Abstract
Recent findings confirm that faster eating rates support higher energy intakes within a meal and are associated with increased body weight and adiposity in children. The current study sought to identify the eating behaviours that underpin faster eating rates and energy intake in children, and to investigate their variations by weight status and other individual differences. Children (N=386) from the Growing Up in Singapore towards Healthy Outcomes (GUSTO) cohort took part in a video-recorded ad libitum lunch at 4.5 years of age to measure acute energy intake. Videos were coded for three eating behaviours (bites, chews and swallows) to derive a measure of eating rate (g/min) and measures of eating microstructure: eating rate (g/min), total oral exposure (minutes), average bite size (g/bite), chews per gram, oral exposure per bite (seconds), total bites and proportion of active to total mealtime. Children’s BMIs were calculated and a subset of children underwent MRI scanning to establish abdominal adiposity. Children were grouped into faster and slower eaters, and into healthy and overweight groups to compare their eating behaviours. Results demonstrate that faster eating rates were correlated with larger average bite size (r=0.55, p<0.001), fewer chews per gram (r=-0.71, p<0.001) and shorter oral exposure time per bite (r=-0.25, p<0.001), and with higher energy intakes (r=0.61, p<0.001). Children with overweight and higher adiposity had faster eating rates (p<0.01) and higher energy intakes (p<0.01), driven by larger bite sizes (p<0.05). Eating behaviours varied by sex, ethnicity and early feeding regimes, partially attributable to BMI. We propose that these behaviours describe an ‘obesogenic eating style’ that is characterised by faster eating rates, achieved through larger bites, reduced chewing and shorter oral exposure time. This obesogenic eating style supports acute energy intake within a meal and is more prevalent among, though not exclusive to, children with overweight.
1.0. Introduction
With childhood obesity rates rising, there is a need to understand the development of eating patterns that promote excessive energy intake and subsequent early weight gain [1]. While the type and quantity of foods consumed and the associated meal patterns that support sustained positive energy balance are important contributors to early weight gain, limited research has focussed on exploring whether children exhibit patterns of food oral processing, or “eating microstructure” patterns [2], that support increased energy intake within a meal. Previous research has shown that faster eating rates support increased energy intake in adults and a meta-analysis of 22 ad libitum feeding trials demonstrated that adults who eat at a faster rate consume more energy [3]. Similar findings have been reported in studies conducted with children [4–9]. We have recently demonstrated that children who ate at a faster rate consumed on average 75% more energy compared to children who ate slower [10]. A better understanding of the specific behaviours through which these faster eating rates are achieved will inform intervention strategies to support weight management and help reduce excessive early childhood weight gain.
Accumulating evidence suggests that certain elements of eating microstructure support increased energy intake through their effects on eating rate. Adults who take fewer chews before swallowing have shorter oral exposure times per bite, eat at faster rates and consume more energy [11–13]. By contrast, more thorough chewing of each bite increases oral exposure, reduces eating rate, reduces food intake and promotes a stronger satiety response for the same calories consumed [14–16]. Whether these same patterns occur in children requires further investigation. Moreover, a larger bite size has also been identified as an element of eating microstructure which underpins faster eating rates and supports energy intake in both adults [17–20] and children [21]. The current evidence suggests that certain features of eating microstructure may promote faster eating rates during the meal, which may support increased energy intakes and over time may lead to weight gain.
There is some evidence suggesting that adults and children with obesity eat at faster rates and this has been linked to features of eating microstructure [8, 22–27]. In our previous study we showed substantial differences in BMI and whole-body adiposity between children who had slower versus faster eating rates [10]. Other features of eating microstructure beyond the eating rate may also differentiate between people with healthy weight and overweight, but results for these behaviours have been less consistent. Previous work suggests that compared to healthy weight people children and adults with obesity take fewer chews before swallowing [5, 16, 28–30], and tend to have a larger average bite size compared to their healthy weight counterparts [31, 32]. However, other studies have failed to find links between elements of eating microstructure and weight status [16, 20, 33].
Beyond comparisons of lean and obese eating styles our knowledge of individual differences in eating microstructure becomes more limited. Gender differences have been documented to show that males eat faster than females [34], and tend to have a higher chewing frequency, shorter chewing time and overall better masticatory performance [35]. Findings from children are limited but suggest similar gender patterns, where boys tend to eat faster than girls, and this gender differentiation tends to increase with age [28]. Early feeding experiences have been associated with food preferences, eating patterns and healthy or unhealthy trajectories of childhood weight gain [36]. Development of eating behaviours that support increased energy intake may be mediated by the development of the microstructural patterns of eating that promote higher habitual energy intakes within meals. It remains unclear why, and whether early feeding experiences such as duration of breastfeeding and age of introduction of solid foods, are linked to microstructural patterns of eating at the later stage of development, independently of weight status.
Understanding the eating microstructure patterns linked to faster eating rates and greater energy intakes in children will provide opportunities for designing intervention strategies to slow eating rates and improve energy intake control. The current study had three aims. Firstly, we wanted to identify specific microstructural patterns of eating that promote faster eating rates, and identify their relative importance in predicting energy intake in a large multi-ethnic cohort of pre-school children. The second aim was to investigate whether these eating patterns differ by children’s weight status. Finally, we explored whether there were patterns of eating microstructure associated with individual differences in sex, ethnicity, gestational age at birth and early feeding history.
2.0. Methods
2.1. Participants
Participants in this study were a subset of 484 parent-child dyads from the Growing Up In Singapore Towards Healthy Outcomes cohort (GUSTO; N=1,247), who took part in the a video recorded ad libitum buffet lunch at 4.5 years of age (54± 2 months; for a full description of the GUSTO cohort see [37]). Some videos were removed (n=97) for quality purposes (e.g. child leaving the room with food in their mouth, or child outside the camera view), leaving a total of 386 children included in the final sample for analyses. Flow chart of study participants is presented in Appendix A.
Children in the sample represented the three dominant ethnic groups in Singapore, namely Chinese (n= 210), Indian (n= 68) and Malay (n= 108), and a comparable number from both sexes (n=202 boys and n=184 girls). To investigate the relationship between eating rates and a range of individual differences, the subset (n=386) were characterised by BMIz score, abdominal adiposity, breastfeeding history, gestational age at birth and age of complementary food introduction. Information on gestational age at birth, breastfeeding status at 6 months and complementary food introduction were collected during the first 12 months of life by trained researchers. Children’s breastfeeding status at 6 months was used to group the sample into predominantly breastfed (n=51) and predominantly bottle-fed (n=294), which included those fed using expressed breast milk as a primary interest of the current study was in potential learning differences, rather than nutritional value of breastmilk. Children were also divided into three groups based on the age of introduction of complementary foods; the early introduction group consisted of children who received complementary foods before 4 months of age (n=70), typical group who began complementary feeding between 4-6 months of age (n=204), and late group, who were introduced to complementary foods after 6 months of age (n=70). Typical introduction was defined as 4-6 months of age as cross-sectional data suggests that the majority of infants world-wide are introduced to complementary foods between 4-6 months of age, despite WHO recommendations to begin complementary feeding at 6 months [38]. Children who began complementary feeding after the typical age of introduction were classified as a “late group”. Children were also grouped according to their gestational age at birth into preterm (<37 weeks; n=91) and full-term (37+ weeks; n=295; [39]). The study was approved by the Institutional Review Boards of the hospitals involved (clinical trials registry: NCT01174875).
