Abstract
Background
The purpose of this study was to describe how the up-front transoral robotic surgery (TORS) approach could be used to individually tailor adjuvant therapy based on surgical pathology.
Methods
Between January 2009 and December 2013, 76 patients received TORS for oropharyngeal squamous cell carcinoma (OPSCC). Clinical predictors of adjuvant therapy were analyzed and comparisons were made between recommended treatment guidelines for up-front surgery versus definitive nonsurgical approaches.
Results
Advanced N classification, human papillomavirus (HPV)-positive tumor, extracapsular spread (ECS; 26 of 76), perineural invasion (PNI; 14 of 76), and positive margins (7 of 76) were significant predictors of adjuvant chemoradiotherapy (CRT) (p < .05). Up-front TORS deintensified adjuvant therapy; 76% of stage I/II and 46% of stage III/IV patients avoided CRT. Conversely, pathologic staging resulted in 33% of patients who would have received radiotherapy (RT) alone based on clinical staging, to be intensified to receive adjuvant CRT.
Conclusion
The TORS approach deintensifies adjuvant therapy and provides valuable pathologic information to intensify treatment in select patients. TORS may be less effective in deintensification of adjuvant therapy in patients with clinically advanced N classification disease.
Keywords: transoral robotic surgery (TORS), adjuvant therapy, oropharyngeal squamous cell carcinoma (OPSCC), oropharynx, decision-making
INTRODUCTION
In 2009, the Food and Drug Administration expanded the indications for the intuitive surgical system to include transoral otolaryngology procedures for benign and early T classification malignancies.1 Studies have suggested that primary transoral robotic surgery (TORS) offers excellent oncologic and survival outcomes2–4 as well as potential benefit in patient-reported quality of life (QOL), compared to primary radiotherapy (RT) or chemoradiotherapy (CRT). In patients who had transoral laser microsurgery plus adjuvant therapy, swallowing function declines sharply at 3 months postsurgery, presumably this is during RT, and then improves so that almost 90% of patients have good swallowing function as marked by a Functional Outcome Swallowing Scale score of 0 to 2, a year after treatment.5 Similarly, in patients with TORS alone, there are acceptable 1-month QOL scores that improve over 6, 12, and 24-month follow-up intervals.6 The 2010 National Comprehensive Cancer Network (NCCN) guidelines describe indications for postoperative adjuvant therapy for oropharyngeal squamous cell carcinoma (OPSCC), including extracapsular spread (ECS), positive margins, pT3 or pT4, N2 or N3 nodal disease, level IV/V nodal disease, perineural invasion (PNI), and vascular embolism.4 Currently, however, there are no TORS-specific adjuvant therapy guidelines. Subsequently, there exist institution-dependent practice patterns for the choice of adjuvant therapy. The potential variability in how adjuvant therapy is chosen after TORS may allow treatment deintensification but can also potentially expose patients to trimodality therapy with an increased treatment burden. Examining clinical factors that drive postoperative adjuvant therapy after TORS could be helpful in counseling patients before surgery. In this study, we examined a variety of preoperative and postoperative clinicopathologic parameters for their association with the adjuvant therapy treatment decision after TORS. The purpose of the study was to better characterize the role of TORS in both the deintensification and intensification of adjuvant therapy.
MATERIALS AND METHODS
Approval for this study was obtained from the Institutional Review Board of the University of Pittsburgh Medical Center. A retrospective review was conducted for patients who received TORS for primary treatment for OPSCC from January 2009 and December 2013. All patients were clinically staged using physical examination, contrasted CT of the neck and chest, as well as positron emission tomography/CT for selected cases. Patients were separated based on a >10 pack-year smoking history and similarly patients were identified as heavy drinkers according to the Centers for Disease Control description (8 or more drinks per week for women and 15 or more drinks per week for men). Attributable causes for positive margin were extracted from operative notes. Surgical site and defect (base of tongue vs palatine tonsil vs combined) were extracted to test its association with adjuvant therapy. Those who received therapy before TORS, TORS for biopsy only, and no therapeutic neck dissection were excluded from this study. A total of 92 patients were identified and 76 met criteria.
