Abstract
Two-pore channels are endolysosomal Ca2+ release channels involved in proper trafficking to and from those organelles. They are the likely targets for the Ca2+-mobilizing messenger NAADP, and are further regulated by a variety of mechanisms including phosphoinositide levels and Rab proteins. As discussed here, recent studies highlight a role for these channels in the pathomechanism(s) underlying Parkinson’s disease, with important implications for possible alternative treatment strategies.
Keywords: TPCN1, TPCN2, NAADP, Parkinson’s Disease, LRRK2, Rab Proteins, Calcium, Iron
Introduction
Endosomes and lysosomes play crucial roles for many aspects of cellular physiology including signalling, protein transport and digestion of macromolecules. Endolysosomal trafficking is a highly regulated process whereby cargoes are delivered to their respective destinations. For example, macromolecules can be sorted for degradation by the fusion of autophagosomes or phagosomes with lysosomes, which contain acidic hydrolases required for degradation. Cell surface receptors can be endocytosed into early endosomes, and from there can be targeted for degradation after reaching the lysosome via the late endosome. Alternatively, receptors can be recycled back to the cell surface either directly from early endosomes or via trafficking through recycling endosomes. Late endosomes/lysosomes receive newly synthesized hydrolytic enzymes from the trans-Golgi network (TGN), and can fuse with the plasma membrane to mediate plasma membrane repair/secretion of lysosomal content via a process termed lysosomal exocytosis. Finally, lysosome-related organelles (LROs) are a heterogeneous set of organelles derived from the endolysosomal system and usually contain lysosomal proteins. They exist in various specialized cells such as lytic granules in cytotoxic T lymphocytes, dense granules in platelets, melanosomes in melanocytes, osteoclast granules in osteoclasts and lamellar bodies in lung epithelial type II cells (Maxfield and McGraw, 2004; Luzio et al., 2007; Raposo et al., 2007).
Interfering with proper endolysosomal trafficking underlies a variety of lysosomal storage diseases which in most cases are associated with neurodegeneration (Platt et al., 2012). Conversely, a variety of neurodegenerative diseases such as Alzheimer’s disease, amyotrophic lateral sclerosis and Parkinson’s disease (PD) seem to involve endolysosomal deficits as an underlying molecular mechanism (Nixon, 2013; Abeliovich and Gitler, 2016). Therefore, a detailed understanding of the endolysosomal system has become crucial towards developing therapies able to restore normal cellular function for a range of neurodegenerative diseases with worldwide socioeconomic impact.
Two-pore channels (TPCNs) form a subfamily of the voltage-gated ion channel superfamily, with two distinct gene products expressed in humans (Patel and Cai, 2015). They are localized to endosomes, lysosomes and lysosome-related organelles (LROs), suggesting that they may contribute to the proper functioning of the endolysosomal system. Recent studies have revealed that these channels may be intimately linked to at least some pathomechanism(s) underlying PD.
Regulators of Endolysosomal Trafficking
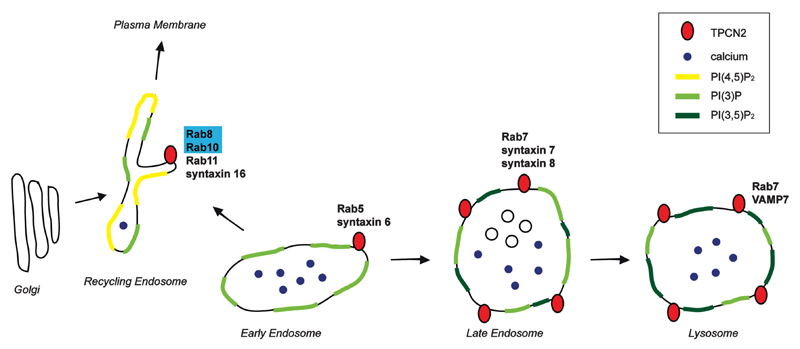
Early endosomes, recycling endosomes, late endosomes and lysosomes are characterized by differences in the protein and lipid composition of their membranes as well as by their intraluminal content, which together contribute to the proper trafficking through the endolysosomal system (Fig. 1). The actual fusion event between two lipid bilayers is mediated by distinct soluble N-ethylmaleimidesensitive fusion protein (NSF) attachment protein receptors (SNAREs). SNARE complexes form a highly stable four-helix bundle mediated by three Q-SNAREs on one membrane and one R-SNARE on the opposing membrane (Südhof and Rothman, 2009). A variety of SNARE proteins have been described across the endolysosomal system (Kümmel and Ungermann, 2014; Luzio et al., 2009), including the R-SNARE VAMP4 on early endosomes and VAMP7 on lysosomes, the Q-SNAREs syntaxin 6/13 on early endosomes, syntaxins 7/8 on early/late endosomes, or syntaxins 12/16 on recycling endosomes, respectively (Fig. 1). Membrane fusion within the endolysosomal system also seems to require an increase in juxta-organellar Ca2+ mediated by Ca2+ release channels and sensed by Ca2+-sensing proteins, such as synaptotagmin VII on lysosomes or synaptotagmin IX on recycling endosomes, respectively (Luzio et al., 2007).
Figure 1.
Schematics of different endocytic compartments and the distribution of PIPs and TPCN2-associated proteins. TPCN2-reported interactors are in bold. Interactors which also have been reported to be substrates for the kinase activity of LRRK2 are shaded in blue. The endocytic recycling compartment may contain TPCN2, and several of the TPCN2 interactors and LRRK2 kinase substrates are localized to this compartment. Luminal Ca2+ concentration in the different compartments is estimated to be in the micromolar to millimolar range, whilst Ca2+ concentration in the recycling compartment remains to be precisely determined. Note that regulation of TPCN2 by PI(3,5)P2 is thought to occur in the late endosome and lysosome, but not in the endocytic recycling compartment which is devoid of this PIP. For further information see text.
