Abstract
Purpose
The clinical swallowing evaluation (CSE) represents a critical component of a comprehensive assessment of deglutition. Although universally utilized across clinical settings, the CSE demonstrates limitations in its ability to accurately identify all individuals with dysphagia. There exists a need to improve assessment and screening techniques to improve health outcomes, treatment recommendations and ultimately mortality in individuals at risk for dysphagia. The following narrative review provides a summary of currently used validated CSE’s and examines the potential role of cough testing and screening in the CSE.
Recent findings
Recent evidence highlights a relationship between objective physiologic measurements of both voluntarily and reflexively induced cough and swallowing safety status across several patient populations. Although more research is needed across a wider range of patient populations to validate these findings; emerging data supports the consideration of inclusion of cough testing during the CSE as an index of airway defense mechanisms and capabilities in individuals at risk for aspiration.
Summary
The sensorimotor processes of cough and swallowing share common neuroanatomical and functional substrates. Inclusion of voluntarily or reflexively induced cough testing in the CSE may aide in the identification of dysphagia and reduced airway protection capabilities.
Keywords: Swallowing, Cough, Clinical swallowing evaluation, Screen, Assessment, Dysphagia
Introduction
Dysphagia (impaired swallowing) impacts the ability of an individual to consume oral intake safely and efficiently. Dysphagia accounts for approximately 7 % of hospital admissions in the United States [1, 2] and is estimated to have a total economic burden of approximately $547 million dollars per year [3]. Impairments in swallowing efficiency refer to difficulties transporting foods and liquids from the oral cavity into the stomach, that result in residues in the oral cavity, pharynx, and esophagus, and are linked to malnutrition [4]. Impairments in airway safety occur when ingested foods or liquids enter the airway (i.e., penetration or aspiration) and are linked to increased pneumonia risk [5]. The inability to eat or drink by mouth is associated with reductions in mental well-being, quality of life, and increased caregiver burden [3, 6–10]. These life-threatening and psychosocial sequelae of dysphagia necessitate timely and accurate identification of swallowing impairment in at-risk individuals to optimize safe oral intake, pulmonary health, and quality of life.
Evaluation of swallowing begins with a “bedside” or clinical swallow examination (CSE). The CSE typically includes a review of patient history, patient-reported symptoms, assessment of the oral mechanism, and observation of liquid and food swallowing trials [11]. Subsequent instrumental evaluation may be performed at the clinician’s discretion, if clinical signs or symptoms warrant further evaluation, and pending the availability of resources. The Videofluoroscopic Swallow Study (VFSS) represents the gold standard instrumental swallowing assessment. It constitutes the only type of assessment with direct visualization of both the oral and pharyngeal phases of swallowing to confirm specific impairments in swallowing that maybe suspected during the CSE, and affords the ability to determine specific contributing mechanisms of oral, pharyngeal, and often esophageal stage impairments [12]. Although VFSS represents the gold standard instrument, many clinicians may rely solely on the CSE given limited or no access to VFSS [13]. Another instrumental evaluation technique, the fiberoptic endoscopic evaluation of swallowing (FEES), is a useful tool in providing a 3-dimensional visualization of the pharyngeal stage of swallowing. FEES is noted to provide superior and direct imaging of pharyngeal anatomy, secretions, and vocal fold movement; however, it is limited in its application in many settings due to access to equipment and skill level of the clinician.
Since the CSE does not permit direct visualization of the swallowing process, its ability to accurately identify individuals who ‘silently’ aspirate (i.e., no cough in response to material entering airway) has been identified as a major limitation [14, 15, 16•]. For example, one study documented that the CSE identified only 30 % of radiographically confirmed aspirators in 107 hospitalized patients [17]. Considering this limitation, research has focused on determining the sensitivity and specificity of various validated clinical tools, screeners, and clinical signs to identify dysphagia or aspiration in order to improve the utility of the CSE [18–21]. For example, can tasks identifying poor lingual movement discriminate safe versus unsafe swallowing? Determining components of the CSE that accurately detect swallowing safety and efficiency during swallowing is a significant research initiative and may reduce error during CSEs.
Although the American Speech-Language-Hearing Association (ASHA) provides guidelines for performing an instrumental evaluation of swallowing [22], only practice recommendations (and no published guidelines) exist for the CSE [13]. As a result, current CSE protocols vary widely and might constitute use of a validated CSE tool (see Table 1) or combinations of various standardized assessments. Further, procedural policies for conducting a standardized CSE are limited and, given the variability in clinical practice patterns, dysphagia recommendations and management strategies also vary [23].
Table 1.
Summary of published clinical swallowing evaluation protocols and screening tools with reference to first author, patient population validated against, protocol items, and test sensitivity and specificity for detecting swallowing impairment and/or aspiration (as specified)
| First author year | Protocol | Administrator | Patient population Number of participants (N) Validation instrument utilized |
Summary of protocol items | Sensitivity: Swallow safetya Dysphagiab |
Specificity: Swallow safetya Dysphagiab |
Volitional cough test included? |
|---|---|---|---|---|---|---|---|
| Martino et al. (2009) [53] | The toronto bedside swallowing screening test (TOR-BSST) | Nurse speech- language pathologist | Inpatient stroke: Acute*; Rehabilitation** (N = 311) VFSS |
Kidd 50 cc water swallow test; tongue movement; general dysphonia; voice quality before and after 50 mL liquid trial | Dysphagiab 91.3 % | Dysphagiab 66.7 % | No |
| Mann (2002) [51] | Mann assessment of swallowing ability (MASA) | Speech-language pathologist | Acute stroke VFSS |
Ratings across the following items: Alertness; cooperation; auditory comprehension; respiration; respiratory rate during swallowing; dysphasia, dyspraxia; dysarthria; saliva; lip seal; tongue movement/strength/co-ordination; gag; palate; voluntary cough; reflexive cough during swallowing; voice; tracheotomy; pharyngeal response; diet recommendations; fluid recommendations | Aspirationa 93 % | Aspirationa 63 % | Yes |
| Antonios et al. (2010) [52] | Modified mann assessment of swallowing ability (MMASA) | Physician Neurologist 1 Neurologist 2 |
Acute stroke* (N = 150) Comprehensive clinical assessment based on MASA |
Items rated? 1–12: alertness; cooperation; respiration; expressive dysphagia; auditory comprehension; dysarthria; saliva; tongue movement; tongue strength; gag; voluntary cough; palatal movements | Dysphagia (N 1) 92 % (N 1) 87 % |
Dysphagia (N 1) 86 % (N 1) 84 % |
Yes |
| Trapl et al. (2007) [55] | Gugging swallowing screen (GUSS) | Speech-language pathologist (Gp 1) Nurse (Gp 2) |
Acute stroke* (N = 50) Group 1 n = 20 Group 2 n = 30 FEES |
Indirect swallow test: laryngeal elevation; saliva swallow; vigilance; voluntary cough; throat clearing Direct swallow test: six ½ tsp. puree, 3, 5, 10, 20, 50 cc thin liquid, small piece dry bread ×5 trials |
Aspirationa PAS = 4 and/or 5 Group 1: 100 % Group 2:100 % |
Aspirationa PAS = 4 and/or 5 Group 1: 50 % Group 2: 69 % |
Yes |
| Edmiaston et al. (2010) [50] | Acute stroke dysphagia screen (ASDS) | Nurse | Acute stroke* (N = 300) MASA |
Glasgow Coma Scale; facial asymmetry; weakness of the face/tongue/palate; 3 oz. water swallow test | Aspirationa 95 % | Aspirationa 68 % | No |
| Edmiaston et al. (2014) [49] | Barnes-jewish hospital Stroke dysphagia Screen (BJH-SDS) |
Nurse | Acute stroke* (N = 225) VFSS |
Glasgow coma scale; asymmetry; weakness; face/tongue/palate function; 3 oz. water swallow | Aspirationa 95 % Dysphagia 91 % |
Aspirationa 50 % Dysphagia 74 % |
No |
| Hinds and wiles (1998) [54] | Timed swallow test | Physician | Acute stroke* (N = 93) |
Swallow questionnaire; 5–10 cc and 100–150 cc thin liquids serial timed swallowing trials | Not assessed | Not assessed | No |
| Logemann et al. (1999) [58] | Northwestern dysphagia Patient check sheet |
Not specified | Heterogeneous*** (N = 200) VFSS |