Expected definitive versus adjuvant treatment with RT or CRT was defined by the 2010 NCCN guidelines for OPSCC as well as findings from European Organisation for Research and Treatment of Cancer 22931. At our institution, T1N1 patients without adverse features are managed with adjuvant RT alone. Additionally, comparisons were then made between the actual postoperative adjuvant treatment received versus the recommended 2010 NCCN OPSCC adjuvant therapy guidelines. Based on this comparison, a patient was defined as having received “more” adjuvant therapy than expected if they received CRT when RT would have sufficed based on the guidelines, the converse is true for those who received “less” adjuvant therapy.
Statistical analysis
Patient demographics and clinical and pathological information were tested for individual associations with the type of therapy received using chi-square tests, Fisher’s exact tests, or an analysis of variance, as appropriate. A Cochran Armitage test was used to test for a trend in the proportion of human papillomavirus (HPV)-positive patients with increasing N classification. T classification and nodal classification were each tested for change between the time periods using t tests. Individual statistical tests were not adjusted for multiple testing and all p values were 2-sided. Statistical analyses were performed using SAS/STAT statistical software version 9.4 (SAS Institute, Cary, NC) and R version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient characteristics
Seventy-six patients were identified who met study criteria (Table 1). The mean patient age was 59 years. Of 76 patients, 15 (20%) had pathologic stage I/II and 56 (74%) were HPV-positive. The majority of patients were men and smokers. Additionally, there was a trend in the HPV-positive group to have higher N classifications than the HPV-negative group (p = .03; Table 1). It should be noted that not all patients had combined complete clinical staging because of 14 unknown primary cases. Upstaging of the primary site was noted when clinical T classification was compared to pathologic T classification. Much of this effect was due to the repartitioning of an unknown primary lesion into other T classifications, resulting in an overall upstaging (Figure 1A). Similarly, there was an upstaging of N classification when clinical classification was compared to pathologic classification; N0 and N1 were more accurately reclassified as higher classifications, postoperatively. TORS plus neck dissection increased the nodal classification in 36% of patients, and was a significant driver for adjuvant therapy (p < .05; Figure 1B). When we examined overall staging, 14 patients had unknown primaries, and they were all pathologically staged as III and IV. Of the 62 patients available with clinical staging, 37% were upstaged, 57% remained the same stage, and only 6% were downstaged with final pathologic analysis. Of 25 stage I/II patients, 11 were upstaged to stage III/IV (Figure 1C). A separate analysis of the 7 patients with positive margins revealed no trend toward increased T classification (all T1 and T2 lesions).
TABLE 1.
Patient demographics.
| Demographics | All No. of patients | Adjuvant therapy
|
||||||
|---|---|---|---|---|---|---|---|---|
| No adjuvant therapy
|
RT
|
CRT
