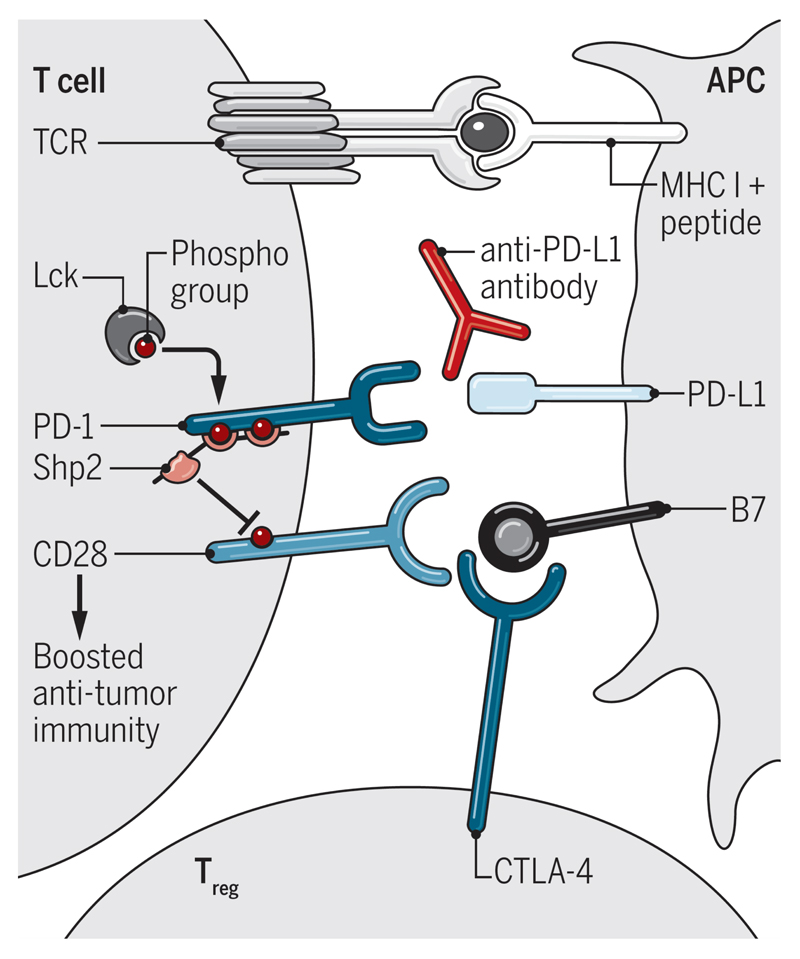
PD-1-centered therapies rely on CD28 activation to promote anti-tumor immunity
Drugs targeting PD-1 and CTLA-4 have been the trailblazers of cancer immunotherapy and have distinct response rates and side effect profiles. However, recent work published in Science (1, 2) suggests that these two molecules may have more in common than we thought. The new studies reveal that PD-1 primarily works by inhibiting signaling through CD28, an important costimulatory receptor that promotes T cell activation. This is intriguing given that CTLA-4 also regulates the CD28 pathway.
To elucidate the molecular interactions mediated by PD-1, Hui et al (1) employed an elegant cell free system. They displayed the cytoplasmic tail of PD-1 on liposomes and used a Fluorescence Resonance Energy Transfer (FRET)-based assay to show Lck is the main kinase that phosphorylates it. Next, they explored which SH2-domain-containing proteins bind to phosphorylated PD-1. Shp2 (but not Shp1) was efficiently recruited to phosphorylated PD-1, with both tyrosines in the PD-1 cytoplasmic tail contributing to this recruitment. Armed with the identity of the main PD-1 effector molecule, the authors then addressed a key outstanding question in the field - which mediators of T cell activation does PD-1 actually inhibit? They modified their liposome system to incorporate an array of the usual suspects including TCR, CD28, CD3ζ, and ZAP70 and assessed how efficiently PD-1-Shp2 dephosphorylated them. Strikingly, PD-1 extinguished CD28 phosphorylation with the highest efficiency. While TCR signaling components were also dephosphorylated, this required many more PD-1 molecules to be present. In a direct comparison between CD28 and CD3ζ, it was found that both were dephosphorylated by PD-1-Shp2, but the re-phosphorylation of CD3ζ by Lck was more efficient. The net consequence was that PD-1-Shp2 could rapidly decrease the number of phosphorylated CD28 molecules while barely affecting CD3ζ molecules. Consistent with regulation of CD28, TIRF microscopy revealed tight co-clustering of PD-1 and CD28 in T cells as they interacted with their ligands on a supported lipid bilayer. These data build on the previous identification of PD-1-Shp2 inhibitory microclusters (3) but reveal a preference for CD28 as the target for inhibition.
