl K, Hoffmann T, Busslinger M, Martinez J. The mammalian tRNA ligase complex mediates splicing of XBP1 mRNA and controls antibody secretion in plasma cells. EMBO J. 2014;33:2922–2936. doi: 10.15252/embj.201490332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu YY, Liang FX, Wang XZ. A Synthetic Biology Approach Identifies the Mammalian UPR RNA Ligase RtcB. Mol Cell. 2014;55:758–770. doi: 10.1016/j.molcel.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shinya S, Kadokura H, Imagawa Y, Inoue M, Yanagitani K, Kohno K. Reconstitution and characterization of the unconventional splicing of XBP1u mRNA in vitro. Nucleic Acids Res. 2011;39:5245–5254. doi: 10.1093/nar/gkr132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sidrauski C, Cox JS, Walter P. tRNA ligase is required for regulated mRNA splicing in the unfolded protein response. Cell. 1996;87:405–413. doi: 10.1016/s0092-8674(00)81361-6. [DOI] [PubMed] [Google Scholar]
- 27.Foti DM, Welihinda A, Kaufman RJ, Lee AS. Conservation and divergence of the yeast and mammalian unfolded protein response. Activation of specific mammalian endoplasmic reticulum stress element of the grp78/BiP promoter by yeast Hac1. J Biol. Chem. 1999;274:30402–30409. doi: 10.1074/jbc.274.43.30402. [DOI] [PubMed] [Google Scholar]
- 28.Korennykh A, Walter P. Structural Basis of the Unfolded Protein Response. Ann. Rev. Cell Dev. Biol. 2012;28:251–277. doi: 10.1146/annurev-cellbio-101011-155826. [DOI] [PubMed] [Google Scholar]
- 29.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollien J, Weissman JS. Decay of Endoplasmic Reticulum-Localized mRNAs During the Unfolded Protein Response. Science. 2006;313:104. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 31.Han D, Lerner AG, Walle LV, Upton J-P, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR. IRE1α Kinase Activation Modes Control Alternate Endoribonuclease Outputs to Determine Divergent Cell Fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oikawa D, Tokuda M, Hosoda A, Iwawaki T. Identification of a consensus element recognized and cleaved by IRE1 alpha. Nucleic Acids Res. 2010;38:6265–6273. doi: 10.1093/nar/gkq452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maurel M, Chevet E, Tavernier J, Gerlo S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends. Biochem. Sci. 2014;39:245–254. doi: 10.1016/j.tibs.2014.02.008. [DOI] [PubMed] [Google Scholar]
- 34.Wang M, Kaufman RJ. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.