Medical history; behavioral variables; gross motor function; oral motor exam; swallow trials of: 1 cc thin, 1 cc pudding, ¼ Lorna Doone cookie | Aspirationa & Dysphagia Reported for 28 individual items |
Aspirationa & Dysphagia Reported for 28 individual items |
Yes |
| Suiter and Leder (2008) [59] | 3-oz. Water Swallow test (WST) | Speech-Language Pathologist | Heterogeneous*** (N = 3000) FEES |
90 cc sequential liquid swallow trial. Fail: cannot complete, wet vocal quality, cough/throat clear within one minute after completion |
Aspirationa 96.5 % | Aspirationa 48.7 % | No |
| Suiter et al. (2014) [56] | Yale swallow protocol | Speech-language Pathologist | Heterogeneous*** (N = 25) VFSS |
Cognitive screen; brief oral mechanism examination and liquid swallow trial of 90 cc of water. Patient instructed to drink continuously without stopping | Aspirationa 100 % | Aspirationa 64 % | No |
| Clave et al. (2008) [57] | Volume-viscosity Swallow test (V-VST) | Speech swallow therapist | Heterogeneous*** Control N = 12 Patients N = 85 VFSS |
Swallow trials including 5, 10, and 20 cc of nectar thick liquid, thin liquid, and pudding. | Penetration 83.7 % Aspiration 100 % |
Penetration 64.7 % Aspiration 28.8 % |
No |
cc cubic centimeter; FEES fiberoptic endoscopic evaluation of swallowing; mL milliliter; oz.ounce; VFSS: videofluoroscopic swallow study
Acute stroke defined as patients admitted to hospital immediately following a stroke;
Rehabilitation stroke is defined as Patients in a sub-acute rehabilitation facility following stroke;
Heterogeneous population by author includes the following: Logemann [58]: single stroke, multiple strokes, head and neck cancer, spinal cord injury, other; Suiter [59]: cardiothoracic surgery, esophageal surgery, head and neck surgery, neurosurgery, medical, pulmonary, cancer, other, left stroke, right stroke, brainstem stroke, Parkinson’s disease, dementia, other neurological; Suiter [56]: esophageal surgery, head and neck cancer, neurosurgery, medical, neurological (stroke, multiple sclerosis, traumatic brain injury); Clave [57]: cerebrovascular disease, chronic pneumopathy, diabetes, geriatric diseases, neurodegenerative diseases (amyotrophic lateral sclerosis, multiple sclerosis, Parkinson’s disease, Alzheimer’s disease, Huntington’s disease, Duchenne muscular dystrophy, and other), Zenker’s diverticulum, cricopharyngeal bar, post surgical, and tracheotomy
Swallow Safety: Sensitivity and specify outcome measures in reference to swallow safety status and the presence of penetration or aspiration
Dysphagia: Outcome measures in reference to the presence or absence of general swallowing impairment (dysphagia)
Cough function has been an area of increasing interest in the evaluation, management, and treatment of dysphagia [16•]. This is not surprising given the crucial role cough that serves in defending the airway during swallowing. Indeed, recent research highlights a close relationship between voluntary and reflexive cough airflow measures and airway safety status during swallowing; emerging data suggest that cough airflow measures may serve as a useful physiologic metric to index airway defense capabilities in at-risk individuals [16•, 24•, 25•, 26•, 27, 28, 29•, 30]. The purpose of this narrative review is to examine the relationship between cough and swallow, summarize current validated CSEs, and review the discriminant capacity of both voluntary and reflexive cough testing to detect unsafe swallowing.
Relationship Between Cough and Swallow
Cough is a sensorimotor behavior involved in airway protection to forcefully eject foreign material from the laryngeal vestibule and lower airways [25•, 31, 32, 33]. An effective cough is therefore critical in removing aspirate material from the airway during swallowing, particularly in patients with additional co-morbidities who are more susceptible to developing pulmonary sequelae. In many neurogenic populations, dystussia (impaired cough) and dysphagia are present in parallel [16•, 24•, 26•, 29•, 34], a finding that is not surprising given the shared neural and anatomical substrates of respiration, cough, and swallowing function [31, 35, 36•].
Central pattern generators (CPGs) in the brainstem regulate the processes of eupnea (unlabored respiration), swallow, and cough [37]. The nucleus ambiguus, dorsal respiratory group, and ventral respiratory group located within the brainstem are associated with the neural control of the behaviors of respiration, cough, and swallow [32, 35, 38]. Vagal afferent nerves that are both chemically and mechanically sensitive and non-myelinated c-fibers across multiple afferent beds [39] provide sensory feedback during swallowing (e.g., bolus volume consistency and volume, the presence of aspirate material in the airway) which then informs the swallow central CPG [31]. The CPGs are inherently flexible in their connectivity to allow for rapid, on-line modification between the behaviors of cough, breathing, and swallowing, such as increasing apnea duration due to a larger swallowed bolus or the execution of a rapid and protective cough in response to aspirated material during swallowing [40]. Changes in respiratory muscle activation occur as the swallow CPG is informed about characteristics of the swallow (i.e., safe vs. unsafe, sequential vs. single sip) [41]. Higher order cortical processing or supramedullary input such as sensory integration and motor planning also provides vital input modulating both cough and swallowing behaviors. Computational modeling studies performed to determine neural networks of both cough and swallowing have elucidated shared efferent and afferent pathways involved for breathing, swallowing, and cough [31, 37].
Evidence from human studies suggests that supramedullary input is involved in both voluntary and reflexive cough, with neural processing of stimuli prior to the act of cough resulting in what Davenport et al. [43] have termed as the “urge to cough” [42–45]. This cortical regulatory component in humans is supported by the voluntary suppression of a reflexive cough response [44]. In addition to the established shared central neurologic substrates, functions of respiration, swallow, and cough also peripherally share anatomical structures of the upper airway, pharynx, and oral and nasal cavities. Troche et al. [44] conceptualized a framework for understanding the shared neural and anatomical substrates of cough and swallow in a comprehensive review on this topic [36•]. This conceptual framework presents swallowing and cough along a ‘spectrum of airway protective behaviors,’ with swallowing at one end of the spectrum (protective function) and cough at the opposite end (defensive function) [36•]. Thus, these two sensorimotor acts have highly co-ordinated and reciprocal functions with shared anatomical and neurologic underpinnings that provide a mechanistic, anatomical, and neurologic foundation for considering the role of cough during a clinical swallow examination.
Clinical Swallowing Evaluation
The main components of the CSE include the following: a thorough medical history review; patient and caregiver interview of symptoms; physical inspection of the integrity of swallow anatomy at rest and during movement; and observation of performance on food and liquid swallowing trials [12]. The CSE is typically completed by a certified Speech-Language Pathologist (SLP) and performed across a variety of healthcare settings that include but are not limited to the following: acute, sub-acute, and rehabilitation hospitals; specialized outpatient clinics’ skilled nursing homes; home health care; and assisted living facilities. The objective of the CSE is to obtain information from the patient’s history, self-reported symptoms, and presenting clinical signs to make best clinical judgments regarding swallowing safety and efficiency, and to provide dietary and treatment recommendations. The CSE plays an important role in patient care, and it is critical to accurately identify patients who may have compromised swallow efficiency and airway safety. Dysphagia screening is typically implemented more broadly to asymptomatic patients in order to detect a possible condition [11]. At-risk patient groups (e.g., stroke) are often targeted for dysphagia screening.
Given the previously identified limitations of the CSE to identify all individuals with dysphagia, and barriers to use, there exists a critical need for sensitive screening tools to be incorporated during the CSE [16•, 18, 46]. Given the shared neurologic, anatomic, and mechanic roles of cough and swallow, the potential utility of cough testing in the CSE has been a recent topic of interest to provide information regarding mechanisms of airway safety and the physiologic ability of an individual to defend their airway [16•, 24•, 25•, 29•, 30, 47]. Currently, however, cough testing is not routinely incorporated in the CSE across all settings. There is substantial variability in current practice patterns in the evaluation of swallowing function [48].