2.2. Buffet lunch
Foods served during the meal were provided ad-libitum in a buffet and comprised 9 commercially available foods and 3 drinks, selected as familiar and accepted products for this age group based on food frequency questionnaire data from the same cohort. The foods and drinks served were: white bread (Gardenia; 2.6 kcal/g), Honey Stars cereal (Nestle; 3.8 kcal/g), pancakes (Aunty Jemima; 3 kcal/g), chocolate cake (Sara Lee; 4.3 kcal/g), cheese (Cowhead; 2.9 kcal/g), chicken cocktail sausage (Fairprice; 2.9 kcal/g), chicken nuggets (CP; 2.3 kcal/g), apple slices (0.44 kcal/g), canned corn (Hosen; 0.81 kcal/g), apple juice (Marigold; 0.5 kcal/ml), full cream milk (Marigold; 0.65 kcal/ml) and water. Energy content for food items was derived from the Health Promotion Board of Singapore food composition tables [40].
The test room was equipped with child appropriate furniture and utensils to recreate the child’s normal lunchtime environment, with video cameras positioned in three corners of the room to record the consumption. Cameras enabled high resolution video capture of all aspects of the food choice and consumption, and it was possible to zoom in from different angles up to 400% without the loss of resolution, for the behavioural coding of specific eating behaviours. Children were instructed to consume their normal breakfast at home and abstain from consuming foods for the minimum of 3 hours before the meal. Children and their mothers consumed the lunch with no other people present in the test room. Prior to the meal, mothers were requested not to prompt or interfere with children’s food choices, portion size selection or to encourage them to eat, but otherwise were free to interact with their child in the usual way. Participants were told that they could eat as much or as little as they wished during the meal and that they would be given 20 minutes to eat. Extensions of 10 minutes were granted to finish the meal if they ran out of time. All products served were weighed before and after the meal and intake for each food was recorded (g) and later converted to energy consumed using each foods energy density (kcals) [41].
2.3. Behavioural Coding Analysis
Oral processing behaviours were coded using ELAN 4.9.1 (Max Planck Institute for Psycholinguistics, The Language Archive, Nijmegen, The Netherlands). Video-coding of oral processing behaviours has previously been validated to show good correspondence with EMG recordings of oral muscle activity during eating [42]. A behavioural coding scheme was developed to record the frequencies of four ‘point’ events (frequency counts of bites, chews, swallows and sips) and duration of a single ‘continuous’ event (total oral exposure time in minutes). A number of eating parameters were derived from these coded parameters based on a previously published approach [11, 12, 18, 43]. Total oral exposure time (minutes) was calculated as the cumulative time food spent in mouth between the bite and the swallow throughout the meal. Total oral exposure time was then divided by the total number of bites, to derive the average oral exposure time per bite (seconds). Average eating rate was calculated by dividing the grams consumed by the total oral exposure time (g/min). Average bite size was calculated by dividing total number of grams consumed by the total number of bites (g/bite). Total number of chews were divided by the total weight of food consumed (grams) to derive the average number of chews per gram. Total oral exposure time was divided by the total lunch duration and multiplied by 100, to derive a percentage of the time that each child spent actively consuming food, in proportion to the total duration of their meal [44]. This measure provides information about the length of pauses between bites, to distinguish between children who ate continuously from those taking breaks between eating bouts. Values closer to 100% indicate that children spent a higher percentage of time eating, and values closer to 0% indicate that children spent less time actively eating relative to the amount of time spent in the room. An example of the coding scheme used to derive the main behavioural outcomes is shown in a previous publication (Figure 1 from Forde et al., 2013a) [18]. Behavioural video coding was completed by a single trained video-coder and 10% of all coded videos were later blind-validated by the second trained video-coder through standard reliability measures, to achieve an acceptable agreement (≥80%) on all ‘point events’ and the continuous (time in mouth) event, in line with the previously published recommendations [45].
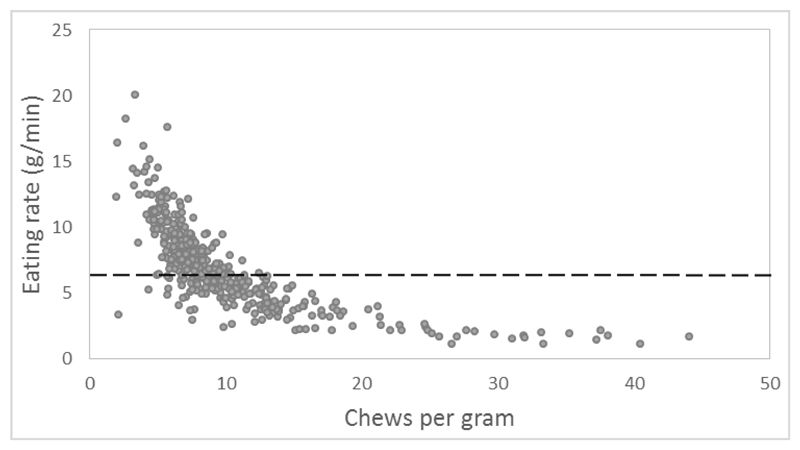
Figure 1.
Relationship between children’s chewing behaviour and eating rate. The median eating rate used to split children into slower and faster eating groups is shown; below the line represents slower eating rates and above the line represents faster eating rates.
2.4. Anthropometric Measures
Children’s weight and height were recorded during a separate session at the same time point. Height and weight were transformed to BMI z-scores (BMIz) corrected for age and sex according to WHO child growth standards [46]. These were used to classify children into a healthy weight (BMIz≤1.04; n=314) and overweight group (BMIz>1.04; n=64), which is equivalent to 85th percentile [47], commonly used cut-off for defining overweight among children [48] and previously used in studies with a similar design [9].