Before the actual membrane fusion event, a variety of pre-fusion steps assure fidelity of trafficking across the endolysosomal system, and they are mediated by Rab proteins, Rab effectors, tethering factors and phosphoinositides (PIPs) (Di Paolo and De Camilli, 2006; Hutagalung and Novick, 2011; Jean and Kiger, 2012). Rab proteins are small GTPases which in their active, GTP-bound form are able to recruit specific effector proteins to define organelle identity, trafficking specificity and vesicle motility. Rab proteins localized across the endolysosomal system include Rab5 on early endosomes, Rab7 on late endosomes/lysosomes or Rab8 and Rab11 involved in vesicular transport to and from recycling endosomes (Fig. 1). Targeting Rab proteins to their correct membrane compartments and regulation of their activity is a complex process also involving PIPs (Di Paolo and De Camilli, 2006). PI(3)P is a crucial determinant of early endosome membrane identity and required for almost every aspect of endosomal membrane trafficking and fusion, including autophagy and phagocytosis. It is generated from PI either by VPS34, or by sequential dephosphorylation of PI(3,4,5)P3 by inositol phosphatases specific for the 5 and 4 positions. PI(3)P is then phosphorylated at the 5 position by the Fab1/PIKfyve kinase, which is localized to late endosomes/lysosomes and generates PI(3,5)P2 (Fig. 1). Whilst generally of low abundance, microdomains of PI(3,5)P2 are thought to be important in regulating a variety of events along the late endocytic pathway including trafficking from late endosomes to lysosomes or autophagosome-lysosome fusion, and PI(3,5)P2 is important for late endosomal/lysosomal vesicular fission events as well (Jean and Kiger, 2012). Dephosphorylation of PI(3,5)P2 can be brought about by various phosphatases at the 5 position as well as by myotubularin/myotubularinrelated protein, a 3-phosphatase (Di Paolo and De Camilli 2006). Interestingly, mutations in PI(3,5)P2-metabolizing enzymes cause a variety of neurodegenerative diseases including amyotrophic lateral sclerosis and Charcot-Marie-Tooth disease (Bolino et al., 2000; Chow et al., 2007; Chow et al., 2009), and mutations in the polyphosphoinositide phosphatase synaptojanin 1 have recently been associated with autosomal recessive, early-onset Parkinsonism (Quadri et al., 2013), highlighting a mechanistic link between abnormal PIP metabolism and PD.
The above-mentioned components important for trafficking through the endolysosomal system undergo additional complex and mutual regulation in various ways. For example, SNAREs can regulate Rab effectors and Ca2+ channels, and PIPs can modulate SNAREs as well as Ca2+ release channels (Shen et al., 2011). In addition, there is intricate crosstalk between PIPs and Rab GTPases. For example, PIPs regulate Rab GTPase activity by recruiting and modulating the activity of guanine nucleotide exchange factors (GEFs) or GTPase activating proteins (GAPs) which are known to stimulate Rab GTP loading or GTP hydrolysis, respectively. PIPs also often function as co-receptors with Rab proteins to recruit effectors to specific membrane compartments. Finally, several PIP-metabolizing enzymes have been described as Rab protein effectors. Such positive and negative feedback loops between Rab proteins and PIPs are thought to allow for the establishment of precise identity codes amongst membranes and membrane domains, and help define vesicular identity to assure proper directionality in membrane trafficking (Di Paolo and De Camilli, 2006; Jean and Kiger, 2012).
Endolysosomal structures also display distinct subcellular positioning which is important for their proper functioning. Lysosomes are localized either peripherally or are clustered in a perinuclear area dependent on cellular nutrient status (Korolchuk et al., 2011), whilst lysosomes undergoing lysosomal exocytosis for plasma membrane repair seem to be localized peripherally in a manner dependent on Rab3a (Encarnação et al., 2016). Early endosomes are generally peripheral structures, whilst recycling endosomes are located deeper inside the cell around the microtubule-organizing center and involved not only in endocytic, but also exocytic vesicle trafficking events (van IJzendoorn, 2006). Finally, the different endocytic compartments display distinct intraluminal pH, with early endosomes (pH 5.9–6), late endosomes (pH 5.0–6.0) and lysosomes (pH 5.0–5.5) acidic, LROs generally displaying an acidic pH as well, but recycling endosomes (pH 6.4–6.5) somewhat more alkaline in comparison (van IJzendoorn, 2006; Raposo et al., 2007).
Endolysosomal CA2+ and CA2+ Release Channels
Endosomes, lysosomes and LROs contain significant amounts of the intracellular signaling ion Ca2+ (Patel and Muallem, 2011). Endolysosomal Ca2+ can be mobilized from these stores, and Ca2+-induced Ca2+ release from the ER can then generate physiologically relevant and spatiotemporally complex Ca2+ signals (Cancela, et al., 1999; Churchill et al., 2002; Galione, 2015). Importantly, apart from regulating global Ca2+ signaling, vesicular fusion events along the endolysosomal system also depend on luminal Ca2+ release from these organelles (Luzio et al., 2007).
A variety of endolysosomal Ca2+ channels have been described, including members of the transient receptor potential (TRP) ion channel family, ATP-gated ionotropic receptors (P2X) and TPCNs (Patel and Cai, 2015). TPCNs are largely ubiquitously expressed, with TPCN1 more broadly distributed across the endolysosomal system, and TPCN2 more prominent in lysosomes (Fig. 1) (Calcraft et al., 2009; Brailoiu et al., 2009; Patel and Cai, 2015) as well as in various LROs (Davis et al., 2012; Ambrosio et al., 2015; Ambrosio et al., 2016). Interestingly, TPCN2 has also been described to regulate vesicular trafficking dependent on recycling endosomes, even though it currently remains unknown whether this correlates with a localization of the channel to those organelles (Ruas et al., 2010). TPCNs can conduct distinct ions including Ca2+, Na+, H+ and Fe2+ (Wang et al., 2012; Cang et al., 2013; Jha et al., 2014; Patel, 2015; Fernández et al., 2016; Penny et al., 2016). Their conductance is further modulated by PI(3,5)P2 as well as by nicotinic acid adenine dinucleotide phosphate (NAADP), the most potent Ca2+-mobilizing messenger known to date (Patel, 2015). Whilst NAADP has been reported to also activate select TRP channels and ryanodine receptors (Lee et al., 2015; Gerasimenko et al., 2015), most data are consistent with NAADP most prominently regulating the Ca2+ conductance of TPCN channels (Ruas et al., 2015). Thus, NAADP and select antagonists (Naylor et al., 2009) allow for molecular dissection of the role of TPCNs in endolysosomal functioning.