Validated Clinical Swallow Evaluations and Screening Tools
Commonly utilized validated clinical swallow protocols include the following: Barnes-Jewish Hospital Stroke Dysphagia Screen (BJH-SDS) [49, 50]; Mann Assessment of Swallowing Ability [51]; Modified Mann Assessment of Swallowing Ability (MMASA) [52]; Toronto Bedside Swallowing Test (TOR-BSST) [53]; Timed Swallow Test [54]; Acute Stroke Dysphagia Screen (ASDS) [49, 50]; Gugging Swallow Screen (GUSS) [55]; Yale Swallow Protocol [56]; Volume-Viscosity Test [57]; Northwestern Dysphagia Patient Check Sheet [58]; and the 3-oz. Water Swallow [59]. Table 1 provides a summary of these published, validated CSEs, with reference to the patient population the tool was validated for, tool administration, inclusion of cough testing, and any published statistical data regarding its discriminant ability to identify dysphagia or unsafe swallowing.
Of the 11 validated CSEs commonly used, 6 were designed to be administered specifically by Speech-Language Pathologists, 3 to be administered by trained nursing staff, and 1 by physicians (Logemann et al. [58]). Review of published reports indicates that the highest levels of sensitivity (>85 %) for detecting aspiration is provided by the BJH-SDS [49, 50], Acute Stroke Dysphagia Screen (ASDS) [49, 50], Yale Swallow Protocol [56], Volume-Viscosity Test [57], Northwestern Dysphagia Patient Check Sheet [58], and the 3-oz. Water Swallow Test [59]. However, none of the protocols reach the highest level (>85 %) of reported overall specificity for detecting aspiration. The Modified Mann Assessment of Swallowing Ability, a physician-administered protocol, provides the highest levels of sensitivity and specificity for detecting global swallowing impairment (i.e., 92 and 87 %, respectively).
Of the 11 validated CSEs, 4 (36 %) incorporate some form of cough testing. Description of cough testing methodology varies within the context of each examination. Upon careful inspection of the published protocols that include cough assessment, specific instructions for eliciting the cough task are vague, and the subjective perceptual measures of cough vary between protocols. The MMASA (same tasks as the MASA for cough testing) contains the most detailed instruction for cough elicitation and perceptual cough judgment. Per protocol, the physician or administrator asks the patient to ‘cough as strong as possible’ [52]. Judgments of cough strength and clarity are rated, with an outcome score being assigned corresponding to one of the following: no abnormality, cough attempted but is hoarse in quality, attempt inadequate, no attempt, or unable to perform. Logemann et al. [58] described a subjective cough assessment in the Northwestern Dysphagia Patient Check Sheet, in which administrators judge either a voluntary cough, or throat-clearing maneuver, and perceptually rated the strength of the behavior. A strong cough/throat clear was judged as ‘safe’, and weak cough/throat clear was judged as ‘unsafe’ [58]. The GUSS includes an assessment of ‘voluntary cough’ without reference to specific cough task instruction; the cough task is rated based on a weak or absent response [55].
Laciuga and colleagues recently investigated relationships between perceptual ratings of cough and objective airflow measures of cough [60]. Thirty clinicians (speech-language pathologists, otolaryngologists, and neurologists) rated the subjective parameters of strength, duration, quality, quantity, and overall ‘effectiveness’ of ten audio recordings of cough containing specific airflow characteristics. Objective physiological aerodynamic parameters of cough airflow were associated with the clinical perception of cough strength and effectiveness. The specific parameters that were clinically perceived as strong and effective included the following: compression phase duration, peak expiratory flow rate, peak expiratory flow rise time, cough volume acceleration, and total expired volume. Interestingly, only 4 CSE protocols reviewed here currently utilize perceptual judgment of cough as part of the swallowing examination, and none include physiologic measures of cough airflow.
Utility of Voluntary Cough Testing in Dysphagia
Voluntary or volitional cough testing involves asking a patient to cough (typical instructions are “as hard as you can” or “like have something stuck in their throat”). The resulting motor output can then be assessed either subjectively by listening, or objectively with specialized equipment. For a complete review of the physiologic components of cough, we refer readers to Smith-Hammond et al. [25•]. Briefly, cough is characterized by three distinct phases:
Inspiratory phase: composed of contraction of the external intercostal muscles elevating the anterior rib cage and drawing down the diaphragm as it contracts [61], while laryngeal muscle activation allows for passage of air through the glottis resulting in a negative pressure drawing air into the lungs [61, 62].
Compression phase: during which adduction of the vocal folds builds and maintains subglottic pressure generation.
Expiratory phase: composed of a forceful and rapid abduction of the vocal folds.
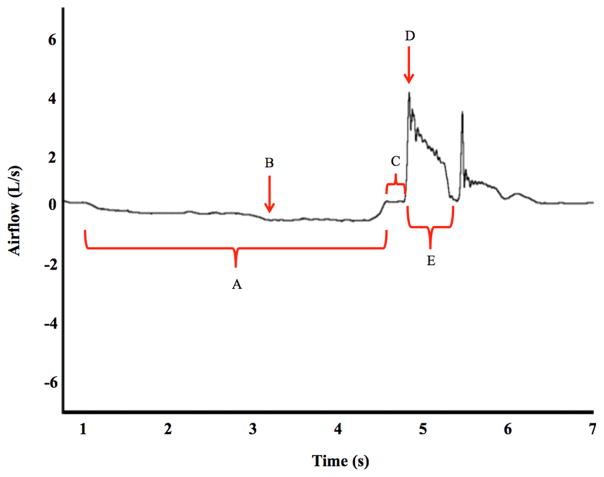
Physiologic cough testing using the gold standard pneumotachograph measures airflow signals across all three phases that can be subsequently analyzed using specialized software. Objective cough flow measures can be derived and are illustrated in Fig. 1 with definitions provided in Table 2.
Fig. 1.
Example of voluntary cough waveform measured with cough spirometry. Selected derived objective measures are delineated on the waveform and referenced in Table 2 including a inspiratory phase duration, b inspiratory peak flow, c compression phase duration, d peak expiratory flow rate, and e cough expired volume. Expiratory rise time is calculated by subtracting time at end of compression from peak expiratory flow time. Cough volume acceleration is not depicted but is calculated by dividing peak expiratory flow rate by the expiratory rise time
Table 2.
Definitions of objective voluntary cough airflow measures with reference to illustrative cough waveform depicted in Fig. 1
| Figure reference | Objective cough measure | Description |
|---|---|---|
| Inspiratory phase | ||
| A | Inspiratory phase duration (s) | Time from onset of inspiration at 0 L/s to the beginning of glottic closure [1–3] or the start of the expiration onset if there is no appreciable compression phase [3] |
| B | Inspiratory peak flow rate (L/s) | Peak inspiratory flow during the inspiratory phase [1–3] |
| Compression phase | ||
| C | Compression phase duration (s) | Time to glottic opening measured from the end of the inspiratory phase to the beginning of the expiratory phase [1–4] |
| Expiratory phase | ||
| D | Peak expiratory flow rate (L/s) | Peak expiratory airflow during the expiratory phase of the cough [1–4] |
| E | Cough expired volume (%) | Percent of total expired volume of air expired during a cough epoch [4] |
| Expiratory rise time (s) | Time from the beginning of the expiratory phase to the peak of the expiratory flow [1–3] | |
| Cough volume acceleration (L/s/s) | A ratio measure derived by dividing expiratory peak flow by expiratory rise time. Proposed to be a measure of cough effectiveness [1–4] | |
Specific references of published studies utilizing each measure are also provided
Several investigators have examined relationships between voluntary cough airflow measures and swallow safety status to elucidate the clinical utility of voluntary cough spirometry testing in several patient populations [16•, 24•, 25•, 30]. These are summarized in Table 3 and reviewed below.
Table 3.