2.5. MRI of Abdominal Adiposity
A subset of participants (N=158) underwent MRI scanning to measure their subcutaneous adipose tissue (SAT; Appendix A). Magnetic resonance images (MRI) of the abdomen were obtained from a 3T MR scanner (Siemens Skyra, VE11A). Sixty axial slices with 5 mm slice thickness and in-plane resolution of 0.94 × 0.94 mm were acquired using a water suppressed HASTE sequence (TR=1000 ms, TE=95 ms) and body matrix coil. A fully automated graph theoretic segmentation algorithm was used to segment and quantify the SAT depots between the top of liver and top of sacrum [49]. The first step of the algorithm employed intensity thresholding to remove non-fat tissues from the fat tissues and create the fat mask. The SAT compartment was then segmented from the mask using graph cuts. The number of voxels in SAT were summed and multiplied by the resolution to get the SAT volume. Based on SAT volume, the children were split into lower (n=79) and higher (n=79) subcutaneous adiposity groups using median split (median=433.09).
3.0. Data analysis
The cumulative frequency of food choice from the ad-libitum buffet was compared across the group and showed that all foods were chosen with similar frequency, with the exception of apples and corn, which were chosen with lower frequency. The frequency of foods chosen was compared within the cohort across healthy weight and overweight children, and faster and slower eaters (chi-square analysis). Previous analysis of the dataset demonstrated a strong positive relationship between eating rate and energy consumed during the ad-libitum meal (Pearson’s r; r=0.61, p<0.001; see [10]). The current study investigated elements of eating microstructure through which these faster eating rates were achieved using correlations (Pearson’s r). A median split of eating rate (Median=6.41 g/min) was used to divide children into slower (n=192) and faster eating styles (n=194), and all elements of eating microstructure were compared between these two groups (t-test). The relative contribution of eating microstructure parameters in explaining energy intake was assessed using two separate regression analyses for the slower and faster eating groups. Variables were all entered in one block to test their independent contribution to energy intake. Elements of eating microstructure (excluding eating rate due to large covariance) were used as predictors and energy intake was used as an outcome variable.
Comparisons were also drawn between healthy weight and overweight groups and between children with lower and higher abdominal adiposity levels, to explore differences in eating microstructure associated with body composition (t-test). Children with overweight and healthy weight were additionally grouped into slower and faster eating styles by median split of eating rates within each weight category, and eating microstructure was compared across the four groups of slower and faster eaters with healthy and overweight status (ANOVA; Bonferroni correction). A Chi-squared analysis was performed to test the odds of children with overweight being categorised as faster eaters. Individual differences in eating microstructure were investigated using t-tests to examine binomial groups and ANOVAs for polynomial groups. Significant differences between groups were re-examined adjusting for BMI, to dissociate group differences linked directly to children’s BMI, from those pertaining to other factors.
4.0. Results
4.1. Food Choice from the Ad libitum Buffet
Children were free to choose from any of the 9 food items served and at a group level chose all foods with similar frequency, with the exception of apple slices and corn, which were chosen less frequently. Children with healthy weight and overweight did not differ in the frequency of the foods they chose (χ2≤1.5, p>0.10) or in the variety (number) of foods consumed (t=0.49, p=0.62). Children who ate faster consumed a slightly larger variety of foods (Δ0.5 food item; t= 3.55, p<0.001) and chose all foods more frequently and some significantly more frequently (corn, nuggets, apple and cheese; (χ2≥4.2, p<0.001) though these differences in choice were not biased towards foods with higher energy density. Children who ate a slower rate selected Honey Stars more frequently (χ2=36.7, p<0.001). Differences in energy intake were therefore not driven by selection frequency of individual food items. Comparison of the eating rates of the different foods demonstrated that seven of the nine foods in the sample were consumed at a similar rate (Range: 9.7-11.6 g/min), while Honey Stars were consumed at a slightly slower rate (5.8 g/min) and cheese slices at a slightly faster rate (15.1 g/min). Foods consumed slightly slower and slightly faster were of similar energy density (3.8 vs. 2.95 respectively) and these small differences in eating rate were not believed to unduly bias overall energy intakes within the group.
4.2. Eating Microstructure Patterns in Faster and Slower Eating Styles
Table 1 reports the correlations between eating rate and the associated eating microstructure. Results show that children who ate at a faster rate tended to take a greater total number of bites, had a larger average bite size, used fewer chews per gram and had shorter oral exposure time per bite. Faster eaters also showed a tendency to take fewer sips of drinks throughout the meal. Eating behaviours showed large co-variance such that several eating behaviours were correlated with eating rate, but also co-varied with one another. All behaviours associated with eating rate showed linear dependence, with the exception of chews per gram which was exponentially associated with the rate of eating (Figure 1).
Table 1.
Correlations between eating rate and eating behaviours observed during the meal.
| Sips | Oral exposure | Bites | mealtime | Oral exposure per bite | Bite size | Chews per gram | |
|---|---|---|---|---|---|---|---|
| ER | -0.10* | -0.05 | 0.15** | -0.02 | -0.25*** | 0.55*** | -0.71*** |
| Sips | 0.04 | <0.01 | 0.08 | 0.02 | -0.08 | 0.06 | |
| OE | 0.54*** | 0.33*** | 0.02 | -0.01 | -0.11* | ||
| Bites | 0.11* | -0.58*** | -0.42*** | -0.18*** | |||
| m. | 0.16*** | 0.17*** | -0.05 | ||||
| OE per bite | 0.54*** | 0.14** | |||||
| Bite size | -0.46*** |
*p<0.05, **p<0.01, ***p<0.001; ER= Eating rate; OE= Oral exposure; m. = mealtime
4.3. Comparison of eating microstructure between slower and faster eating styles
Characteristics of eating microstructure across the slower and faster eating styles are presented in Table 2. Faster eating rates supported larger ad-libitum energy intake and this was achieved through a greater total number of bites, larger average bite size (g), fewer chews per gram and shorter oral exposure time per bite (seconds). The average bite size associated with faster eating style was 70% larger than the average bite size associated with slower eating style. Those bites were also tended to be processed on average 4 seconds faster and using 50% fewer chews compared to oral processing associated with slower eating rates.
Table 2.