So what are the physiological implications of these findings? Dovetailing with this article, a study from Kamphorst et al. (2) probed the interplay between the PD-1 and CD28 systems in vivo. Following chronic lymphocytic choriomeningitis virus (LCMV) infection in mice, they show that anti-PD-L1 mediated rescue of T cell exhaustion depends on CD28 costimulation. By blocking the CD28 ligands (B7.1, B7.2), or using CD28-deficient virus-specific T cells, the authors could virtually negate the actions of anti-PD-L1 antibodies. Might the absence of costimulation have interfered with the capacity of the T cells to become exhausted in the first place? To circumvent this possibility, the authors used an inducible model that allowed CD28 to be ablated from a fraction of T cells once exhaustion had been established. Again, T cells that had lost CD28 were refractory to anti-PD-L1 treatment. One link between the PD-1 and CD28 pathways is that PD-L1 can bind B7.1, however similar results were obtained using antibodies directed against PD-1, arguing against a role for this partnership here. CD28 costimulation was also found to be required for the anti-tumor effects of anti-PD-L1 in a colon carcinoma model. After anti-PD-L1 treatment, only 1 out of 9 mice showed tumor progression, but if B7 ligands were also blocked tumors progressed in 8 out of 10 mice. Thus, for PD-1 pathway inhibition to unleash the immune response to tumor, a functional B7-CD28 costimulatory axis is required.
Turning from mice to humans, the authors studied blood samples from lung cancer patients receiving immunotherapy. After PD-1 blockade, an increase in proliferating CD8 T cells was seen in ~50% of patients and crucially nearly all of these cells expressed CD28. While CD28 is normally found on most T cells, it can be downregulated by chronic stimulation, including in the tumor microenvironment (2). Whether the ratio of CD28+/CD28- T cells changes following immunotherapy, and how this relates to response rates, are tantalizing future questions.
The archetypal regulator of the CD28 pathway is the inhibitory protein CTLA-4. CTLA-4 shares ligands with CD28 but binds them with higher affinity: it therefore serves as a ligand competitor and can also downregulate B7 expression on antigen presenting cells (4). Since CTLA4 governs access to the B7 ligands, CTLA-4-mediated immune regulation is strictly focused on the CD28 pathway. This is elegantly demonstrated by the finding that immune hyperactivation in CTLA-4-deficient mice depends on signaling motifs in the CD28 cytoplasmic tail (5). Thus, CTLA-4 regulates the ability of CD28 to bind its ligands while PD-1 regulates CD28 signaling (Figure 1). An implication of this work is that combinations of drugs targeting CTLA-4 and PD-1 may work synergistically since blocking CTLA-4 increases availability of CD28 ligands and blocking PD-1 increases T cell sensitivity to CD28 ligation.
Figure 1. Extrinsic and intrinsic regulation of CD28.
CTLA-4 regulates CD28 ligand-binding while PD-1 inhibits CD28 signaling. CD28 costimulation is required for anti-PD-L1 antibodies to enhance immunity.