Review of six research studies investigating the significant differences in voluntary swallowers in dysphagic populations including Stroke, Parkinson’s disease, and Amyotrophic cough measures between unsafe (penetrators and/or aspirators) and safe (non-aspirators) Lateral Sclerosis
| First author year | Patient population Number of participants (N) Swallowing safety groups |
Swallowing assessment method Testing stimuli Statistical comparison |
Significant outcomes: summary of results |
|---|---|---|---|
| Smith-Hammond et al. (2001) [25•] | Stroke N = 43 Stroke; 18 control Three airway safety groups: Severe aspirators (asp’ on all bolus trials) Mild aspirators (asp’ on one or two bolus consistencies) Non-aspirators (no asp’ across trials) |
VFSS or FEES (group n is not specified) 5 mL, 15 mL, and unregulated cup sips of thin liquid, ensure plus, and ‘thickened liquid’ (250–300 cP). (Liquid prepared to match available drinks to inpatients) Between groups comparison (severe aspirators vs. non-aspirators) |
Severe aspirators (vs. non-aspirators) demonstrated the following: Lower peak inspiratory flow rate (770.60 vs. 1120 mL/s) Lower peak expiratory flow rate (−875.13 vs. −1,884.14 mL/s) Higher expiratory rise times (0.34 vs. 0.09 s) Lower cough volume acceleration (5.49 vs. 27.84 mL/s/s) |
| Pitts et al. (2008) [24•] | Parkinson’s disease N = 20 Safe: PAS score 1 Unsafe: PAS score 2–8 |
VFSS 30 mL liquid Between groups comparison (safe vs. unsafe) |
PD patients with unsafe PD swallowing demonstrated the following: Longer compression phase durations (0.36 vs. 0.16 s) Higher expiratory rise times (0.41 vs. 0.21 s) Lower peak expiratory flow rate (6.17 vs. 8.94 L/s) Lower cough volume acceleration (17.02 vs. 45.24 L/s/s) |
| Smith-Hammond et al. (2009) [30] | Stroke N = 96 Non-aspirators: PAS score 1–4 Aspirators: PAS score 5–8 |
VFSS (n = 91) or FEES (n = 5) Pearson correlation coefficient to determine associations between aspiration risk (PAS ≥ 5) and objective cough measures |
Stroke patients who aspirated demonstrated the following: Lower inspiration phase volume (0.45 vs. 0.69 L) Lower inspiration peak flow (−0.82 vs. −1.44 L/s) Lower peak expiratory flow rate (1.98 vs. 5.62 L/s) Higher expiratory rise times (161.50 vs. 14.05 ms) Lower cough volume acceleration (23.49 vs. 136.15 L/s/s) |
| Pitts et al. (2010) [29•] | Parkinson’s disease N = 58 Safe: PAS score 1 Unsafe: PAS score 2–8 |
VFSS 30 mL liquid Receiver operator characteristic analysis |
Discriminant ability of voluntary cough airflow measures to detect penetration/aspiration: Compression phase duration: CP: 0.2 s, sensitivity: 95.8 %, specificity: 64.7 %, LR: 2.7, AUC: 0.83 Expiratory phase rise time: CP: 70.8 ms, sensitivity: 70.8 %, specificity: 64.7 %, LR: 2.7, AUC: 0.71 Expiratory phase peak flow: CP: 7.5 L/s, sensitivity: 87.5 %, specificity: 50 %, LR: 1.8, AUC: 0.69 Cough volume acceleration: CP: 84.5 s/s, sensitivity: 54.5 %, specificity: 97.1 %, LR: 18.4, AUC: 0.72 |
| Hegland et al. (2014) [26•] | Parkinson’s disease N = 40 Safe: PAS score 1–2 Unsafe: PAS score 3–8 |
VFSS ~5 mL thin liquid; cup sip thin liquid; two sequential sips thin liquid; spoon-sized pudding bolus; cookie coated in barium Between groups comparison among cough parameters and penetrator/aspirator vs. non-P/A |
On the first cough of the epoch, PD patients with safe vs. unsafe (PAS ≥ 4) swallowing demonstrated the following: Longer compression phase durations (0.45 vs. 0.22 s) Lower peak expiratory flow rates (5.51 vs. 4.19 L/s) Lower amount of air expired during the sequential cough (49 vs. 42 %) |
| Plowman et al. (2016) [16•, 46] | Amyotrophic lateral sclerosis N = 70 Safe: PAS score 1–2 Unsafe: PAS score 3–8 |
VFSS 20 mL liquid Between group comparisons and receiver operator characteristic analysis |
Unsafe ALS patients demonstrated the following: Lower cough volume acceleration (33.21 vs. 103.71 L/s/s) Longer peak expiratory rise times (159.20 vs. 78.80 ms) Lower peak expiratory flow rate (2.88 vs. 5.31 L/s) Discriminant ability of voluntary cough airflow measures to detect penetration/aspiration: Cough volume acceleration: CP: 45.3 s/s, sensitivity: 91.3 %, specificity: 82.2 %, LR: 5.1, AUC: 0.85 Expiratory rise time: CP: 80 ms, sensitivity: 82.6 %, specificity: 73.9 %, LR: 3.2; AUC: 0.81 Peak expiratory flow: CP: 3.98 L/s, sensitivity: 73.9 %, specificity: 78.3 %, LR: 3.4; AUC: 0.78 |
FEES fiberoptic endoscopic evaluation of swallowing; PAS Penetration–Aspiration Scale; VFS: videofluoroscopic swallow study; CP cut point; AUC area under the curve value; PPV positive predictive value, LR likelihood ratios
Stroke
Smith-Hammond et al. [25•] first examined the relationship between objective voluntary cough airflow measures and swallowing, and noted significant relationships between expulsive rise times and aspiration status (p < 0.001) in 43 stroke patients [34]. Subsequently, Smith-Hammond et al. [30] expanded these preliminary findings in a larger cohort of 96 stroke patients who underwent cognitive testing, a CSE, voluntary cough spirometry testing, cough sound pressure level testing (dB SPL), and an instrumental swallow evaluation (either FEES or VFSS). Swallow safety status was objectively defined using the Penetration–Aspiration Scale (PAS) score [30], with participant groups delineated into non-aspirators (PAS ≥4) versus aspirators (PAS ≥5). Clinical indications such as absent swallow initiation, difficulty with secretions, and elicitation of postprandial reflexive cough had an overall sensitivity of 53 % and specificity of 83 %, indicating poor sensitivity and moderate specificity in relation to the clinical assessment measures. Acoustic cough testing demonstrated clinical utility, with mean cough sound pressure levels significantly lower in aspirators compared to non-aspirators (83.7 vs. 96.4, dB SPL, p < 0.0001). There were significant differences in several cough airflow measures between the groups. Specifically, non-aspirators demonstrated lower inspiration phase volume (0.45 vs. 0.69L, p < 0.05), lower inspiration peak flow (−0.82 vs. −1.44 L/s, p < 0.0001), lower peak expiratory flow rate (1.98 vs. 5.62 L/s, p < 0.0001), higher expiratory rise times (161.50 vs. 14.05 ms, p < 0.0001), and lower cough volume acceleration (23.49 vs. 136.15 L/s/s, p < 0.0001). These authors concluded that, in addition to instrumental swallowing assessment techniques, objective measures of voluntary cough spirometry may be useful in identifying airway safety status in individuals post stroke [30].
Parkinson’s Disease
Pitts et al. [24•] first documented relationships between voluntary cough airflow measures and swallowing airway safety status in 20 individuals with Parkinson’s disease (PD). Unsafe PD swallowers (PAS ≥2) demonstrated longer compression phase durations (0.36 vs. 0.16 s, p < 0.001), longer peak expiratory rise times (0.41 vs. 0.21 s, p < 0.001), lower peak expiratory flow rates (6.17 vs. 8.94 L/s, p < 0.001), and lower cough volume accelerations (17.02 vs. 45.24 L/s/s, p < 0.001).
In a larger follow-up investigation, Pitts and colleagues [29•] evaluated the discriminant ability of voluntary cough airflow measures for detecting unsafe swallowing in 58 individuals with PD. Results of this work indicated that the same four cough measures that were reported to be different in their earlier study demonstrated good discriminant ability to detect unsafe PD swallowers [29•].