Comparison of eating behaviours among slow and fast eaters in the sample (Mean ± SEM; t-tests).
| Slow eaters (n= 192) | Fast eaters (n=194) | t | Sig | |
|---|---|---|---|---|
| Bites (#) | 57.7±2.5 | 68.4±2.5 | 3.04 | 0.003 |
| Oral exposure per bite (s) | 20.1±0.9 | 15.6±0.5 | 4.11 | <0.0001 |
| Bite size (g/bite) | 1.4±0.1 | 2.4±0.1 | 9.17 | <0.0001 |
| Chews per gram | 13.9±0.5 | 6.7±0.1 | 13.30 | <0.0001 |
| Sips (#) | 8.6±0.5 | 8.2±0.5 | 0.54 | 0.58 |
| Mealtime (%) | 75.0±1.0 | 76.0±1.0 | 0.56 | 0.57 |
| Total oral exposure (min) | 15.1±0.4 | 15.2±0.4 | 0.08 | 0.93 |
| Kcal | 175.3±6.09 | 306.8±9.9 | 11.28 | <0.0001 |
4.4. Contribution of Eating Microstructure to Energy Intake
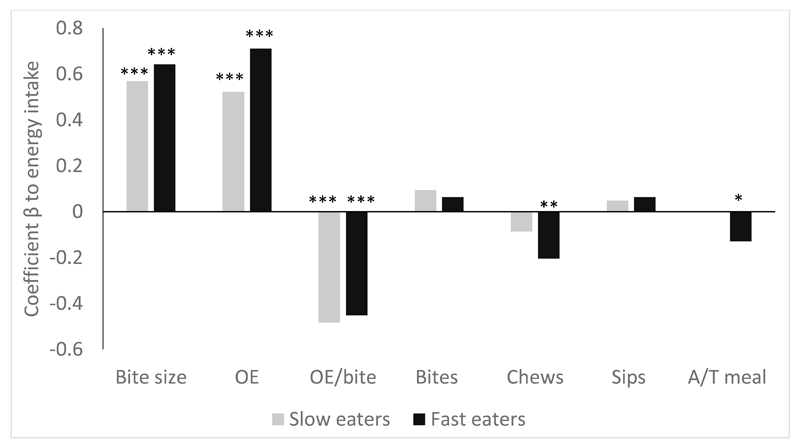
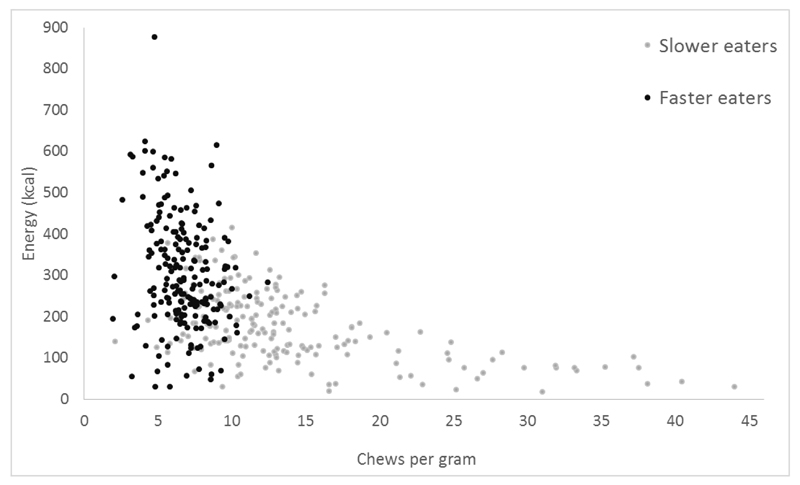
Figure 2 shows the beta-values from the regression model of each specific elements of eating microstructure independent contribution to energy intake among slower and faster eaters. The three strongest predictors of energy intake were the same for slower and faster eaters and included bite size, total oral exposure time and oral exposure time per bite. Among the slower eaters there were no other behaviours that had substantial linear impact on energy consumed (R2=0.67; F(7, 189) = 53.48, p<0.001), whereas among children with faster eating rates, chews per gram and proportion of active to total mealtime were additional linear independent predictors of energy ingested (R2 = 0.56; F(7, 192) = 34.21, p<0.001). As chews per gram showed an exponential relationship with eating rate, two additional regression analyses were conducted with exponential curve estimation to predict energy intake from the chews per gram consumed, separately for slower and faster eaters. Number of chews per gram was a significant predictor of energy intake for both slower (F(1, 190)= 96.64, p<0.0001) and faster eaters (F(1, 192)= 4.02, p=0.046). There were large differences in the range of chews per gram between the two groups with faster eaters chewing in a narrower range of between 2-12 chews per gram, whereas slower eaters chewed anywhere from 2-44 chews per gram (Figure 2). Among the slower eaters, the link between chews per gram and energy consumed tended to be exponential, whereas among faster eaters it was linear (Figure 3). In summary, a greater total number of bites and larger bite size in combination with longer total oral exposure time, shorter oral exposure per bite and fewer chews per gram were associated with higher energy intake during the meal among children eating in both slower and faster eating groups.
Figure 2.
Independent contribution of elements of eating microstructure to energy intake, presented separately for slower and faster eating children. Significant contributors to the model are marked with: ***p<0.001, **p<0.01 and *p<0.05. OE= Total oral exposure; OE/bite= oral exposure per bite; A/T meal= Proportion of active mealtime to total meal duration.
Figure 3.
Relationship between chews per gram and energy consumed during the meal, presented separately for slower and faster eating children.
4.5. Comparison of eating microstructure among children with healthy weight and overweight
Eating microstructure patterns were compared between children with healthy weight and overweight, and between children with lower and higher levels of abdominal subcutaneous adiposity, and are summarised in Table 3. Among children with BMI in the healthy range, 79 had lower levels of SAT and 65 had higher levels of SAT. Among children whose BMI was in the overweight range, all children had higher levels of SAT. As reported previously, children with overweight tended to eat at a faster rate (g/min) than healthy weight children [10]. Subsequent analysis of specific differences in eating microstructure showed that faster eating rates among children with overweight and among children with higher abdominal adiposity were attributable to larger bite size.
Table 3.
Differences in eating behaviours (Mean ± SEM) between children with healthy weight and overweight/obesity and between children with lower and higher levels of abdominal adiposity (t-test).