|
||||||
| No. of patients | (%) | No. of patients | (%) | No. of patients | (%) | p value | ||
| Sex | ||||||||
| Male | 62 | 21 | (33) | 13 | (20) | 28 | (45) | |
| Female | 14 | 8 | (57) | 2 | (14) | 4 | (28) | |
| Age, mean [range], y | 76 | 59 [43–74] | 58 [44–74] | 60 [30–85] | .85 | |||
| Heavy alcohol use | .373 | |||||||
| Yes | 20 | 6 | (30) | 4 | (20) | 10 | (50) | |
| No | 38 | 14 | (37) | 10 | (26) | 14 | (37) | |
| NA | 18 | 9 | (50) | 1 | (6) | 8 | (44) | |
| >10 pack-year smoker | .57 | |||||||
| Yes | 49 | 16 | (34) | 11 | (22) | 22 | (44) | |
| No | 21 | 9 | (43) | 4 | (19) | 8 | (38) | |
| NA | 6 | 4 | (67) | 0 | 0 | 2 | (33) | |
| HPV status | .04 | |||||||
| Positive | 56 | 18 | (32) | 14 | (25) | 24 | (42) | |
| Negative | 17 | 10 | (58) | 7 | (41) | |||
| NA | 3 | 1 | (33) | 1 | (33) | 1 | (33) | |
| Overall clinical stage | .0006 | |||||||
| 1 | 13 | 9 | (69) | 1 | (7) | 3 | (23) | |
| 2 | 12 | 9 | (75) | 3 | (25) | |||
| 3 | 20 | 6 | (30) | 6 | (30) | 8 | (40) | |
| 4 | 17 | 1 | (5) | 4 | (23) | 12 | (70) | |
| NA | 14 | 4 | (28) | 4 | (28) | 6 | (42) | |
| T classification, clinical | .45 | |||||||
| T1 | 34 | 14 | (41) | 8 | (23) | 12 | (35) | |
| T2 | 25 | 11 | (44) | 2 | (8) | 12 | (48) | |
| T3 | 2 | 1 | (50) | 1 | (50) | |||
| T4 | 1 | 1 | (100) | |||||
| TX | 14 | 4 | (28) | 4 | (28) | 6 | (42) | |
| N classification, clinical | .0014 | |||||||
| N0 | 27 | 18 | (66) | 2 | (7) | 7 | (25) | |
| N1 | 27 | 9 | (33) | 7 | (25) | 11 | (40) | |
| N2A | 5 | 2 | (40) | 3 | (60) | |||
| N2B | 16 | 2 | (12) | 3 | (18) | 11 | (68) | |
| N2C | 1 | 1 | (100) | |||||
| Overall pathologic staging | < .0001 | |||||||
| 1 | 11 | 10 | (90) | 1 | (9) | |||
| 2 | 4 | 4 | (100) | |||||
| 3 | 23 | 13 | (56) | 3 | (13) | 7 | (30) | |
| 4 | 38 | 2 | (5) | 12 | (31) | 24 | (63) | |
| T classification, pathologic | .34 | |||||||
| T1 | 42 | 20 | (47) | 9 | (21) | 13 | (30) | |
| T2 | 27 | 8 | (29) | 5 | (18) | 14 | (51) | |
| T3 | 6 | 1 | (16) | 1 | (16) | 4 | (66) | |
| T4 | 1 | 1 | (100) | |||||
| N classification, pathologic | < .0001 | |||||||
| N0 | 17 | 14 | (82) | 1 | (5) | 2 | (11) | |
| N1 | 21 | 13 | (61) | 2 | (9) | 6 | (28) | |
| N2A | 10 | 1 | (10) | 4 | (40) | 5 | (50) | |
| N2B | 24 | 1 | (4) | 7 | (29) | 16 | (66) | |
| N2C | 4 | 1 | (25) | 3 | (75) | |||
| ECS | < .0001 | |||||||
| Yes | 26 | 2 | (7) | 3 | (11) | 21 | (80) | |
| No | 47 | 24 | (51) | 12 | (25) | 11 | (23) | |
| NA | 3 | 3 | (100) | |||||
| PNI | .03 | |||||||
| Yes | 14 | 4 | (28) | 10 | (71) | |||
| No | 61 | 24 | (39) | 15 | (24) | 22 | (36) | |
| NA | 1 | 1 | (100) | |||||
| Positive margins | .005 | |||||||
| Yes | 7 | 7 | (100) | |||||
| No | 69 | 29 | (42) | 15 | (21) | 25 | (36) | |
| Cancer site | .18 | |||||||
| Tonsil | 35 | 17 | (48) | 7 | (20) | 11 | (31) | |
| BOT | 36 | 9 | (25) | 8 | (22) | 19 | (52) | |
| Overlap | 5 | 3 | (60) | 2 | (40) | |||
| Primary site procedure | .81 | |||||||
| Lateral oropharyngectomy | 16 | 8 | (50) | 3 | (18) | 5 | (31) | |
| BOT resection | 12 | 4 | (33) | 3 | (25) | 5 | (41) | |
| Combined | 48 | 17 | (35) | 9 | (18) | 22 | (45) | |
Abbreviations: RT, radiotherapy; CRT, chemoradiotherapy; NA, not available; HPV, human papillomavirus; ECS, extracapsular spread; PNI, perineural invasion; BOT, base of tongue.
FIGURE 1.