Hegland et al. [26•] most recently demonstrated that sequential voluntary cough is associated with airway safety status in individuals with PD [26•]. Airflow measures were recorded and objective cough spirometry measures, including percent cough expired volume (%CEV), were obtained across two trials of sequential voluntary coughs. Significant differences between safe (PAS ≤2) vs. unsafe (PAS ≥3) swallowing groups were noted for the following: compression phase duration, expiratory peak flow, and percent cough expired volume (p < 0.05). PD patients with safe swallowing demonstrated coughs with higher peak expiratory flow rates, cough volume acceleration, and percent cough expired volume (i.e., significantly different in the first and third expiratory effort). Further, Hegland and colleagues noted that differences in cough expired volumes between safe and unsafe swallow groups provided evidence of unco-ordinated sequential cough patterns in the unsafe swallow PD subjects [26•].
Amyotrophic Lateral Sclerosis
Plowman et al. [16•] studied voluntary cough spirometry airflow measures and airway safety status in 70 individuals with amyotrophic lateral sclerosis (ALS). Participants completed both voluntary cough airflow testing and a VFSS, and were grouped into safe (PAS ≤2) or unsafe (PAS ≥3) ALS swallowers. Similar to the findings in stroke and PD patient populations, significant differences were observed across a number of measures. ALS patients with unsafe swallowing demonstrated lower cough volume acceleration (33.21 vs. 103.71 L/s/s, p = 0.00001), longer peak expiratory rise times (159.20 vs. 78.80 ms, p = 0.003), and lower peak expiratory flow rates (2.88 vs. 5.31 L/s, p = 0.00005). Further, these three expiratory phase measures showed a good discriminant ability to detect the presence of penetration and/or aspiration (see Table 3 for full results) [16•]. Sensitivity and specificity were highest for cough volume acceleration (91.3 and 82.2 %, respectively), and ALS patients whose cough volume acceleration was below 45.28L/s/s were 5.12 times more likely to penetrate/aspirate. These authors concluded that impairment in the expiratory phase of voluntary cough may be related to degeneration of laryngeal, respiratory, and upper aerodigestive tract musculature, which compromises the ability to build ballistic force generation needed for an effective expiration phase [16•]. Recommendations were made for the consideration of cough flow testing in the clinical screening of individuals with ALS, and the use of their published cut points as references when considering airway safety risk status [16•].
These studies, across three different neurogenic patient populations, highlight the potential utility of voluntary cough assessment during the clinical evaluation of swallowing. Several limitations exist, however, regarding the practical implementation of such testing protocols. First, the equipment required to perform such testing is expensive and likely cost prohibitive in most clinical settings. Second, specialized software and training of personnel are required to analyze cough waveforms, and the analyses are labor and time intensive. Finally, this equipment is not easily portable, posing a barrier to access in certain patient populations. A potential alternative to the gold standard pneumotachograph airflow testing techniques utilized in the aforementioned studies is the use of a handheld digital, or analog, peak cough flow meter capable of measuring peak cough flow (L/s) and forced expiratory volume (FEV1, L) in real time without the need for waveform analysis or cost prohibitive equipment. Indeed, Silverman et al. [63] recognized this need and studied the concordance of several handheld digital and analog peak cough flow devices to quantify peak cough airflows compared to the gold standard pneumotachograph method. Silverman et al. [63] indicated that both digital and analog devices (the Mini Wright peak flow meter, and Mini Wright digital peak flow meter) demonstrated good concordance with the gold standard method for measuring peak cough flow in healthy males and older female PD patients. The analog peak airflow device was reported to demonstrate a higher level of concordance for cough strength in both healthy and disease states [63]. It is important to note, however, that these devices do not provide the detailed measurement parameters offered by cough spirometry testing. Additionally, there is contraindicating evidence that documents have a poor agreement between portable peak flow meter readings and the peak cough flow as measured by the gold standard physiologic assessment (i.e., pneumotachograph) [64]. Further research is necessary to determine the validity of voluntary cough testing using such handheld devices in several patient populations and healthy controls.
An additional consideration regarding the utility of voluntary cough testing in the evaluation of swallowing function is the fact that evaluating a volitional cough (i.e., asking a patient to cough) does not provide direct information on the nature of a protective cough response to aspirated material during swallowing (i.e., triggered by afferent stimuli in the airway). Additionally, voluntary cough production is highly dependent on instruction. That is, airflow patterns and perceived “strength” of a cough have been noted to change in a graded manner based upon the instruction provided [43]. A testing method that more closely models an airway protective cough response is the reflexive cough testing method, which will be discussed next.
Reflexive or Induced Cough Testing
Another method of testing cough is to perform reflexive cough testing to induce or elicit a cough response and measure response profiles. Using this method, an individual inhales an aerosolized irritant such as capsaicin, citric acid aerosols, fog, tartaric acid, acetic acid, or hypertonic solutions [65] that can be delivered at different concentrations through a nebulizer or face mask. A patient’s response profile can then be measured and their cough threshold determined and compared to normative values. Outcomes can be as simple as a binary measure (present/absent cough response), or airflow parameters can be measured using the cough spirometry techniques previously discussed. In addition to measuring the motor output of the cough response, the afferent aspect of this sensorimotor behavior can be probed by asking the patient their perceived ‘urge to cough’ using a modified Borg scale across each cough trial [43]. Cough output is affected by irritant type, concentration, volume and duration of exposure, order of presentation, placebo trials, nasal afferent stimulation, and lung volume at the start of cough initiation [36•]. These variables impact cough flow rates, number of coughs produced, urge to cough (self-report), amplitude and duration of expiratory muscle activation, and time to initiation of a cough response [36•]. Similar to voluntary cough testing, several investigators have examined the potential discriminant ability of reflexive cough testing in determining swallowing safety status, which will now be highlighted. A summary of these studies is provided in Table 4.
Table 4.
Summary of published reports investigating the discriminant ability of reflexive cough testing to detect swallowing safety
| First author year | Patient population Number of participants (N) Swallowing assessment method |
Reflex cough testing protocol | Summary of results and conclusions |
|---|---|---|---|
| Sato et al. (2012) [28] | Heterogeneous* (N = 141) FEES |
Oral inhalation of citric acid (1 % w/v) via nebulizer delivered for 1 min or until first cough Outcome: Time (seconds) from start of inhalation to first cough Patients divided into cough response groups based on response time (in 5 s intervals) |
Results: discriminant ability of reflex cough testing to distinguish: Silent aspirators vs. non-silent aspirators: A cut value of 30 s to elicit first cough to stimuli distinguished silent aspirators vs. aspirators (sensitivity: 92 %, specificity: 94 %, PPV: 97 %, NVP: 83 %) Silent aspirators vs. non-silent and safe swallowers: A cut value of 60 s to elicit first cough to stimuli distinguished silent aspirators vs. non-silent and safe swallowers. (sensitivity: 81 %, specificity: 65 %, PPV: 45 %, NVP:91 %) Conclusions: The ability to detect silent aspiration (true positive) in this heterogeneous patient population was high (0.81); however, there were a high number of false positives (0.65); therefore, this methodology may over identify individuals with unsafe swallowing |
| Miles et al. (2013) [14] | Heterogeneous* (N = 181) VFSS or FEES |
Citric acid (0.4, 0.6 & 0.8 mol/L) and placebo of 0.9 % sodium chloride. Each concentration delivered from low to high for 15 s via facemask and placebo administered between citric acid doses Outcome: The presence/absence of a positive cough response of cough during 15 s delivery. ** |
Results: Discriminant ability of reflexive cough test to detect aspiration in individuals undergoing VFSS: 0.4 mol/L: sensitivity = 77 %, specificity = 57 % 0.6 mol/L: sensitivity = 71 %, specificity = 71 % 0.8 mol/L: sensitivity = 59 %, specificity = 86 % Discriminant ability of reflexive cough test to detect aspiration in individuals undergoing FEES: 0.4 mol/L: sensitivity = 69 %, specificity = 81 % 0.6 mol/L: sensitivity = 50 %, specificity = 93 % 0.8 mol/L: sensitivity = 44 %, specificity = 96 % Conclusions: Sensitivity and specificity of reflex cough testing varied dependent upon the specific dose of citric acid administered. Sensitivity was higher at lower doses while specificity was higher at higher doses of citric acid |