| BMI z-score | Abdominal Adiposity (cc*) | |||||
|---|---|---|---|---|---|---|
| Healthy weight (n=314) | Overweight (n=64) | p | Lower (n=79) | Higher (n=79) | P | |
| Eating rate (g/min) | 6.7±0.2 | 8.0±0.4 | 0.002 | 6.7±0.4 | 7.8±0.4 | 0.029 |
| Bite size (g/bite) | 1.8±0.06 | 2.2±0.2 | 0.040 | 1.8±0.1 | 2.2±0.2 | 0.045 |
| Sips (#) | 8.5±0.4 | 7.7±0.8 | 0.36 | 8.9±0.8 | 8.0±0.8 | 0.41 |
| Bites (#) | 63.1±1.99 | 64.6±4.2 | 0.76 | 64.1±4.2 | 67.1±4.0 | 0.61 |
| Oral exposure (min) | 15.1±0.3 | 15.6±0.8 | 0.52 | 14.9±0.6 | 15.6±0.5 | 0.39 |
| Oral exposure per bite (s) | 18.1±0.6 | 16.6±0.9 | 0.32 | 18.2±1.5 | 17.2±1.1 | 0.59 |
| Chews per gram | 10.5±0.4 | 9.1±0.8 | 0.12 | 10.7±0.8 | 9.0±0.7 | 0.11 |
| Mealtime (%) | 76±0.1 | 75±0.2 | 0.76 | 75±0.1 | 75±0.1 | 0.88 |
| Kcal | 230.5±6.7 | 298.0±21.8 | 0.004 | 235.0±14.5 | 277.2±17.1 | 0.063 |
cc= cubic centimetres
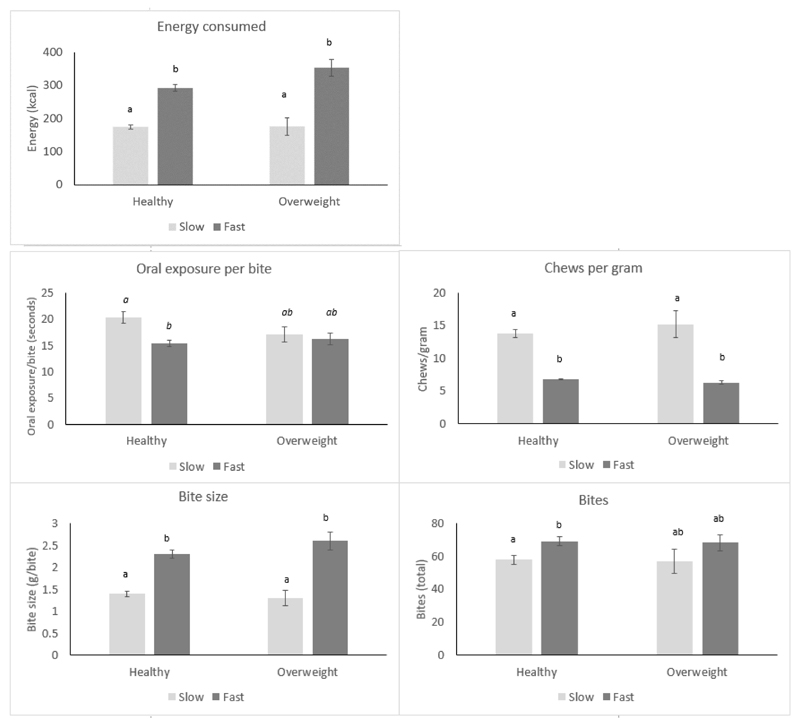
Large differences in energy consumed were recorded between children with healthy weight and overweight, which were underpinned by differences in eating rate and bite size. These differences were re-examined among slower and faster eaters who were healthy weight and overweight, to compare stability of the relationship between eating microstructure patterns and intake across the two weight status groups. Participants were divided into four groups: healthy weight slower (n= 166) and faster eaters (n=148), and overweight slower (n= 20) and faster eaters (n=44). The four groups differed in the total number of bites (p=0.022), bite size (p<0.0001), chews per gram (p<0.0001), oral exposure per bite (p<0.0001) and energy consumed during the meal (p<0.0001). Group differences are presented in Figure 4 (a-e). Faster eating overweight children consumed the most kilocalories, which equated to over 100% more energy than slower eaters with healthy weight (Δ=178.7 kcal). Faster eaters had larger bite size and took fewer chews per gram, independently of their weight status. These patterns were largely replicated when children were split by abdominal adiposity status rather than BMI. Overweight faster eaters showed similar eating microstructure patterns to faster eaters who were healthy weight, indicating that the same microstructure of larger bites and fewer chews per gram supported energy intakes among both children with healthy weight and overweight. Children who were of healthy weight were equally likely to be slower or faster eaters, but children with overweight status were 2.5 times more likely to be classified as faster eaters (χ2=9.93, p=0.002), albeit with unbalanced groups. Analysis of body composition among children with healthy weight showed that faster eaters with healthy weight still had higher BMIz scores (Δz=0.2, p=0.02) and more abdominal adiposity (Δ=46.6cm3, p=0.046) compared to slower eaters with healthy weight.
Figure 4.
(a-e). Group differences in energy consumed (a), oral exposure per bite (b), chews per gram (c), bite size (d) and bites (e), between slower and faster eaters split, by their weight status. Superscript annotation of a and b indicates groups which are significantly different at p<0.001. Groups with the same annotation are not different. *p<0.05, ***p<0.001.
4.6. Associations between Children’s Individual differences and their Eating microstructure
Variations in eating microstructure across the sample were explored and are summarised in Table 4. Initial comparisons revealed faster eating rates to be more prevalent among boys, children of Indian origin and children who were born preterm. However, after controlling for BMI, only sex differences in eating rate remained. There were no differences in eating rates between children with different early feeding history such as age of introduction of complementary foods, or being breast or bottle fed. Some minor differences in eating microstructure were observed. For example, children who began complementary feeding earlier had a larger average bite size, and these children also tended to have the longest oral exposure per bite, which remained stable after controlling for BMI. Breastfed children were more likely to take more bites and had shorter oral exposure time per bite, compared to bottle-fed children.
Table 4.
Individual differences in eating behaviours, energy intake and body composition (mean± SEM).