Pathogenic Events Underlying PD
PD is an age-related neurodegenerative movement disorder with unknown etiology, characterized by the progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta, and by the presence of intracellular protein aggregates rich in α-synuclein, which are called Lewy bodies, in surviving neurons. The molecular and cellular mechanisms underlying the disease have largely remained unknown. The majority of PD cases appear to be idiopathic, with age as major risk factor, and environmental insults and genetic predisposition seem to play a contributing role as well. Studies into the sporadic forms of PD have centered on abnormal protein degradation, toxic protein accumulation and cellular alterations leading to oxidative stress. However, over the past years, studies into genetic forms of the disease have greatly helped in deciphering the possible mechanisms underlying cellular demise. Autosomal-dominant missense mutations in LRRK2 comprise the most common monogenic form of PD, which clinically and pathologically resembles the sporadic form of the disease. Furthermore, genomewide association studies have shown that variations in the LRRK2 gene comprise an important risk factor for sporadic PD. These findings suggest that LRRK2 plays a role for the entire PD disease spectrum, which allows for the establishment of well-defined cellular and animal models to define disease mechanisms (Gómez-Suaga et al., 2014; Cookson, 2016; Abeliovich and Gitler, 2016).
Similar to sporadic PD, pathogenic LRRK2-mediated events seem to converge on altered endolysosomal functioning leading to impaired autophagic clearance of damaged organelles and aggregated proteins, and alterations in intracellular calcium homeostasis, both known to be crucial for the proper functioning of dopaminergic neurons (Gómez-Suaga et al., 2014; Cookson, 2016; Abeliovich and Gitler, 2016; Surmeier et al., 2017). Whilst the precise mechanism(s) by which LRRK2 causes such deficits remain to be properly deciphered, recent studies imply an involvement of TPCN channels in this process.
PD and Aberrant TPCN Functioning
Whilst there is currently no evidence that mutations in TPCNs are associated with familial forms of PD, a recent transcriptomic meta-analysis and network analysis of blood microarrays from patients with PD identified several deregulated genes including nicotinamide phosphoribosyltransferase (NAMPT) and TPCN2 (Santiago et al., 2016). Interestingly, NAMPT is involved in NAD biosynthesis which serves as a precursor for NAADP, suggesting that both TPCN2 and its biological activator NAADP may be altered in sporadic PD patients.
TPCNs are expressed in dopaminergic neurons of the substantia nigra (Lutas et al., 2016), and additional links between TPCNs and PD have recently emerged. Pathogenic LRRK2 was found to cause defects in autophagic flux associated with a decrease in the number of acidic, functional lysosomes, without a change in total lysosome number. These alterations were mimicked by NAADP and reverted by Ned19, an NAADP antagonist (Naylor et al., 2009), as well as by overexpression of dominant-negative TPCN2 (Gómez-Suaga et al., 2012). In agreement with those data, overexpression of TPCN2 has been found to inhibit autophagosome-lysosome fusion via alkalinizing lysosomal pH (Lu et al., 2013), suggesting that increased TPCN2 functioning may underlie the autophagic deficits associated with pathogenic LRRK2. Similarly, aberrant lysosomal morphology was described in fibroblasts from PD patients carrying LRRK2 mutations as compared to healthy controls, and such altered morphology was reversed by molecular silencing of TPCN2 or by NAADP antagonists (Hockey et al., 2015). NAADP stimulation was further shown to cause increased global Ca2+ signals in fibroblasts from LRRK2-PD patients as compared to controls. The lysosomal morphology alterations were reversed by acute Ca2+ chelation, suggesting that the observed enlargement of lysosomes may be due to excessive TPCN2-mediated and Ca2+-dependent fusion events amongst these organelles (Hockey et al., 2015). Altogether, these data are consistent with the idea that pathogenic LRRK2 increases TPCN2 functioning, and thus that regulating TPCN channel activity (Rahman et al., 2014), or generally modulating intracellular Ca2+ levels (Rivero-Ríos et al., 2016; Surmeier et al., 2017) may be a promising therapeutic strategy for at least LRRK2-related PD.
The precise downstream cellular consequences of the altered lysosomal morphology for endomembrane trafficking and lysosomal functioning remain unclear. The enlarged endolysosomal structures may display deficits in proper tubulation and lysosome reformation, a process recently shown to be dependent on TRPML1, another endolysosomal Ca2+ channel (Li et al., 2016), with downstream consequences for a variety of vesicular trafficking events to and from lysosomes. Furthermore, the increase in endolysosomal pH (Gomez-Suaga et al., 2012) is expected to cause deficiencies in proper lysosomal degradation, as the activities of the lysosomal hydrolases depend on an adequately acidic environment. A proper acidic luminal environment may additionally contribute to the appropriate directional movement and maturation of organelles along the endolysosomal pathway (Hu et al., 2015).
In metazoans, the mechanism ofr Ca2+ uptake into acidic organelles has been difficult to dissect, but seems to be mediated by a variety of mechanisms which may or may not require endolysosomal acidification (López et al., 2005; Demaegd et al., 2013; Melchionda et al., 2016; Garrity et al., 2016). Therefore, the observed alkalinization of lysosomal pH may not necessarily cause altered intraluminal Ca2+ content. Rather, it may be a reflection of enhanced TPCN2 functioning, as it is known that NAADP-mediated Ca2+ release causes a concomitant increase in endolysosomal pH (Morgan and Galione, 2007).
How LRRK2 may cause enhanced TPCN2 functioning remains unknown as well. Since the observed cellular effects are dependent on LRRK2 kinase activity (Gómez-Suaga et al., 2012; Hockey et al., 2015), they must be associated with alterations in the phosphorylation of select LRRK2 kinase substrates. TPCN2 has been shown to be regulated by various kinases (Cang et al., 2013; Jha et al., 2014) and is able to interact with LRRK2 (Gómez-Suaga et al., 2012), but whether kinase-mediated effects are via direct phosphorylation of the channel remains to be determined.