| Hegland et al. (2016) [66] | Healthy volunteers (N = 49) Parkinson’s disease (N = 21) VFSS |
Capsaicin (200 μM, 1 inhalation) and distilled water (fog, continuous for one min or until a cough occurred) delivered via nebulizer Three trials of each irritant administered (total 6 trials) Outcome: Total number of coughs within 30 s and a binary response/no response outcomea |
Results: Binary responder/non-responder data: The discriminant ability of cough reflex testing to detect penetration and/or aspiration in PD patients: Capsaicin: sensitivity of 44.4 % and specificity of 100 % Fog: sensitivity of 77.8 % and specificity of 90.9 % Unsafe PD swallowers produced fewer coughs to both fog and capsaicin. Single-breath inhalation of 200 μM of capsaicin yielded a high false positive rate and thus may not be the correct methodology to implement to rule out aspiration in this population. Authors report the difference in response to fog vs. capsaicin suggests possible differences in neural control of cough regulation |
FEES fiberoptic endoscopic evaluation of swallowing; NPV negative predictive value; OR odds ratio; PAS penetration aspiration scale; PPV positive predictive value; SCT simplified cough test (reflexive cough test); VFSS videofluoroscopic swallow study
Heterogeneous sample Sato et al. [28] included 141 consecutive patients; 89 individuals post stroke, 22 disuse syndrome, 8 neuromuscular, 14 respiratory, 3 cancer, 2 cervical spine injury, 3 miscellaneous. Neuromuscular disease included Parkinson’s disease, corticobasal degeneration, multiple systems atrophy, and spinocerebellar degeneration. Miles et al. [14] included Stroke, Head and neck cancer, respiratory, progressive neurological, other neurological, and other
Positive cough response defined as two or more consecutive coughs triggered
Binary classification based on total number of coughs produced was as follows: “responders” were defined as those who produced at least 2 coughs on 2/3 trials for each irritant type independently (fog and capsaicin)
Sato et al. [28] evaluated 141 consecutively referred patients with non-specific complaints of dysphagia. Primary medical diagnoses included stroke, neuromuscular disease, deconditioning, respiratory disease, cancer, cervical spinal injury, and ‘miscellaneous.’ FEES was utilized to determine airway safety status, yielding 53 unsafe swallowers (aspirators) and 88 safe swallowers (no aspiration). Reflex cough testing was performed using a citric acid–saline solution (1 % weight/volume (w/v)) to induce a reflexive cough with time from citric acid administration to elicitation of the first cough, the primary metric of interest. Results indicated that time to first cough demonstrated excellent discriminant ability for identifying silent aspirators in this cohort. Specifically, a value of 30 s post-irritant administration to the first cough demonstrated a sensitivity and specificity for detection of silent aspiration of 92 and 94 %, respectively. When including all aspirators, however, a cutoff of 60 s for cough reaction time yielded a sensitivity and specificity for detection of aspiration at 81 and 65 %, respectively. These results suggest that subtle differences in cough reaction time affect the accuracy of detecting silent aspiration.
Miles et al. [14] examined the utility of reflexive cough testing for identification of silent aspiration in 181 consecutively referred inpatients with diagnoses including stroke, head and neck cancer, ‘respiratory disease,’ progressive neurologic disease, and ‘other.’ All individuals were evaluated with reflexive cough testing and an instrumental swallowing evaluation (either FEES or VFSS). Swallowing safety status was determined by a blinded SLP who rated either the FEES or VFSS using the PAS scale, and patients were grouped by the following: no aspiration, aspiration with cough (not specified if it was an effective cough), trace silent aspiration, and silent aspiration.
Cough thresholds were evaluated using randomly administered citric acid solutions (0.4, 0.6, and 0.8 mol/L, placebo) via facemask nebulizer on a continuous flow. The primary outcome measure was the presence or absence of cough following each 15-s interval. The trial was considered a “positive” response if the patient coughed two or more times at a given concentration. Additionally, researchers perceptually rated subjective cough response strength (weak or strong). The concentration of 0.6 mol/L was shown to have the highest level of accuracy for discriminating between safe and unsafe swallowers on the VFSS (sensitivity of 71 %, specificity of 60 %). However, these values are considered below ideal for a good screening tool.
More recently, Hegland et al. [66] investigated cough response profiles to varied irritant types in both healthy controls and individuals with PD. Patients underwent VFSS and were categorized into safe (non-aspirators, PAS ≤4) vs. unsafe (aspirators, PAS ≥5) swallowing groups. Irritant stimuli included diluted capsaicin (200 μM dissolved in vehicle solution of 80 % physiologic saline and 20 % ethanol) and aerosolized water (fog). Both irritants were delivered through a nebulizer (Omron Micro-Air NE U22 V) for 60 s, and the mean number of coughs produced within a 30 s time frame and categorical ‘responders’ and ‘non-responder’ data was collected. For binary responder/non-responder outcomes, there were differences in response to irritant type with regards to the sensitivity and specificity for detecting laryngeal penetration and/or aspiration. Specifically, capsaicin yielded a sensitivity of 44.4 % and specificity of 100 %, and fog yielded a sensitivity of 77.8 % and specificity of 90.9 %. Additionally, there were significant differences in the number of coughs produced between safe and unsafe swallowers, with unsafe swallowers producing fewer coughs to both fog and capsaicin.
Hegland and colleagues reported poor sensitivity (20 %) but good specificity (95.9 %) for detecting unsafe swallowing with reflexive cough testing (using capsaicin) in PD [66] and concluded that the high false negative (not detecting an impairment) may indicate that the single inhalation may not be the correct methodology to implement to rule out aspiration in this population. The authors also reported that a difference in response to fog versus capsaicin suggests possible differences in neural control of cough regulation.
Kallesen et al. [67] investigated the clinical utility of reflexive cough testing for assessment of swallowing impairment in 106 recently extubated intensive care unit patients [67]. Patients underwent FEES evaluation and reflexive cough testing with concentrations of 0.4, 0.6, and 0.8 mL/L nebulized citric acid mixed with 0.9 % sodium within 24 h of extubation. The PAS was used to differentiate penetrators (PAS ≤5) versus aspirators (PAS ≥6), yielding 13 aspirators, 9 of which were identified as silent aspirators (69 %). Concentrations of 0.4, 0.6, and 0.8 mL/L demonstrated sensitivity values of 100, 100, and 88 %, and a specificity of 42, 49, and 58 % for detecting aspiration, respectively. Kallesen and colleagues concluded that reflex cough testing over-identified aspiration in this patient population.
Multiple variables can be manipulated when performing reflexive cough testing and, thus, may result in drastically different patient responses. These studies highlight the potential utility of reflexive cough evaluation for the assessment of aspiration status and also provide complimentary literature to the voluntary cough testing. Cough reflex testing methodology may be more practical as part of a screening assessment as the methodology is inexpensive, quick to administer, and objective outcomes relatively are simple to interpret. However, the lack of consensus for testing protocols and scarce data in multiple patient populations highlight an important gap in the literature. This leads to the inability to provide cohesive practice recommendations in regards to the optimal irritant type and strength of solution, length of delivery, and outcome measures. Although these articles provide an excellent foundation, more research is warranted to provide guidelines to practicing clinicians.
Limitations and Future Directions
Although an emerging and promising dataset supports the use of cough testing in the clinical evaluation of swallowing, current data are limited and restricted to only a few patient populations with a critical need for more data to validate these promising findings in other patient populations. Practical limitations of objective voluntary cough testing procedures necessitate the need for further studies to examine the discriminant ability of simple and inexpensive cough testing using handheld peak flow meters, similar to the work of Silverman and colleagues [63]. Reflexive cough testing represents a relatively simple, inexpensive, and relatively quick method of testing that is currently being utilized clinically by Dr. Karen Hegland in a busy clinic for individuals with Parkinson’s disease, with binary cough threshold testing and urge to cough screens performed routinely at every patient visit (Hegland, personal communications).