| Eating rate (g/min) | Bite size (g/bite) | Oral exposure (min) | Oral exposure per bite (s) | Bites (#) | Chews per gram | Sips (#) | Mealtime (%) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | 7.4±3.4 | 2.1±0.2 | 14.5±0.4 | 9.7±0.5 | |||||
| Female |
6.3±2.8 p<0.001 |
1.8±0.1 p=0.011 |
15.9±0.3 p=0.007 |
10.8±0.5 p=0.084 |
||||||
| Ethnicity | Chinese | 6.6±2.3a | 1.8±0.1a | 16.2±0.3a | 69.5±2.5a | 9.4±0.5a | 77.0±0.1a | |||
| Malay |
6.8±3.1 |
2.1±0.1b, p=0.049 |
14.3±0.5b, p<0.001 |
55.7±3.0b |
7.2±0.6b, p=0.01 |
74.0±0.1 | ||||
| Indian | 7.7±3.4b p=0.031 |
2.1±0.1b, p=0.068 |
13.2±0.9b p<0.001 |
54.7±3.7b p<0.0001 |
7.5±0.8 |
72.0±0.2b, p=0.012 |
||||
| Feeding | Breastfed | 15.5±1.0 | 71.0±5.5 | |||||||
| Bottlefed |
18.4±0.7 p=0.084 |
61.2±1.99 p=0.058 |
||||||||
| Weaning | Early | 2.1±0.2a | 19.9±1.6a | |||||||
| Typical | 2.0±0.1 | 17.8±0.7 | ||||||||
| Late |
1.7±0.1b, p=0.048 |
15.4±0.7b, p=0.024 |
||||||||
| GA | Preterm | 7.4±3.1 | 9.3±0.6 | |||||||
| Full-term | 6.7±3.2 p=0.09 |
10.6±0.4 p=0.086 |
||||||||
Values in bold indicate significant or near significant group differences, which were still present after controlling for BMI. Values not in bold indicate significant group differences which were no longer significant after controlling for BMI. Missing fields indicate no significant group differences
5.0. Discussion
The current study described the eating microstructure patterns that contribute to faster eating rates and support increased energy intakes within a meal among a multi-ethnic group of Singaporean children. Children who exhibited a faster eating style took more bites during the meal, had a larger average bite size, took fewer chews per gram of food, and had a shorter oral exposure time per bite. These children also consumed the most energy. Children with overweight status and children with larger abdominal adiposity were more likely to eat at a faster rate than healthy weight children, driven by larger average bite size. Although this faster eating style was more prevalent among children with overweight, a large number of children within the healthy weight category were also faster eaters. Healthy weight children who exhibited the faster eating style tended to have a higher BMI and greater abdominal adiposity compared to their slower eating counterparts. Analysis of individual differences in eating microstructure highlighted robust differences in eating style across sexes, and some variation across early feeding regimes, independently of weight status.
The three elements of eating microstructure that showed the strongest associations with eating rate and energy intake were children’s average bite size, chews per gram and oral exposure per bite, which is consistent with past findings linking these behaviours to faster eating rates and higher energy intake [14, 31, 33, 50, 51]. There was a greater variation in the number of chews taken for each gram of food among children with the slower eating style compared to children who ate faster. These results support the idea that chewing behaviour is an important contributor to energy intake within a meal and suggest that children may show more variation in their chewing compared to chewing patterns seen among adults, who typically show linear associations between chewing and energy intake [15]. Other aspects of eating microstructure such as sipping rate have been less studied, however this too can affect eating rate and energy intake. Achieving a sufficient breakdown of structure and degree of bolus lubrication is required for swallowing and is linked to the time a food is chewed, as previously described by the ‘oral process model’ of Hutchings and Lillford [52].
There were clear differences in the microstructural patterns of eating that characterised slower and faster eating styles, and these behaviours were observed in children with healthy weight and overweight status. Previous research demonstrated that children with overweight status tend to eat at faster rates compared to healthy weight children [5, 6, 8, 9, 22]. However, there has been less agreement on the differences in other microstructural patterns of eating between people with healthy weight and overweight status, with some studies finding differences [7, 28, 53] and other studies that did not [33, 54, 55]. The faster eating style was found across both overweight and healthy weight children, and many of the fast eating healthy weight children were heavier than their slower eating counterparts, though still within the healthy range. This suggests that this eating style may support the increased energy intakes required for weight gain and the transition from healthy weight to overweight. We propose that this eating style and associated microstructural oral processing behaviours can be considered as ‘obesogenic’, as they support higher energy intakes, and likely facilitate the transition from healthy weight to overweight when combined with other dietary factors, such as higher dietary energy density and larger portions. The ‘obesogenic’ eating style is characterised by faster eating rates that are achieved through taking larger and more frequent bites that are chewed less and held in the oral cavity for a shorter time.
There were clear differences in the microstructure of eating between slower and faster eating children, and these paralleled in some cases two-fold differences in energy consumed during the meal. These faster eating behaviours were spontaneous as participants were not strictly time restricted and were instructed to eat in their normal way. The current study attempted to explore some of the possible factors that may be linked to this obesogenic eating style, with a specific focus on differences in eating rate across sex, ethnicity, gestational age at birth and early feeding history. Consistent with previous literature, we found that boys ate faster than girls and this was achieved through larger bite size, shorter oral exposure time and reduced chewing [34, 35]. Children introduced to solid foods before 4 months of age tended to exhibit larger bite size; however, they also had the longest oral exposure time per bite and as a result these children did not show higher eating rates. Overall there were no differences in eating rates between breastfed and bottle fed children, but the cumulative influence of breastfeeding and weaning time were beyond the scope of the current study and should be further examined in the future. Differences in eating microstructure may reflect underlying differences in appetitive traits such as eating for pleasure, food reward or satiety responsiveness [56]. Links between eating rate and differences in motivation to eat, self-regulation and impulsivity also merit further exploration. Eating behaviours are learned through early experiences with foods, parental feeding practices and the food environment within which each child is developing [36]. A better understanding of the key early experiences that influence the development of eating microstructure patterns requires further investigation if we are to set guidelines on best practice approaches to encourage appropriate eating styles to sustain normal growth and development, rather than the obesogenic style characterised in the current paper. In addition there may be elements of the current food environment that promote faster eating rates such as time pressure around meals and snacks, and the widespread availability of softly textured energy dense foods. Understanding how the food environmental helps promote this obesogenic eating style is key for the development of strategies to tackle behaviours that encourage increased or excessive energy intakes.
The results of the current study highlight an opportunity to design eating behaviour interventions that target specific features of eating microstructure, to reduce eating rate and energy intake. Past research has demonstrated the efficacy of several different approaches to manipulating eating rate. Manipulation of bite size may be achieved through changing the shape of foods [57], using utensils that support smaller bites [51] and by changing the textures of foods to reduce eating rate and energy intake [11, 58, 59]. Changes to eating rate can be produced by prompting the eater via external monitors of eating rate; this has been shown to have some success in children and adults [60, 61], and has been shown to be a successful weight reduction strategy among children with overweight [62] and adolescents [63]. Similarly it is possible to change eating rate indirectly through the use of food textures that are equally liked, but require longer chewing [11, 13, 18, 43, 58]. Although many studies have focused on the impact of timing of introduction and food texture quality on the development of children’s eating behaviour [38, 64, 65], to date the efficacy of using food texture to produce sustained reductions in children’s eating rates, and subsequent reduction in energy intake has not been demonstrated.
The current study surveyed a large multi-ethnic sample of young children using a comprehensive objective assessment of features of eating microstructure. However, despite the large sample size, there was an unbalanced comparison between children with healthy weight and overweight status, as there were only a small number of children with BMI in the overweight range in the cohort. Further studies are required with larger samples of children with overweight to definitively conclude on the consistency of these eating behaviours among different weight categories. The current study focussed on a cross-sectional comparison of eating behaviours at a single time point. To determine whether faster eating rates help support future weight gain and adiposity, further studies are needed to explore the stability of these eating behaviours longitudinally within the same children, to ascertain the impact of faster eating rates on energy intake, body weight and adiposity over time.