LRRK2 may impact upon the modulators of TPCN2 activity, and the recent report of LRRK2-mediated phosphorylation of synaptojanin I (Islam et al., 2016) raises the possibility that pathogenic LRRK2 may regulate TPCN2 channel functioning through increasing PI(3,5)P2 levels. TPCN2 has also been shown to interact with a variety of Rab proteins including Rab1, Rab5, Rab7, Rab8, Rab10, Rab11, Rab14 and Rab21, various SNARE proteins including syntaxins 6, 7, 8, 16 and 18, as well as VAMP7 (Fig. 1) (Lin-Moshier et al., 2014; Grimm et al., 2014). Some of those interactions may occur during the biosynthetic trafficking of TPCN2, such as interactions with Rab1, localized to ER exit sites and the pre-Golgi intermediate compartment, or interactions with syntaxin 18, localized mainly at the ER (Stenmark, 2009). Other reported interactors such as Rab8 and Rab11, or syntaxin 12/16, play roles in the endocytic recycling compartment. Whilst not acidic in nature, this compartment has been shown to contain Ca2+ as well as TRPML2 (Shen et al., 2011), raising the possibility that TPCN2 may also play a role in vesicular trafficking to and from the recycling compartment (Ruas et al., 2010). Finally, the TPCN2 interactor Rab14 is known to be involved in the recycling pathway between the Golgi and endosomal compartments, whilst other interactors such as Rab7, VAMP7, syntaxins 7 and 8 are localized to late endosomes and lysosomes, thus overlapping with the most prominent steady-state localization of TPCN2 (Fig. 1).
Analysis of the regulation of TPCN2 functioning by the late endosomal/lysosomal protein Rab7 revealed that the N-terminus of TPCN2 is important, with a triple-mutation in the N-terminus displaying reduced interaction and reduced channel activity (Lin-Moshier et al., 2014). In addition, a competitive nucleotide binding inhibitor of Rab7 (Agola et al., 2012) decreased TPCN2 functioning, altogether suggesting that TPCN2 is modulated by Rab7 activity.
LRRK2 has been reported to cause deficits in endolysosomal trafficking associated with decreased Rab7 activity when assayed either from transfected cells or from fibroblasts of mutant LRRK2 PD patients as compared to controls (Gómez-Suaga et al., 2014). At first glance, such decreased Rab7 activity would be expected to decrease TPCN2 function, contrary to what is observed in pathogenic LRRK2-expressing cells (Gómez-Suaga et al., 2014; Hockey et al., 2015). However, a recent study reported that Ca2+ release through the endolysosomal P2Xa channel causes downregulation of Rab11a activity and vacuole fusion, and this was mediated by a Ca2+-dependent increase in Rab11a GAP activity (Parkinson et al., 2014). These data suggest a model whereby Rab proteins, when in their active form, signal the correct membrane apposition to endolysosomal Ca2+ release channels which, upon Ca2+ release and membrane fusion, inactivate the respective Rab protein to correctly terminate the process. In this context, it is tempting to speculate that even though capable to be modulated by Rab7, the enhanced TPCN2 activity in the presence of pathogenic LRRK2 may cause a concomitant Ca2+-mediated decrease in overall Rab7 activity.
A recent phosphoproteomics screen has identified a subset of Rab proteins as bona fide LRRK2 kinase substrates (Steger et al., 2016). Rab7 was not identified as LRRK2 kinase substrate, again suggesting that the LRRK2-mediated effects on Rab7 activity may be indirect. However, a set of Rab proteins were identified as LRRK2 kinase substrates including Rab8 and Rab10 (Fig. 1). Phosphorylation of these Rab proteins caused decreased interaction with GDI1/2 as well as with GEFs, suggesting that phosphorylated Rab proteins may accumulate in membranes in their inactive, GDP-bound forms (Steger et al., 2016). Therefore, further studies are warranted to determine the role for TPCN2 in trafficking to and from the recycling compartment, its regulation by Rab8 and/or Rab10, and possible alterations in TPCN2 activity upon LRRK2-mediated phosphorylation of these Rab proteins.
Additional scenarios by which LRRK2 kinase activity may impact upon TPCN2 functioning are possible as well. For example, LRRK2 has been shown to phosphorylate NSF, causing an increased rate of SNARE complex disassembly (Belluzzi et al., 2016), which may in turn regulate TPCN2 functioning via differential interactions with the reported SNARE proteins. Finally, TPCN2 channels have been reported to be permeable to iron as well (Fernández et al., 2016). Iron is an important transition metal playing a crucial role for many chemical reactions, but can also cause hydroxyl radical production via the Fenton reaction, leading to oxidative stress and eventual cellular demise. During healthy ageing, iron is known to accumulate especially in the substantia nigra, and a further increase in iron accumulation is observed in brains of PD patients as compared to healthy aged-matched controls (Ward et al., 2014). Thus, aberrant conductance of TPCN2 channels may contribute to iron-associated cellular alterations and cell death of neurons in the substantia nigra in sporadic PD patients.
At least with respect to pharmacological approaches targeting LRRK2-related PD, special attention to its role in peripheral tissues is warranted as well. LRRK2 is highly expressed in some peripheral tissues such as lung, and genetic or pharmacological kinase inhibition has been shown to lead to alterations in lamellar body surfactant secretion in alveolar type II epithelial cells (Baptista et al., 2013; Miklavc et al., 2014; Fuji et al., 2015). Since lamellar bodies are another type of LROs, and it will be interesting to determine whether they contain TPCN2 channels, and if so, whether the reported safety liability issues around LRRK2 kinase inhibition can be reverted by selectively targeting TPCN2 channel activity in peripheral tissues.