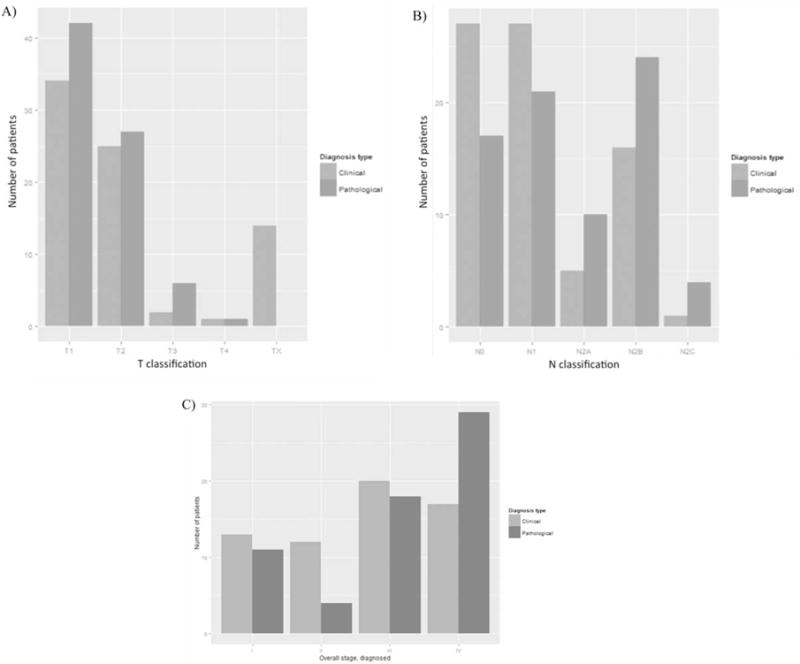
Comparison of clinical and pathologic staging.
Predictors of adjuvant therapy
Predictors associated with adjuvant CRT were clinical N classification, clinical overall stage, pathologic N classification, and pathologic overall stage (p < .05). Presence of ECS (26 of 76), PNI (14 of 76), a positive margin (7 of 76), and HPV-positive status were significant predictors of CRT (p < .05), whereas surgical site and defect (base of tongue vs palatine tonsil vs combined) were not (p = .40). As expected, higher clinical stage predicted more intensive adjuvant therapy (p value < .0001). Venn diagrams were used to represent the interaction among ECS, PNI, and a positive margin in the decision-making process for CRT. Six stage I/II patients received adjuvant CRT; there was no clear trend in risk factors (Figure 2A). When study patients of all stages are considered, 32 received CRT, 44% for ECS, 13% for a positive margin, 9% for PNI, 9% for high T or N classification, and the remaining 25% of patients for some combination of these factors (Figure 2B). There was a relative risk of 2.12 (p = .03) of a stage III/IV patient receiving adjuvant chemotherapy. Of the stage I/II and stage III/IV patients, 76% and 46% were able to avoid adjuvant chemotherapy, respectively (see Figure 3). When expected therapy (based on NCCN guidelines) was compared to observed adjuvant therapy, there was variability in implementation of treatment plans. Patients received expected adjuvant therapy in 78% of cases, 13% received more, and 9% received less than expected (Table 2).
FIGURE 2.
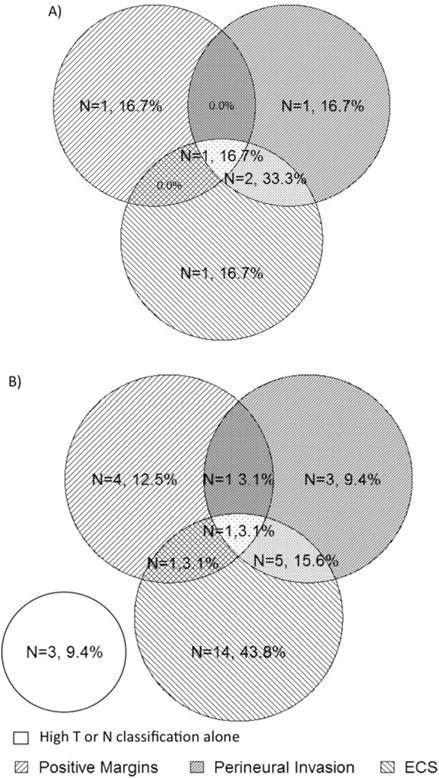
Venn diagram of adverse histologic features that led to a recommendation for adjuvant chemoradiotherapy. Clinical stage I/II (A) and all stages (B) with number of patients (%).
FIGURE 3.
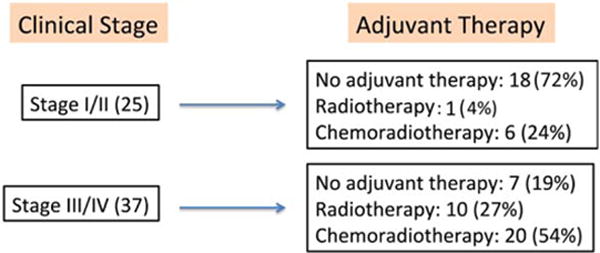
Overall clinical staging and adjuvant therapy. Number of patients (%). ECS, extracapsular spread. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
TABLE 2.