If PD-1 and CTLA-4 both regulate the CD28 pathway, does that make them functionally equivalent? There are several reasons why this is not the case. Arguably the most important distinction is the mode of operation of the two molecules. Extensive evidence indicates that CTLA-4 functions primarily in a cell-extrinsic manner - i.e. that CTLA-4 expressed on one T cell regulates the activation of another T cell, most likely by competing for and downregulating B7 ligands (reviewed in (4)). PD-1 on the other hand acts directly on the T cells that express it. This simple distinction means that even if both regulatory pathways converge on CD28, the circumstances in which regulation is elicited are likely to be very different. CTLA-4 is highly expressed in regulatory T cells (Treg) where it can be used to control the priming of naive T cells. It is also upregulated on activated T cells where, similar to the situation in Treg, it can operate in a cell-extrinsic manner to regulate the CD28-dependent activation of other T cells. Conversely, PD-1 is not thought to regulate T cell priming since it is absent from resting T cells. Although expressed on Treg, PD-1 is dispensable for their suppressive function (6). Accordingly, CTLA-4-deficient mice resemble Treg-deficient mice whereas PD-1-deficient mice do not. Instead, PD-1 directly regulates antigen-experienced T cells and is a characteristic marker of the exhausted CD8 T cells that arise during chronic stimulation. The regulation of T cell exhaustion is complex, however recent studies suggest the transcription factor TCF1 can define less exhausted and more exhausted populations (TCFhigh and TCFlow respectively) (7) with exciting data indicating it is the former population that responds to PD-1 blockade (8). New findings from the autoimmunity field provide additional support for the notion of heterogeneity within exhausted populations: anti-CD3 immunotherapy was shown to induce partial exhaustion in CD8 cells that could be further deepened upon TIGIT engagement (9). Thus, definitions of the populations influenced by PD-1 engagement are still emerging and an increased understanding of exhausted states will doubtless illuminate this issue further. Finally, although PD-1 preferentially regulates CD28, TCR signaling components do not completely escape its reach (1). Thus, while CTLA-4-based regulation is strictly CD28-dependent, PD-1 can also regulate other pathways, particularly in cell types that do not express CD28 such as B cells (10).
Collectively the new studies underscore the pivotal role of the CD28 pathway in T cell immunity and highlight the diverse mechanisms that have evolved to regulate it: CTLA-4 controlling access to B7 ligands, PD-1 controlling CD28 signaling and CD28 downregulation vetoing use of this pathway altogether. They also illustrate how mechanistic understanding of inhibitory receptor function could be used to guide selection of combination therapies. One could envisage teaming a cell-extrinsic regulator with a cell-intrinsic one, or a ligand-competitor with a signaling inhibitor. Moreover, combining agents that require CD28 signaling (such as PD-L1 antibodies) with drugs that promote CD28 re-expression could represent an attractive strategy to improve responses to immunotherapy.
References
- 1.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, Vale RD. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science. 2017;355:1428–1433. doi: 10.1126/science.aaf1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, Sica GL, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science. 2017;355:1423–1427. doi: 10.1126/science.aaf0683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yokosuka T, Takamatsu M, Kobayashi-Imanishi W, Hashimoto-Tane A, Azuma M, Saito T. Programmed cell death 1 forms negative costimulatory microclusters that directly inhibit T cell receptor signaling by recruiting phosphatase SHP2. J Exp Med. 2012;209:1201–1217. doi: 10.1084/jem.20112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011;11:852–863. doi: 10.1038/nri3108. [DOI] [PubMed] [Google Scholar]
- 5.Tai X, Van Laethem F, Sharpe AH, Singer A. Induction of autoimmune disease in CTLA-4-/- mice depends on a specific CD28 motif that is required for in vivo costimulation. Proc Natl Acad Sci U S A. 2007;104:13756–13761. doi: 10.1073/pnas.0706509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang B, Chikuma S, Hori S, Fagarasan S, Honjo T. Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model. Proc Natl Acad Sci U S A. 2016;113:8490–8495. doi: 10.1073/pnas.1608873113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu T, Ji Y, Moseman EA, Xu HC, Manglani M, Kirby M, Anderson SM, Handon R, Kenyon E, Elkahloun A, Wu W, et al. The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Science Immunology. 2016;1 doi: 10.1126/sciimmunol.aai8593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, Sharpe AH, et al. Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature. 2016;537:417–421. doi: 10.1038/nature19330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long SA, Thorpe J, DeBerg HA, Gersuk V, Eddy JA, Harris KM, Ehlers M, Herold KC, Nepom GT, Linsley PS. Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Science Immunology. 2016;1:eaai7793. doi: 10.1126/sciimmunol.aai7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okazaki T, Maeda A, Nishimura H, Kurosaki T, Honjo T. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]