Conclusions
This narrative review highlights the shared neural and anatomical substrates mediating cough and swallowing, as well as the co-occurring presence of dystussia and dysphagia. Additionally, the role of cough in defending the airway and rationale for providing a physiologic index of airway defense in patients at risk for dysphagia has been delineated. A small but growing body of literature supports the inclusion of cough testing in the CSE to provide an index of overall function and capacity of airway defense mechanisms to aide in clinical and diagnostic decision-making and assessment of potential risk of impairments in swallowing safety. Clearly, more data are needed to validate these findings, in addition to using practical, inexpensive, and efficient methods that can be easily implemented in busy clinical settings to provide valid and reliable results across practice settings.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest Emily K. Plowman reports R21HD075327 from the National Institute of Child Health and Development. Stephanie A. Watts and Lauren Tabo declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
- 1.Altman KW. Dysphagia evaluation and care in the hospital setting: the need for protocolization. Otolaryngol Head Neck Surg. 2011;145(6):895–8. doi: 10.1177/0194599811415803. [DOI] [PubMed] [Google Scholar]
- 2.Altman KW, Yu GP, Schaefer SD. YConsequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136(8):784–9. doi: 10.1001/archoto.2010.129. [DOI] [PubMed] [Google Scholar]
- 3.Cichero JA, Altman KW. Definition, prevalence and burden of oropharyngeal dysphagia: a serious problem among older adults worldwide and the impact on prognosis and hospital resources. Nestle Nutr Inst Workshop Ser. 2012;72:1–11. doi: 10.1159/000339974. [DOI] [PubMed] [Google Scholar]
- 4.Moreira NC, Krausch-Hofmann S, Matthys C, Vereecken C, Vanhauwaert E, Declercq A, Bekkering GE, Duyck J. Risk factors for malnutrition in older adults: a systematic review of the literature based on longitudinal data. Adv Nutr. 2016;7(3):507–22. doi: 10.3945/an.115.011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabre M, et al. Oropharyngeal dysphagia is a risk factor for readmission for pneumonia in the very elderly persons: observational prospective study. J Gerontol A. 2014;69(3):330–7. doi: 10.1093/gerona/glt099. [DOI] [PubMed] [Google Scholar]
- 6.Plowman-Prine EK, et al. The relationship between quality of life and swallowing in Parkinson’s disease. Mov Disord. 2009;24(9):1352–8. doi: 10.1002/mds.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tabor L, et al. Defining swallowing-related quality of life profiles in individuals with amyotrophic lateral sclerosis. Dysphagia. 2016;31(3):376–82. doi: 10.1007/s00455-015-9686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paris G, et al. Clinical screening of oropharyngeal dysphagia in patients with ALS. Ann Phys Rehabil Med. 2012;55(9–10):601–8. doi: 10.1016/j.rehab.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Maclean J, Cotton S, Perry A. Dysphagia following a total laryngectomy: the effect on quality of life, functioning, and psychological well-being. Dysphagia. 2009;24(3):314–21. doi: 10.1007/s00455-009-9209-0. [DOI] [PubMed] [Google Scholar]
- 10.Leow LP, et al. The impact of dysphagia on quality of life in ageing and Parkinson’s disease as measured by the swallowing quality of life (SWAL-QOL) questionnaire. Dysphagia. 2010;25(3):216–20. doi: 10.1007/s00455-009-9245-9. [DOI] [PubMed] [Google Scholar]
- 11.Suiter DM. Dysphagia screening and the clinical swallow evaluations. Atlanta, GA: American Speech Language and Hearing Association Convention; 2012. [Google Scholar]
- 12.Logemann JA. Evaluation and treatement of swallowing disorders. NSSLHA Journal. 1984;12:38–50. [Google Scholar]
- 13.American Speech-Language-Hearing Association. Clinical indicators for instrumental assessment of dysphagia (guidelines) ASHA Suppl. 2000;20:18–9. [Google Scholar]
- 14.Miles A, et al. Comparison of cough reflex test against instrumental assessment of aspiration. Physiol Behav. 2013;118:25–31. doi: 10.1016/j.physbeh.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Smith Hammond C. Cough and aspiration of food and liquids due to oral pharyngeal dysphagia. Lung. 2008;186(Suppl 1):S35–40. doi: 10.1007/s00408-007-9064-4. [DOI] [PubMed] [Google Scholar]
- 16•.Plowman EK, et al. Voluntary cough airflow differentiates safe versus unsafe swallowing in amyotrophic lateral sclerosis. Dysphagia. 2016 doi: 10.1007/s00455-015-9687-1. Study identifies swallow and cough impairemnt in ALS as well as provides cut-off measures for peak expiratory flows valuable for clinical use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Splaingard ML, Hutchins B, Sulton LD, Chaudhuri G. Aspiration in rehabilitation patients: videofluoroscopy vs bedside clinical assessment. Arch Phys Med Rehabil. 1988;69(8):637–40. [PubMed] [Google Scholar]
- 18.McCullough GH, Wertz RT, Rosenbek JC. Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord. 2001;34:55–72. doi: 10.1016/s0021-9924(00)00041-1. [DOI] [PubMed] [Google Scholar]
- 19.McCullough GH, Rosenbek JC, Wertz RT, McCoy S, Mann G, McCullough K. Utility of clinical swallowing examination measures for detecting aspiration post-stroke. J Speech Lang Hearing Res. 2005;48:1280–93. doi: 10.1044/1092-4388(2005/089). [DOI] [PubMed] [Google Scholar]
- 20.McCullough GH, Martino R. Clinical Evaluation of Patients with Dysphagia: Importance of History Taking and Physical Exam. Newyork: Springer; 2013. pp. 11–30. [Google Scholar]
- 21.Rosenbek JC, McCullough GH, Wertz RT. Is the information about a test important? applying the methods of evidence-based medicine to the clinical examination of swallowing. J Commun Disord. 2004;37(5):437–50. doi: 10.1016/j.jcomdis.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Karen Dikeman JG, Hiss S, chair, Inman A, Kelchner L, Lazarus C, Miller C. Guidelines for speech-language pathologists performing videofluoroscopic swallowing studies. ASHA Special Interest Division 13, Swallowing and Swallowing Disorders (Dysphagia) 2003 [Google Scholar]
- 23.Mathers-Schmidt BA, Kurlinski M. Dysphagia evaluation practices: inconsistencies in clinical assessment and instrumental examination decision-making. Dysphagia. 2003;18(2):114–25. doi: 10.1007/s00455-002-0094-z. [DOI] [PubMed] [Google Scholar]
- 24•.Pitts T, et al. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23(3):297–301. doi: 10.1007/s00455-007-9144-x. Study identifying swallow and cough impairemnt in patients with PD; provided insight to potential mechanisms of disorder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56:502–6. doi: 10.1212/wnl.56.4.502. Early study on cough and swallow relationships in patients post stroke. [DOI] [PubMed] [Google Scholar]
- 26•.Hegland KW, Okun MS, Troche MS. Sequential voluntary cough and aspiration or aspiration risk in Parkinson’s disease. Lung. 2014;192(4):601–8. doi: 10.1007/s00408-014-9584-7. Study highlights importance of evaluating multiple coughs for detection of swallow impairment and introduces the cough expired volume meaure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miles A, Moore S, McFarlane M, Lee F, Allen J, Huckabee ML. Comparison of cough reflex test against instrumental assessment of aspiration. Physiol Behav. 2013;118:25–31. doi: 10.1016/j.physbeh.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Sato M, et al. Simplified cough test for screening silent aspiration. Arch Phys Med Rehabil. 2012;93(11):1982–6. doi: 10.1016/j.apmr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 29•.Pitts T, et al. Using voluntary cough to detect penetration and aspiration during oropharyngeal swallowing in patients with Parkinson disease. Chest. 2010;138(6):1426–31. doi: 10.1378/chest.10-0342. Study provides discriminitve ability of cough testing for detection of swallow impairment in patients with PD. [DOI] [PubMed] [Google Scholar]