Conclusion
Children who eat at a faster rate do so by taking more bites, with larger average bite size, chewing less per gram and having a shorter oral exposure time per bite. These behaviours are associated with higher energy intakes and are exhibited in children with overweight and healthy weight status. We propose that these combinations of these microstructural patterns of eating characterise an ‘obesogenic’ eating style that supports greater acute energy intake and may promote sustained positive energy balance and weight gain over time. Individual differences in eating microstructure and eating rate may be associated with specific early food experiences and this requires further exploration. These results highlight an opportunity to design eating behaviour interventions that target specific features of eating microstructure and through this reduce eating rate and acute energy intakes.
Supplementary Material
Acknowledgments
We thank the contributions of study participants, GUSTO study group and all clinical and home-visit staff involved. The GUSTO study group includes Pratibha Agarwal, Arijit Biswas, Choon Looi Bong, Birit FP Broekman, Shirong Cai, Jerry Kok Yen Chan, Yiong Huak Chan, Cornelia Yin Ing Chee, Helen Y. H Chen, Yin Bun Cheung, Audrey Chia, Amutha Chinnadurai, Chai Kiat Chng, Shang Chee Chong, Mei Chien Chua, Chun Ming Ding, Eric Andrew Finkelstein, Doris Fok, Marielle Fortier, Anne Eng Neo Goh, Yam Thiam Daniel Goh, Joshua J. Gooley, Wee Meng Han, Mark Hanson, Christiani Jeyakumar Henry, Joanna D. Holbrook, Chin-Ying Hsu, Hazel Inskip, Jeevesh Kapur, Ivy Yee-Man Lau, Bee Wah Lee, Yung Seng Lee, Ngee Lek, Sok Bee Lim, Yen-Ling Low, Iliana Magiati, Lourdes Mary Daniel, Michael Meaney, Cheryl Ngo, Krishnamoorthy Naiduvaje, Wei Wei Pang, Anqi Qiu, Boon Long Quah, Victor Samuel Rajadurai, Mary Rauff, Salome A. Rebello, Jenny L. Richmond, Anne Rifkin-Graboi, Lynette Pei-Chi Shek, Allan Sheppard, Borys Shuter, Leher Singh, Shu-E Soh, Walter Stunkel, Lin Lin Su, Kok Hian Tan, Oon Hoe Teoh, Mya Thway Tint, Hugo P S van Bever, Rob M. van Dam, Inez Bik Yun Wong, P. C. Wong, Fabian Yap, George Seow Heong Yeo.
Sources of Support: This work is supported by the Translational Clinical Research (TCR) Flagship Program on Developmental Pathways to Metabolic Disease funded by the National Research Foundation (NRF) and administered by the National Medical Research Council (NMRC), Singapore-NMRC/TCR/004-NUS/2008. Additional funding is provided by the Singapore Institute for Clinical Sciences, A*STAR and Nestec SA. KMG is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union's Seventh Framework Programme (FP7/2007-2013), project Early Nutrition under grant agreement n°289346.
Abbreviations
- BMIz
Body Mass Index z-score
- SAT
Subcutaneous Adipose Tissue
- OE
oral exposure
Footnotes
Authors’ Contributions: This study was conceived and designed by CGF, AF, MFFC and LRF. Clinical analyses were performed by SS, SV, AF, ATG, and CGF and data analysis and interpretation were carried out by AF and CGF. AF and CGF prepared the draft manuscript and all authors reviewed and approved the final draft. This study was given ethical approval by ethical review boards of the KK Women’s and Children’s Hospital and National University Hospital in Singapore.
Author disclosures: Keith Godfrey, Lee Yung-Seng and Yap Seng Chong have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. They are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. Lisa Fries is an employee of Nestec SA, working at the Nestlé Research Center. The other authors have no financial or personal conflict of interests.
References
- 1.Gluckman P, Nishtar S, Armstrong T. Ending childhood obesity: a multidimensional challenge. The Lancet. 2016;385(9973):1048–1050. doi: 10.1016/S0140-6736(15)60509-8. [DOI] [PubMed] [Google Scholar]
- 2.Kissileff HR, Guss JL. Microstructure of eating behavior in humans. Appetite. 2001;36(1):70–8. doi: 10.1006/appe.2000.0369. [DOI] [PubMed] [Google Scholar]
- 3.Robinson E, et al. A systematic review and meta-analysis examining the effect of eating rate on energy intake and hunger. Am J Clin Nutr. 2014;100(1):123–51. doi: 10.3945/ajcn.113.081745. [DOI] [PubMed] [Google Scholar]
- 4.Barkeling B, Ekman S, Rössner S. Eating behaviour in obese and normal weight 11-year-old children. International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity. 1992;16(5):355–360. [PubMed] [Google Scholar]
- 5.Chei C, Toyokawa S, Kano K. Relationship between eating habits and obesity among preschool children in Ibaraki Prefecture, Japan. Japanese Journal of Health and Human Ecology. 2005;71(2):73–82. [Google Scholar]
- 6.He Q, et al. Risk factors of obesity in preschool children in China: a population-based case-control study. International journal of obesity. 2000;24(11):1528–1536. doi: 10.1038/sj.ijo.0801394. [DOI] [PubMed] [Google Scholar]
- 7.Laessle RG, et al. Parental influences on laboratory eating behavior in obese and non-obese children. Int J Obes Relat Metab Disord. 2001;25(Suppl 1):S60–2. doi: 10.1038/sj.ijo.0801701. [DOI] [PubMed] [Google Scholar]
- 8.Llewellyn CH, et al. Eating rate is a heritable phenotype related to weight in children. The American Journal of Clinical Nutrition. 2008;88(6):1560–1566. doi: 10.3945/ajcn.2008.26175. [DOI] [PubMed] [Google Scholar]
- 9.Berkowitz RI, et al. Identification of an obese eating style in 4-year-old children born at high and low risk for obesity. Obesity. 2010;18(3):505–512. doi: 10.1038/oby.2009.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogel A, et al. Faster eating rates are associated with higher energy intakes during an ad libitum meal, higher BMI and greater adiposity among 4.5 year old children – Results from the GUSTO cohort. Society for the Study of Ingestive Behaviour Meeting; Porto. 12-16th of July 2016; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forde C, et al. Fast or Slow-Foods? Describing Natural Variations in Oral Processing Characteristics across a Wide Range of Asian Foods. Food & Function. 2016 doi: 10.1039/c6fo01286h. [DOI] [PubMed] [Google Scholar]
- 12.Bolhuis DP, et al. Slow food: Sustained impact of harder foods on the reduction in energy intake over the course of the day. PLoS ONE. 2014;9(4):e93370. doi: 10.1371/journal.pone.0093370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forde CG, et al. Influence of meal texture on eating rate and food intake. Results from three ad-libitum trials. Appetite. 2013;71:474. [Google Scholar]