Adjuvant therapy received compared to National Comprehensive Cancer Network guidelines.
| No. of patients (%) Observed < expected | No. of patients (%) As expected | No. of patients (%) Observed > expected |
|---|---|---|
| 7 (9) | 59 (78) | 10 (13) |
Comparison of definitive nonsurgical approach to up-front transoral robotic surgery
As our series consisted of patients treated with up-front TORS, we examined whether TORS allowed our patients to avoid either RT or CRT. To allow a comparison to nonsurgical therapy, initial clinical staging was used to project what up-front nonsurgical therapy patients would have received based on the NCCN guidelines. According to the NCCN guidelines, 52 patients were projected to have received RT and 24 patients were projected to have received CRT as the definitive nonsurgical therapy if TORS had not been utilized. Instead, these 24 patients were treated with primary surgery and accordingly: 15 underwent TORS 1 adjuvant chemoradiotherapy; 7 underwent adjuvant RT; and 2 received TORS alone. Ninety-two percent of those patients who would have required up-front CRT still underwent adjuvant therapy (either RT or CRT) after TORS. The other 52 patients would have received up-front radiation alone based on clinical staging. Similarly, these 51 patients were treated with up-front surgery instead and 17 patients had TORS + CRT; 8 had TORS + RT; and 27 had TORS alone. Our data show that TORS allowed a large percentage of these patients to be treated with single modality surgical therapy. This benefit was most evident in patients with early-stage disease who would have required RT alone if treated with a nonsurgical approach. Our data also show that 37% of patients who would have required up-front concurrent CRT in a nonoperative approach were able to be deintensified to receive no adjuvant therapy or radiation alone after TORS (see Figure 4).
FIGURE 4.
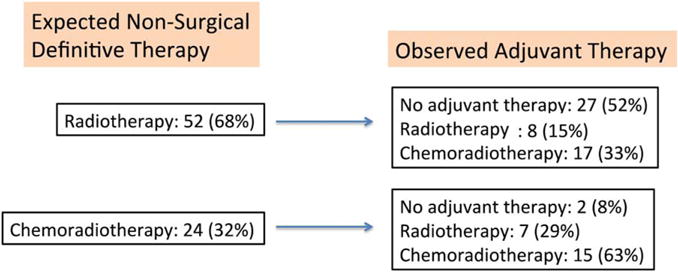
Comparison of therapy received with the National Comprehensive Cancer Network (NCCN) guidelines for nonsurgical definitive therapy. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
DISCUSSION
Adjuvant therapy rates are well known after TORS; however, direct comparison of nonsurgical therapy to up-front TORS is less well-characterized. The purpose of this study was to reexamine the clinical and pathologic variables that contribute to a decision for adjuvant therapy and to evaluate the merit of up-front surgery in providing risk assessment through surgical pathology. Our study population was composed of typical TORS patients when compared with the literature for their staging and HPV-positive status.7 Overall, 61% of patients received adjuvant therapy; of those receiving adjuvant therapy, 68% received CRT. Up-front surgery provided additional staging information of the neck and played a role in locating unknown primary lesions. Overall, as expected, TORS and neck dissection upstaged both the T and N classifications. This more accurate staging modified plans for adjuvant therapy according to today’s best evidence. However, nodal classification may have little prognostic value in HPV-positive disease, especially as currently defined by American Joint Committee on Cancer staging.8,9 For the purposes of this study we defined adding additional treatment modalities as intensification, specifically referring to trimodality therapy. Some may argue that trimodality therapy should be avoided at all costs; however, there may be benefit if surgery allows for reduced radiation dose to the pharyngeal constrictors. Dose to the superior pharyngeal constrictors is a predictor of late radiation associated dysphagia.10
The decision for postoperative adjuvant CRT is primarily driven by ECS. Of the 26 patients with ECS in our study, 80% received CRT. As such, a finding of ECS resulted in intensification of therapy. Currently, ECS seems to be a useful prognostic indicator; in this way, the additional pathologic information allowed for the appropriate addition of chemotherapy to the postoperative regimen in certain patients. However, it should be noted that the role of ECS for prognostication in OPSCC is also debatable.11 Future studies will elucidate the prognostic value of ECS especially in HPV-positive OPSCC. The Adjuvant De-escalation, Extracapsular Spread, P16+, Transoral (ADEPT) trial seeks to answer this question.12
Regarding margin status, there is an evolving understanding of what constitutes clear margins in the oropharynx. Historically, margins of >5 mm have been described for head and neck squamous cell cancer. The anatomy of the lateral oropharynx does not allow that depth of resection; the mean radiographic thickness of the superior constrictor is 2.4 mm. A margin of <2 mm can achieve 99% local control.13 Close cooperation between the surgeon and pathologist may result in improved margin clearance with excellent oncologic results.