Conclusions and Outlook
There are several emerging links between aberrant TPCN2 channel functioning and pathomechanism(s) underlying PD. Whilst the precise downstream cellular consequences of altered TPCN2 functioning remain to be further dissected, they seem to converge on alterations in endolysosomal trafficking and autophagy, both processes known to be affected in PD. Alterations in TPCN2 channel activity may occur by a variety of mechanisms, including phosphorylation-mediated changes of the channel itself, or of PIPs or proteins which modulate channel functioning such as Rab proteins. Indeed, apart from the LRRK2-mediated phosphorylation and concomitant inactivation of a subset of Rab proteins which may contribute to a PD-specific phenotype, other links between Rab proteins and PD have emerged as well. For example, both Rab7L1 and Rab39b have been genetically linked to PD (MacLeod et al., 2013; Beilina et al., 2014; Lesage et al., 2015), and Rab1, Rab3, Rab8 and Rab11 are known to modulate α-synuclein-mediated aggregation and toxicity in cellular and animal models of PD (Cooper et al., 2006; Gitler et al., 2008; Breda et al., 2015). Whilst the precise Rab proteins able to regulate TPCN2 channel functioning in the distinct subcellular compartments require further investigation, currently available data highlight the possibility that targeting the activity of endolysosomal TPCN2 channels may provide alternative therapeutic strategies against neurodegeneration underlying PD.
Acknowledgments
Work in the laboratory is supported by grants from FEDER, The Spanish Ministry of Economy and Competitiveness (MINECO; SAF2014-58653-R), the BBVA Foundation and the Michael J. Fox Foundation.
Biographies
 Pilar Rivero-Ríos received her degree in Biology from the University of Granada in 2012, and is currently studying for a Ph.D. in the laboratory of Dr. Sabine Hilfiker in the IPBLN-CSIC (Granada), which is expected to be completed in October 2018. She studies the effects of LRRK2, the main determinant of inherited Parkinson’s disease, on intracellular membrane trafficking, focusing on the link between LRRK2, Rab proteins and endolysosomal calcium channels.
Pilar Rivero-Ríos received her degree in Biology from the University of Granada in 2012, and is currently studying for a Ph.D. in the laboratory of Dr. Sabine Hilfiker in the IPBLN-CSIC (Granada), which is expected to be completed in October 2018. She studies the effects of LRRK2, the main determinant of inherited Parkinson’s disease, on intracellular membrane trafficking, focusing on the link between LRRK2, Rab proteins and endolysosomal calcium channels.
 Belén Fernández received her degree in Biochemistry in 2005, and her Ph.D. in Chemistry from the University of Granada in 2009. She joined Hilfiker’s laboratory to work on cellular mechanisms underlying neurodegeneration as a postdoctoral fellow funded by a prestigious “Juan de la Cierva” fellowship, and then pursued further postdoctoral work to expand on her expertise in chemistry at the University of Granada. She is currently a senior postdoctoral associate in Hilfiker’s laboratory, and her main research focus is on understanding the link between iron dyshomeostasis and endolysosomal dysfunction underlying familial forms of Parkinson´s disease.
Belén Fernández received her degree in Biochemistry in 2005, and her Ph.D. in Chemistry from the University of Granada in 2009. She joined Hilfiker’s laboratory to work on cellular mechanisms underlying neurodegeneration as a postdoctoral fellow funded by a prestigious “Juan de la Cierva” fellowship, and then pursued further postdoctoral work to expand on her expertise in chemistry at the University of Granada. She is currently a senior postdoctoral associate in Hilfiker’s laboratory, and her main research focus is on understanding the link between iron dyshomeostasis and endolysosomal dysfunction underlying familial forms of Parkinson´s disease.
 Jesús Madero-Pérez received his degree in Biochemistry in 2011 and his master’s degree in Regenerative Biomedicine in 2012, both from the University of Granada. He is currently finishing his Ph.D. studies on Parkinson’s disease in Sabine Hilfiker’s laboratory at the Spanish National Research Council (CSIC) and the University of Granada. His work during the Ph.D. has focused on elucidating the cellular and molecular mechanism underlying Parkinson’s disease caused by LRRK2 mutations, also using iPSC-derived neurons as cellular model systems.
Jesús Madero-Pérez received his degree in Biochemistry in 2011 and his master’s degree in Regenerative Biomedicine in 2012, both from the University of Granada. He is currently finishing his Ph.D. studies on Parkinson’s disease in Sabine Hilfiker’s laboratory at the Spanish National Research Council (CSIC) and the University of Granada. His work during the Ph.D. has focused on elucidating the cellular and molecular mechanism underlying Parkinson’s disease caused by LRRK2 mutations, also using iPSC-derived neurons as cellular model systems.
 María Romo Lozano received her degree in Biology from the University of Granada in 2015, and her Master in Regenerative Biomedicine in 2016 from the same University. She is currently a Ph.D. student at the IPBLN, CSIC, in Granada, in Hilfiker’s laboratory. Her project is centered on understanding the role of pathogenic LRRK2 and two-pore channels in retromer-mediated protein trafficking, which may underlie at least some forms of familial Parkinson’s disease.
María Romo Lozano received her degree in Biology from the University of Granada in 2015, and her Master in Regenerative Biomedicine in 2016 from the same University. She is currently a Ph.D. student at the IPBLN, CSIC, in Granada, in Hilfiker’s laboratory. Her project is centered on understanding the role of pathogenic LRRK2 and two-pore channels in retromer-mediated protein trafficking, which may underlie at least some forms of familial Parkinson’s disease.
 Sabine Hilfiker received her M.S. from the University of Basel, Switzerland in 1992 and her Ph.D. in molecular neuroscience from the Rockefeller University, New York in 1998, under the supervision of Paul Greengard, Nobel Laureate 2000 in Medicine or Physiology. Following a short postdoctoral appointment at the Rockefeller University, Dr. Hilfiker was awarded a BBSRC David Phillips Research Fellowship to join the Faculty of Life Sciences at the University of Manchester, UK in 2000. Since 2008, she is a Principal Investigator in the CSIC (Spanish National Research Council) in Spain. Her laboratory is interested in understanding the molecular and cellular pathways underlying Parkinson’s disease. She is recipient of the first Research Prize of the Spanish Federation for Parkinson’s Disease (2008), a member of the LRRK2 Biology consortium of the Michael J. Fox Foundation (USA), and has published widely on cellular mechanisms underlying LRRK2-related Parkinson’s disease, with recent links highlighting the importance of endolysosomal calcium channels in this disease.