- 30.Smith Hammond CA, et al. Predicting aspiration in patients with ischemic stroke: comparison of clinical signs and aerodynamic measures of voluntary cough. Chest. 2009;135(3):769–77. doi: 10.1378/chest.08-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitts T, Morris K, Lindsey B, Davenport P, Poliacek I, Bolser D. Coordination of cough and swallow in vivo and in silico. Exp Physiol. 2012;97(4):469–73. doi: 10.1113/expphysiol.2011.063362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolser DC, Davenport PW. Functional organization of the central cough generation mechansm. Pulm Pharmacol Ther. 2002;15:221–5. doi: 10.1006/pupt.2002.0361. [DOI] [PubMed] [Google Scholar]
- 33.Ross BB, Gramiak R, Rahn H. Physical dynamics of the cough mechanism. J Appl Physiol. 1955;8:264–8. doi: 10.1152/jappl.1955.8.3.264. [DOI] [PubMed] [Google Scholar]
- 34.Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56:502–6. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 35.Gestreau C, Milano S, Bianchi AL, Grélot L. Activity of dorsal respiratory group inspiratory neurons during laryngeal-induced fictive coughing and swallowing in decerebrate cats. Exp Brain Res. 1996;108(2):247–56. doi: 10.1007/BF00228098. [DOI] [PubMed] [Google Scholar]
- 36•.Troche MS, et al. A framework for understanding shared substrates of airway protection. J Appl Oral Sci. 2014;22(4):251–60. doi: 10.1590/1678-775720140132. Article reviews relationship between coughing and swallowing and provides a useful adapted framework which serves as a foundation to shape future studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davenport PW, Bolser DC, Morris KF. Swallow remodeling of respiratory neural networks. Head Neck. 2011;33(Suppl 1):S8–13. doi: 10.1002/hed.21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jean A. Brain stem control of swallowing: neuronal network and cellular mechanisms. Physol Rev. 2001;81(2):929–69. doi: 10.1152/physrev.2001.81.2.929. [DOI] [PubMed] [Google Scholar]
- 39.Widdicombe JG. Afferent Receptors in the airways and cough. Res Physiol. 1998;114:5–15. doi: 10.1016/s0034-5687(98)00076-0. [DOI] [PubMed] [Google Scholar]
- 40.Bianchi AL, Gestreau C. The brainstem respiratory network: an overview of a half century of research. Respir Physiol Neurobiol. 2009;168(1–2):4–12. doi: 10.1016/j.resp.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 41.Pitts T, et al. Coordination of cough and swallow: a meta-behavioral response to aspiration. Respir Physiol Neurobiol. 2013;189(3):543–51. doi: 10.1016/j.resp.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davenport PW, et al. The urge-to-cough and cough motor response modulation by the central effects of nicotine. Pulm Pharmacol Ther. 2009;22(2):82–9. doi: 10.1016/j.pupt.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davenport PW. Urge-to-cough: what can it teach us about cough? Lung. 2008;186(Suppl 1):S107–11. doi: 10.1007/s00408-007-9045-7. [DOI] [PubMed] [Google Scholar]
- 44.Hegland KW, Pitts T, Bolser DC, Davenport PW. Urge to cough with voluntary suppression following mechanical pharyngeal stimulation. Bratisl Lek Listy. 2011;112(3):109–14. [PMC free article] [PubMed] [Google Scholar]
- 45.Widdicombe J, Eccles R, Fontana G. Supramedullary influences on cough. Respir Physiol Neurobiol. 2006;152(3):320–8. doi: 10.1016/j.resp.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 46.Plowman EK, et al. Discriminant ability of the eating assessment tool-10 to detect aspiration in individuals with amyotrophic lateral sclerosis. Neurogastroenterol Motil. 2016;28(1):85–90. doi: 10.1111/nmo.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wheeler Hegland K, et al. Comparison of voluntary and reflex cough effectiveness in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(11):1226–30. doi: 10.1016/j.parkreldis.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McAllister S, Kruger S, Doeltgen S, et al. Implications of variability in clinical bedside swallowing assessment practices by speech language pathologists. Dysphagia. 2016 doi: 10.1007/s00455-016-9724-8. [DOI] [PubMed] [Google Scholar]
- 49.Edmiaston J, et al. A simple bedside stroke dysphagia screen, validated against videofluoroscopy, detects dysphagia and aspiration with high sensitivity. J Stroke Cerebrovasc Dis. 2014;23(4):712–6. doi: 10.1016/j.jstrokecerebrovasdis.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edmiaston J, et al. Validation of a dysphagia screening tool in acute stroke patients. Am J Crit Care. 2010;19(4):357–64. doi: 10.4037/ajcc2009961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mann G. MASA: The Mann assessment of swallowing ability. Clifton (NY): Thomson Learning Inc; 2002. [Google Scholar]
- 52.Antonios N, et al. Analysis of a physician tool for evaluating dysphagia on an inpatient stroke unit: the modified mann assessment of swallowing ability. J Stroke Cerebrovasc Dis. 2010;19(1):49–57. doi: 10.1016/j.jstrokecerebrovasdis.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 53.Martino R, et al. The toronto bedside swallowing screening test (TOR-BSST): development and validation of a dysphagia screening tool for patients with stroke. Stroke. 2009;40(2):555–61. doi: 10.1161/STROKEAHA.107.510370. [DOI] [PubMed] [Google Scholar]
- 54.Hinds NP, Wiles CM. Assessment of swallowing and referral to speech and language therapists in acute stroke. Q J Med. 1998;91:829–35. doi: 10.1093/qjmed/91.12.829. [DOI] [PubMed] [Google Scholar]
- 55.Trapl M, et al. Dysphagia bedside screening for acute-stroke patients: the gugging swallowing screen. Stroke. 2007;38(11):2948–52. doi: 10.1161/STROKEAHA.107.483933. [DOI] [PubMed] [Google Scholar]
- 56.Suiter DM, Sloggy J, Leder SB. Validation of the yale swallow protocol: a prospective double-blinded videofluoroscopic study. Dysphagia. 2014;29(2):199–203. doi: 10.1007/s00455-013-9488-3. [DOI] [PubMed] [Google Scholar]
- 57.Clave P, et al. Accuracy of the volume-viscosity swallow test for clinical screening of oropharyngeal dysphagia and aspiration. Clin Nutr. 2008;27(6):806–15. doi: 10.1016/j.clnu.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Logemann JA, Veis S, Colangelo L. A screening procedure for orophargyngeal dysphagia. Dysphagia. 1999;14:44–51. doi: 10.1007/PL00009583. [DOI] [PubMed] [Google Scholar]
- 59.Suiter DM, Leder SB. Clinical utility of the 3-ounce water swallow test. Dysphagia. 2008;23(3):244–50. doi: 10.1007/s00455-007-9127-y. [DOI] [PubMed] [Google Scholar]
- 60.Laciuga H, Brandimore AE, Troche MS, et al. Analysis of clinicians’ perceptual cough evaluation. Dysphagia. 2016;31(4):521–30. doi: 10.1007/s00455-016-9708-8. [DOI] [PubMed] [Google Scholar]
- 61.West JB. Respiratory Physiology: The Essentials. 5. Baltimore: Williams & Wilkins; 1995. [Google Scholar]
- 62.Bautista TG, Sun QJ, Pilowsky PM. Expiratory-modulated laryngeal motoneurons exhibit a hyperpolarization preceding depolarization during superior laryngeal nerve stimulation in the in vivo adult rat. Brain Res. 2012;1445:52–61. doi: 10.1016/j.brainres.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 63.Silverman EP, et al. Concordance and discriminatory power of cough measurement devices for individuals with Parkinson disease. Chest. 2014;145(5):1089–96. doi: 10.1378/chest.13-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kulnik ST, et al. Accuracy of portable devices in measuring peak cough flow. Physiol Meas. 2015;36(2):243–57. doi: 10.1088/0967-3334/36/2/243. [DOI] [PubMed] [Google Scholar]
- 65.Fontana GA, Widdicombe J. What is cough and what should be measured? Pulm Pharmacol Ther. 2007;20(4):307–12. doi: 10.1016/j.pupt.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Hegland KW, et al. Comparison of two methods for inducing reflex cough in patients with Parkinson’s disease. Dysphagia. 2016;31(1):66–73. doi: 10.1007/s00455-015-9659-5. [DOI] [PubMed] [Google Scholar]
- 67.Kallesen M, Psirides A, Huckabee ML. Comparison of cough reflex testing with videoendoscopy in recently extubated intensive care unit patients. J Crit Care. 2016;33:90–4. doi: 10.1016/j.jcrc.2016.02.004. [DOI] [PubMed] [Google Scholar]