Interestingly, in our patient cohort, HPV-positive patients received more adjuvant therapy than HPV-negative patients. Specifically, 58% of HPV-negative patients had no adjuvant therapy, whereas 32% of HPV-positive patients had no adjuvant therapy. This was primarily driven by a statistically significant higher N classification in HPV-positive patients. Low T classification and higher N classification is typical of HPV-positive OPSCC.14 This clinical presentation agrees with other reports in the literature, and upstaging of nodal classification represents an important driver of adjuvant therapy. The feasibility of deintensification of adjuvant therapy in HPV-positive patients may be further clarified by Eastern Cooperative Oncology Group 3311 and the Mount Sinai Robotic Trial.15
In our case series, there was variability in observed adjuvant therapy when compared to published guidelines. Treatment decisions for adjuvant therapy were decided in a multidisciplinary tumor board setting. This variability may have been due to a lack of specific TORS guidelines for adjuvant therapy. Deviation from guidelines may also be attributed to patient and physician preferences and bias. Specific reasons for withholding chemotherapy, such as performance status, could not be abstracted from the robotic database.
Deintensification of adjuvant therapy is possible when using up-front TORS. Previous reports have described 50% of stage I/II tumors able to avoid adjuvant therapy,7 whereas 72% of stage I/II tumors in our series were able to avoid all adjuvant therapy. In that study, 34% of stage III/IV patients were spared chemotherapy7 and, in our series, 49% of stage III/IV tumors are spared chemotherapy. A systemic review of early T classification OPSCC demonstrates that 26% of patients receive adjuvant RT and 41% receive adjuvant CRT.16 In our series, 20% of patients received adjuvant RT and 42% received adjuvant CRT, which corresponds well with previous studies. Another way of examining the question of the utility of primary surgery is by comparing the nonsurgical treatment course patients could have chosen. This information is valuable as the patient is deciding treatment course, and underscores the difficulty in predicting adjuvant therapy regimens because of the changes in staging after surgery. In approximately one third of patients who would have received definitive nonsurgical RT alone, findings, such as ECS or upstaging N classification, resulted in patients receiving adjuvant CRT. This represents an intensification of therapy in accordance with the current evidenced-based practice. Conversely, more than a third of patients who would have received CRT in the nonsurgical paradigm were able to avoid it. Similarly, 52% of patients who would have received definitive RT as a sole modality avoided it with up-front surgery. These represent 2 instances of deintensification, with possible long-term QOL benefits. Future studies may reveal that ECS and advanced nodal classification are not poor prognostic indicators, especially in HPV-positive disease. This may alter the utility of additional pathologic staging obtained through surgery. At the same time, more patients would be able to be treated with surgery alone and/or avoid chemotherapy.
Another purpose of this study was to identify presurgical factors that can help predict the need for adjuvant therapy. Our study showed that clinical N classification and overall clinical stage were significant factors in predicting the need for adjuvant therapy after TORS. This data suggest that patients with OPSCC would derive most benefit from TORS if the surgery is limited to those with clinical N0 or N1 disease or those with clinical early-stage disease.
CONCLUSION
TORS, as it is practiced today, is a useful tool to deintensify adjuvant therapy in patients with OPSCC. In selected patients, surgery can provide valuable information about pathologic features, such as ECS, and serve to justify the addition of chemotherapy to adjuvant radiation. Prospective trials will better define the role of TORS in treatment deintensification.
Acknowledgments
Contract grant sponsor: This work was supported in part by funds from the Department of Veterans Affairs, BLSR & D, and the PNC Foundation (U.D.).
This work does not reflect the views of the U.S. Government or the Department of Veterans Affairs.
Footnotes
This work was presented at the American Society of Head and Neck Surgery meeting at the Combined Otolaryngology Spring Meeting, Boston, Massachusetts, April 22–26, 2015.