Sabine Hilfiker received her M.S. from the University of Basel, Switzerland in 1992 and her Ph.D. in molecular neuroscience from the Rockefeller University, New York in 1998, under the supervision of Paul Greengard, Nobel Laureate 2000 in Medicine or Physiology. Following a short postdoctoral appointment at the Rockefeller University, Dr. Hilfiker was awarded a BBSRC David Phillips Research Fellowship to join the Faculty of Life Sciences at the University of Manchester, UK in 2000. Since 2008, she is a Principal Investigator in the CSIC (Spanish National Research Council) in Spain. Her laboratory is interested in understanding the molecular and cellular pathways underlying Parkinson’s disease. She is recipient of the first Research Prize of the Spanish Federation for Parkinson’s Disease (2008), a member of the LRRK2 Biology consortium of the Michael J. Fox Foundation (USA), and has published widely on cellular mechanisms underlying LRRK2-related Parkinson’s disease, with recent links highlighting the importance of endolysosomal calcium channels in this disease.
References
- Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–216. doi: 10.1038/nature20414. [DOI] [PubMed] [Google Scholar]
- Agola JO, Hong L, Surviladze Z, Ursu O, Waller A, Strouse JJ, Simpson DS, Schroeder CE, Oprea TI, Golden JE, Aube J, et al. A competitive nucleotide binding inhibitor: In vitro characterization of Rab7 GTPase inhibition. ACS Chem Biol. 2012;7:1095–1108. doi: 10.1021/cb3001099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio AL, Boyle JA, Di Pietro SM. TPC2 mediates new mechanisms of platelet dense granule membrane dynamics through regulation of Ca2+ release. Mol Biol Cell. 2015;26:3263–3274. doi: 10.1091/mbc.E15-01-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio AL, Boyle JA, Aradi AE, Christian KA, Di Pietro SM. TPC2 controls pigmentation by regulating melanosome pH and size. Proc Natl Acad Sci USA. 2016;113:5622–5627. doi: 10.1073/pnas.1600108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptista MA, Dave KD, Frasier MA, Sherer TB, Greeley M, Beck MJ, Varsho JS, Parker GA, Moore C, Churchill MJ, Meshul CK, et al. Loss of leucine-rich repeat kinase 2 (LRRK2) in rats leads to progressive abnormal phenotypes in peripheral organs. PLoS One. 2013;8:1–16. doi: 10.1371/journal.pone.0080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilina A, Rudenko IN, Kaganovich A, Civiero L, Chau H, Kalia SK, Kalia LV, Lobbestael E, Chia R, Ndukwe K, Ding J, et al. Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc Natl Acad Sci USA. 2014;111:2626–2631. doi: 10.1073/pnas.1318306111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi E, Gonnelli A, Cirnaru MD, Marte A, Plotegher N, Russo I, Civiero L, Cogo S, Carrion MP, Franchin C, Arrigoni G, et al. LRRK2 phosphorylates pre-synaptic N-ethylmaleimide sensitive fusion (NSF) protein enhancing its ATPase activity and SNARE complex disassembling rate. Mol Neurodegener. 2016;11:1–16. doi: 10.1186/s13024-015-0066-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolino A, Muglia M, Conforti FL, LeGuern E, Salih MA, Georgiou DM, Christodoulou K, Hausmanowa-Petrusewicz I, Mandich P, Schenone A, Gambardella A, et al. Charcot-marie-tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat Genet. 2000;25:17–19. doi: 10.1038/75542. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breda C, Nugent ML, Estranero JG, Kyriacou CP, Outeiro TF, Steinert JR, Giorgini F. Rab11 modulates a-synuclein-mediated defects in synaptic transmission and behaviour. Hum Mol Genet. 2015;24:1077–1091. doi: 10.1093/hmg/ddu521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancela JM, Churchill GC, Galione A. Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- Cang C, Zhou Y, Navarro B, Sao YJ, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell. 2013;152:778–790. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, Li J, Zhang X, Lupski JR, et al. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow CY, Landers JE, Bergren SK, Sapp PC, Grant AE, Jones JM, Everett L, Lenk GM, McKenna-Yasek DM, Weisman LS, Figlewicz D, et al. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet. 2009;84:85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- Cookson MR. Cellular functions of LRRK2 implicate vesicular trafficking pathways in Parkinson’s disease. Biochem Soc Trans. 2016;44:1603–1610. doi: 10.1042/BST20160228. [DOI] [PubMed] [Google Scholar]
- Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, et al. Alpha-synuclein blocks ER-golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science. 2006;313:324–328. doi: 10.1126/science.1129462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LC, Morgan AJ, Chen JL, Snead CM, Bloor-Young D, Shenderov E, Stanton-Humphreys MN, Conway SJ, Churchill GC, Parrington J, Cerundolo V, et al. NAADP activates two-pore channels on T cell cytolytic granules to stimulate exocytosis and killing. Curr Biol. 2012;22:2331–2337. doi: 10.1016/j.cub.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaegd D, Foulquier F, Colinet AS, Gremillon L, Legrand D, Mariot P, Peiter E, Van Schaftingen E, Matthijs G, Morsomme P. Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc Natl Acad Sci USA. 2013;110:6859–6864. doi: 10.1073/pnas.1219871110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- Encarnação M, Espada L, Escrevente C, Mateus D, Ramalho J, Michelet X, Santarino I, Hsu VW, Brenner MG, Barral DC, Vieira OV. A Rab3a-dependent complex essential for lysosome positioning and plasma membrane repair. J Cell Biol. 2016;213:631–640. doi: 10.1083/jcb.201511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández B, Fdez E, Gómez-Suaga P, Gil F, Molina-Villalba I, Ferrer I, Patel S, Churchill GC, Hilfiker S. Iron overload causes endolysosomal deficits modulated by NAADP-regulated 2-pore channels and RAB7A. Autophagy. 2016;12:1487–1506. doi: 10.1080/15548627.2016.1190072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuji RN, Flagella M, Baca M, Baptista MA, Brodbeck J, Chan BK, Fiske BK, Honigberg L, Jubb AM, Katavolos P, Lee DW, et al. Effect of selective LRRK2 kinase inhibition on nonhuman primate lung. Sci Transl Med. 2015;7:1–12. doi: 10.1126/scitranslmed.aaa3634. [DOI] [PubMed] [Google Scholar]