References
- 1.Loevner LA, Learned KO, Mohan S, et al. Transoral robotic surgery in head and neck cancer: what radiologists need to know about the cutting edge. Radiographics. 2013;33:1759–1779. doi: 10.1148/rg.336135518. [DOI] [PubMed] [Google Scholar]
- 2.White HN, Moore EJ, Rosenthal EL, et al. Transoral robotic-assisted surgery for head and neck squamous cell carcinoma: one- and 2-year survival analysis. Arch Otolaryngol Head Neck Surg. 2010;136:1248–1252. doi: 10.1001/archoto.2010.216. [DOI] [PubMed] [Google Scholar]
- 3.Weinstein GS, O’Malley BW, Jr, Magnuson JS, et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope. 2012;122:1701–1707. doi: 10.1002/lary.23294. [DOI] [PubMed] [Google Scholar]
- 4.Moore EJ, Hinni ML. Critical review: transoral laser microsurgery and robotic-assisted surgery for oropharynx cancer including human papillomavirus-related cancer. Int J Radiat Oncol Biol Phys. 2013;85:1163–1167. doi: 10.1016/j.ijrobp.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 5.Rich JT, Liu J, Haughey BH. Swallowing function after transoral laser microsurgery (TLM) ± adjuvant therapy for advanced-stage oropharyngeal cancer. Laryngoscope. 2011;121:2381–2390. doi: 10.1002/lary.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choby GW, Kim J, Ling DC, et al. Transoral robotic surgery alone for oropharyngeal cancer: quality-of-life outcomes. JAMA Otolaryngol Head Neck Surg. 2015;141:499–504. doi: 10.1001/jamaoto.2015.0347. [DOI] [PubMed] [Google Scholar]
- 7.Hurtuk A, Agrawal A, Old M, Teknos TN, Ozer E. Outcomes of transoral robotic surgery: a preliminary clinical experience. Otolaryngol Head Neck Surg. 2011;145:248–253. doi: 10.1177/0194599811402172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ward MJ, Mellows T, Harris S, et al. Staging and treatment of oropharyngeal cancer in the human papillomavirus era. Head Neck. 2015;37:1002–1013. doi: 10.1002/hed.23697. [DOI] [PubMed] [Google Scholar]
- 9.Spector ME, Gallagher KK, Bellile E, et al. Patterns of nodal metastasis and prognosis in human papillomavirus-positive oropharyngeal squamous cell carcinoma. Head Neck. 2014;36:1233–1240. doi: 10.1002/hed.23438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Awan MJ, Mohamed AS, Lewin JS, et al. Late radiation-associated dysphagia (late-RAD) with lower cranial neuropathy after oropharyngeal radiotherapy: a preliminary dosimetric comparison. Oral Oncol. 2014;50:746–752. doi: 10.1016/j.oraloncology.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maxwell JH, Ferris RL, Gooding W, et al. Extracapsular spread in head and neck carcinoma: impact of site and human papillomavirus status. Cancer. 2013;119:3302–3308. doi: 10.1002/cncr.28169. [DOI] [PubMed] [Google Scholar]
- 12.Post operative adjuvant therapy de-intensification trial for human papillomavirus-related, p16+ oropharynx cancer (ADEPT) 2012 Available at: Clinicaltrials.gov. Accessed June 1, 2015.
- 13.Hinni ML, Zarka MA, Hoxworth JM. Margin mapping in transoral surgery for head and neck cancer. Laryngoscope. 2013;123:1190–1198. doi: 10.1002/lary.23900. [DOI] [PubMed] [Google Scholar]
- 14.Nichols AC, Faquin WC, Westra WH, et al. HPV-16 infection predicts treatment outcome in oropharyngeal squamous cell carcinoma. Otolaryngol Head Neck Surg. 2009;140:228–234. doi: 10.1016/j.otohns.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 15.Eastern Cooperative Oncology Group. NCT01898494. 2014 Available at: http://ecog-acrin.org/active-clinical-trials/e3311-educational-materials. Accessed June 1, 2015.
- 16.de Almeida JR, Byrd JK, Wu R, et al. A systematic review of transoral robotic surgery and radiotherapy for early oropharynx cancer: a systematic review. Laryngoscope. 2014;124:2096–2102. doi: 10.1002/lary.24712. [DOI] [PubMed] [Google Scholar]