- Galione A. A primer of NAADP-mediated Ca(2+) signalling: From sea urchin eggs to mammalian cells. Cell Calcium. 2015;58:27–47. doi: 10.1016/j.ceca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- Garrity AG, Wang W, Collier CM, Levey SA, Gao Q, Xu H. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. eLife. 2016;5:1–18. doi: 10.7554/eLife.15887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko JV, Charlesworth RM, Sherwood MW, Ferdek PE, Mikoshiba K, Parrington J, Peterson OH, Gerasimenko OV. Both RyRs and TPCs are required for NAADP-induced intracellular Ca2+ release. Cell Calcium. 2015;58:237–245. doi: 10.1016/j.ceca.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, Barlowe C, et al. The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci USA. 2008;105:145–150. doi: 10.1073/pnas.0710685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Suaga P, Fdez E, Fernández B, Martínez-Salvador M, Blanca Ramírez M, Madero-Pérez J, Rivero-Ríos P, Fuentes JM, Hilfiker S. Novel insights into the neurobiology underlying LRRK2-linked Parkinson’s disease. Neuropharmacology. 2014;85:45–56. doi: 10.1016/j.neuropharm.2014.05.020. [DOI] [PubMed] [Google Scholar]
- Gómez-Suaga P, Rivero-Ríos P, Fdez E, Blanca Ramírez M, Ferrer I, Aiastui A, López de Munain A, Hilfiker S. LRRK2 delays degradative receptor trafficking by impeding late endosomal budding through decreasing Rab7 activity. Hum Mol Genet. 2014;23:6779–6796. doi: 10.1093/hmg/ddu395. [DOI] [PubMed] [Google Scholar]
- Gómez-Suaga P, Luzón-Toro B, Churamani D, Zhang L, Bloor-Young D, Patel S, Woodman PG, Churchill GC, Hilfiker S. Leucine-rich repeat kinase 2 regulates autophagy through a calcium-dependent pathway involving NAADP. Hum Mol Genet. 2012;21:511–525. doi: 10.1093/hmg/ddr481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Holdt LM, Chen CC, Hassan S, Mueller C, Joers S, Cuny H, Kissing S, Schroeder B, Butz E, Northoff B, et al. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat Commun. 2014;5:1–13. doi: 10.1038/ncomms5699. [DOI] [PubMed] [Google Scholar]
- Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, Patel S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J Cell Sci. 2015;128:232–238. doi: 10.1242/jcs.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YB, Dammer EB, Ren RJ, Wang G. The endosomal-lysosomal system: From acidification and cargo sorting to neurodegeneration. Transl Neurodegener. 2015;4:18. doi: 10.1186/s40035-015-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–149. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MS, Nolte H, Jacob W, Ziegler AB, Putz S, Grosjean Y, Szczepanowska K, Trifunovic A, Braun T, Heumann H, Heumann R, et al. Human R1441C LRRK2 regulates the synaptic vesicle proteome and phosphoproteome in a Drosophila model of Parkinson’s disease. Hum Mol Genet. 2016;25:5365–5382. doi: 10.1093/hmg/ddw352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean S, Kiger AA. Coordination between RAB GTPase and phosphoinositide regulation and functions. Nat Rev Mol Cell Biol. 2012;13:463–470. doi: 10.1038/nrm3379. [DOI] [PubMed] [Google Scholar]
- Jha A, Ahuja M, Patel S, Brailoiu E, Muallem S. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P2 and multiple protein kinases. EMBO J. 2014;33:501–511. doi: 10.1002/embj.201387035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korolchuk VI, Saiki S, Lichtenberg M, Siddiqi FH, Roberts EA, Imarisio S, Jahreiss L, Sarkar S, Futter M, Menzies FM, O’Kane CJ, et al. Lysosomal positioning coordinates cellular nutrient responses. Nat Cell Biol. 2011;13:453–460. doi: 10.1038/ncb2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kümmel D, Ungermann C. Principles of membrane tethering and fusion in endosome and lysosome biogenesis. Curr Opin Cell Biol. 2014;29:61–66. doi: 10.1016/j.ceb.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, Mitchell CH, et al. Presenilin 1 maintains lysosomal Ca(2+) homeostasis via TRPML1 by regulating vATPase-mediated lysosome acidification. Cell Rep. 2015;12:1430–1444. doi: 10.1016/j.celrep.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage S, Bras J, Cormier-Dequaire F, Condroyer C, Nicolas A, Darwent L, Guerreiero R, Majounie E, Federoff M, Heutink P, Wood NW, et al. French Parkinson’s disease genetics study group (PDG) and the international Parkinson’s disease genomics consortium (IPDGC), loss-of-function mutations in RAB39B are associated with typical early-onset Parkinson disease. Neurol Genet. 2015;1:1–3. doi: 10.1212/NXG.0000000000000009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rydzewski N, Hider A, Xhang X, Yang J, Wang W, Gao Q, Cheng X, Xu H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat Cell Biol. 2016;18:404–417. doi: 10.1038/ncb3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López JJ, Camello-Almaraz C, Pariente JA, Salido GM, Rosado JA. Ca2+ accumulation into acidic organelles mediated by Ca2+- and vacuolar H+-ATPases in human platelets. Biochem J. 2005;390:243–252. doi: 10.1042/BJ20050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Moshier Y, Keebler MV, Hooper R, Boulware MJ, Liu X, Churamani D, Abood ME, Walseth TF, Brailoiu E, Patel S, Marchant JS. The two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc Natl Acad Sci USA. 2014;111:13087–13092. doi: 10.1073/pnas.1407004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Hao BX, Graeff R, Wong CW, Wu WT, Yue J. Two pore channel 2 (TPC2) inhibits autophagosomal-lysosomal fusion by alkalinizing lysosomal pH. J Biol Chem. 2013;288:24247–24263. doi: 10.1074/jbc.M113.484253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lutas A, Lahmann C, Soumillon M, Yellen G. The leak channel NALCN controls tonic firing and glycolytic sensitivity of substantia nigra pars reticulata neurons. Elife. 2016;5:1–19. doi: 10.7554/eLife.15271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: Fusion and function. Nat Rev Mol Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Parkinson MD, Gray SR, Bright NA. The delivery of endocytosed cargo to lysosomes. Biochem Soc Trans. 2009;37:1019–1021. doi: 10.1042/BST0371019. [DOI] [PubMed] [Google Scholar]
- MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, Marder KS, Honig LS, Clark LN, Small SA, Abeliovich A. RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron. 2013;77:425–439. doi: 10.1016/j.neuron.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–132. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- Melchionda M, Pittman JK, Mayor R, Patel S. Ca2+/H+ exchange by acidic organelles regulates cell migration in vivo. J Cell Biol. 2016;212:803–813. doi: 10.1083/jcb.201510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklavc P, Ehinger K, Thompson KE, Hobi N, Shimshek DR, Frick M. Surfactant secretion in LRRK2 knock-out rats: Changes in lamellar body morphology and rate of exocytosis. PLoS One. 2014;9:1–10. doi: 10.1371/journal.pone.0084926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ, Galione A. NAADP induces pH changes in the lumen of acidic Ca2+ stores. Biochem J. 2007;402:301–310. doi: 10.1042/BJ20060759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ. Ca2+ dialogue between acidic vesicles and ER. Biochem Soc Trans. 2016;44:546–553. doi: 10.1042/BST20150290. [DOI] [PubMed] [Google Scholar]
- Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, Galione A, et al. Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol. 2009;5:220–226. doi: 10.1038/nchembio.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon RA. The role of autophagy in neurodegenerative disease. Nat Med. 2013;19:983–997. doi: 10.1038/nm.3232. [DOI] [PubMed] [Google Scholar]
- Parkinson K, Baines AE, Keller T, Gruenheit N, Bragg L, North RA, Thompson CR. Calcium-dependent regulation of Rab activation and vesicle fusion by an intracellular P2X ion channel. Nat Cell Biol. 2014;16:87–98. doi: 10.1038/ncb2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrington J, Galione A. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015;34:1743–1758. doi: 10.15252/embj.201490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. Function and dysfunction of two-pore channels. Sci Signal. 2015;8:re7. doi: 10.1126/scisignal.aab3314. [DOI] [PubMed] [Google Scholar]
- Patel S, Cai X. Evolution of acidic Ca2+ stores and their resident Ca2+-permeable channels. Cell Calcium. 2015;57:222–230. doi: 10.1016/j.ceca.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Patel S, Muallem S. Acidic Ca(2+) stores come to the fore. Cell Calcium. 2011;50:109–112. doi: 10.1016/j.ceca.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Penny CJ, Rahman T, Sula A, Miles AJ, Wallace BA, Patel S. Isolated pores dissected from human two-pore channel 2 are functional. Sci Rep. 2016;6:1–11. doi: 10.1038/srep38426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt FM, Boland B, van der Spoel AC. The cell biology of disease: Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J Cell Biol. 2012;199:723–734. doi: 10.1083/jcb.201208152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri M, Fang M, Picillo M, Olgiati S, Breedveld GJ, Graafland J, Wu B, Xu F, Erro R, Amboni M, Pappata S, et al. Mutation in the SYNJ1 gene associated with autosomal recessive, early-onset Parkinsonism. Hum Mutat. 2013;34:1208–1215. doi: 10.1002/humu.22373. [DOI] [PubMed] [Google Scholar]
- Rahman T, Cai X, Brailoiu GC, Abood ME, Brailoiu E, Patel S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci Signal. 2014;7:ra109. doi: 10.1126/scisignal.2005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Marks MS, Cutler DF. Lysosome-related organelles: Driving post-Golgi compartments into specialisation. Curr Opin Cell Biol. 2007;19:394–401. doi: 10.1016/j.ceb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivero-Ríos P, Madero-Pérez J, Fernández B, Hilfiker S. Targeting the autophagy/lysosomal degradation pathway in Parkinson’s disease. Curr Neuropharmacol. 2016;14:238–249. doi: 10.2174/1570159X13666151030103027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Galione A, Parrington J. Two-pore channels: Lessons from mutant mouse models. Messenger (Los Angel) 2015;4:4–22. doi: 10.1166/msr.2015.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Rietdorf K, Arredouani A, Davis LC, Lloyd-Evans E, Koegel H, Funnell TM, Morgan AJ, Ward JA, Watanabe K, Cheng X, et al. Purified TPC isoforms form NAADP receptors with distinct role for Ca(2+) signaling and endolysosomal trafficking. Curr Biol. 2010;20:703–709. doi: 10.1016/j.cub.2010.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruas M, Davis LC, Chen CC, Morgan AJ, Chuang KT, Walseth TF, Grimm C, Garnham C, Powell T, Platt N, Platt FM, et al. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015;34:1743–1758. doi: 10.15252/embj.201490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago JA, Littlefield AM, Potashkin JA. Integrative transcriptomic meta-analysis of Parkinson’s disease and depressin identifies NAMPT as a potential blood biomarker for de novo Parkinson’s disease. Sci Rep. 2016;6:1–10. doi: 10.1038/srep34579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Wang X, Xu H. Pairing phosphoinositides with calcium ions in endolysosomal dynamics. Bioessays. 2011;33:448–457. doi: 10.1002/bies.201000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, Baptista MA, et al. Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife. 2016;5:e12813. doi: 10.7554/eLife.12813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Schumacker PT, Guzman JD, Ilijic E, Yang B, Zampese E. Calcium and Parkinson’s disease. Biochem Biophys Res Commun. 2017;483:1013–1019. doi: 10.1016/j.bbrc.2016.08.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van IJzendoorn SCD. Recycling endosomes. J Cell Sci. 2006;119:1679–1681. doi: 10.1242/jcs.02948. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014;13:1045–1060. doi: 10.1016/S1474-4422(14)70117-6. [DOI] [PMC free article] [PubMed] [Google Scholar]