- 14.Miquel-Kergoat S, et al. Effects of chewing on appetite, food intake and gut hormones: A systematic review and meta-analysis. Physiology & Behavior. 2015;151:88–96. doi: 10.1016/j.physbeh.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y, Hollis JH. Increasing the number of chews before swallowing reduces meal size in normal-weight, overweight, and obese adults. J Acad Nutr Diet. 2014;114(6):926–31. doi: 10.1016/j.jand.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Li J, et al. Improvement in chewing activity reduces energy intake in one meal and modulates plasma gut hormone concentrations in obese and lean young Chinese men. American Journal of Clinical Nutrition. 2011;94(3):709–716. doi: 10.3945/ajcn.111.015164. [DOI] [PubMed] [Google Scholar]
- 17.Zijlstra N, et al. Effect of bite size and oral processing time of a semisolid food on satiation. The American journal of clinical nutrition. 2009;90(2):269–275. doi: 10.3945/ajcn.2009.27694. [DOI] [PubMed] [Google Scholar]
- 18.Forde CG, et al. Oral processing characteristics of solid savoury meal components, and relationship with food composition, sensory attributes and expected satiation. Appetite. 2013;60(0):208–219. doi: 10.1016/j.appet.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Geier AB, Rozin P, Doros G. Unit bias a new heuristic that helps explain the effect of portion size on food intake. Psychological Science. 2006;17(6):521–525. doi: 10.1111/j.1467-9280.2006.01738.x. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel TA, et al. Bite size, ingestion rate, and meal size in lean and obese women. Appetite. 1993;21(2):131–45. doi: 10.1016/0195-6663(93)90005-5. [DOI] [PubMed] [Google Scholar]
- 21.Fisher JO, Rolls BJ, Birch LL. Children’s bite size and intake of an entrée are greater with large portions than with age-appropriate or self-selected portions. The American Journal of Clinical Nutrition. 2003;77(5):1164–1170. doi: 10.1093/ajcn/77.5.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drabman RS, Hammer D, Jarvie GJ. Eating styles of obese and nonobese black and white children in a naturalistic setting. Addictive Behaviors. 1977;2(2–3):83–86. doi: 10.1016/0306-4603(77)90023-5. [DOI] [PubMed] [Google Scholar]
- 23.Tanihara S, et al. Retrospective longitudinal study on the relationship between 8-year weight change and current eating speed. Appetite. 2011;57(1):179–83. doi: 10.1016/j.appet.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki S, et al. Self-reported rate of eating correlates with body mass index in 18-y-old Japanese women. International Journal of Obesity. 2003;27(11):1405–1410. doi: 10.1038/sj.ijo.0802425. [DOI] [PubMed] [Google Scholar]
- 25.Otsuka R, et al. Eating fast leads to obesity: Findings based on self-administered questionnaires among middle-aged Japanese men and women. Journal of Epidemiology. 2006;16(3):117–124. doi: 10.2188/jea.16.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruyama K, et al. The joint impact on being overweight of self reported behaviours of eating quickly and eating until full: Cross sectional survey. BMJ. 2008;337(7678):1091–1093. doi: 10.1136/bmj.a2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohkuma T, et al. Association between eating rate and obesity: a systematic review and meta-analysis. International Journal of Obesity. 2015 doi: 10.1038/ijo.2015.96. [DOI] [PubMed] [Google Scholar]
- 28.Drabman RS, et al. Developmental trends in eating rates of normal and overweight preschool children. Child development. 1979;50(1):211–216. [PubMed] [Google Scholar]
- 29.Zhu Y, Hollis JH. Differences in chewing behaviors between healthy fully dentate young and older adults assessed by electromyographic recordings. International Journal of Food Sciences and Nutrition. 2015;66(4):452–457. doi: 10.3109/09637486.2015.1038222. [DOI] [PubMed] [Google Scholar]
- 30.Gaul DJ, Craighead WE, Mahoney MJ. Relationship between eating rates and obesity. J Consult Clin Psychol. 1975;43(2):123–5. doi: 10.1037/h0076522. [DOI] [PubMed] [Google Scholar]
- 31.Laessle RG, Uhl H, Lindel B. Parental influences on eating behavior in obese and nonobese preadolescents. International Journal of Eating Disorders. 2001;30(4):447–453. doi: 10.1002/eat.1106. [DOI] [PubMed] [Google Scholar]
- 32.Hill SW, McCutcheon NB. Contributions of obesity, gender, hunger, food preference, and body size to bite size, bite speed, and rate of eating. Appetite. 1984;5(2):73–83. doi: 10.1016/s0195-6663(84)80026-4. [DOI] [PubMed] [Google Scholar]
- 33.Spiegel TA. Rate of intake, bites, and chews—the interpretation of lean–obese differences. Neuroscience & Biobehavioral Reviews. 2000;24(2):229–237. doi: 10.1016/s0149-7634(99)00076-7. [DOI] [PubMed] [Google Scholar]
- 34.Chang HS, Roh SM. Comparison with dietary habits, dietary attitudes and nutritional knowledge according to sex of teenagers in Jeonnam province. Korean Journal of Community Nutrition. 2006;11(4):459–468. [Google Scholar]
- 35.Scudine KGdO, et al. Assessment of the differences in masticatory behavior between male and female adolescents. Physiology & Behavior. 2016;163:115–122. doi: 10.1016/j.physbeh.2016.04.053. [DOI] [PubMed] [Google Scholar]
- 36.Birch LL, Fisher JO. Development of eating behaviors among children and adolescents. Pediatrics. 1998;101(Supplement 2):539–549. [PubMed] [Google Scholar]
- 37.Soh SE, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43(5):1401–9. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 38.Nicklaus S, Demonteil L, Tournier C. 8 - Modifying the texture of foods for infants and young children. In: Chen J, Rosenthal A, editors. Modifying Food Texture. Woodhead Publishing; 2015. pp. 187–222. [Google Scholar]
- 39.WHO. Preterm birth. 2015 Available from: http://www.who.int/mediacentre/factsheets/fs363/en/
- 40.Singapore HPB. Food and Nutrient Composition Database. 2016 Retrieved 2016, from Health Promotion Board. HPB. http://focos.hpb.gov.sg/eservices/ENCF/
- 41.Health Promotion Board Singapore. Recommended Dietary Allowances. 2015. [Google Scholar]
- 42.Hennequin M, et al. Clinical evaluation of mastication: validation of video versus electromyography. Clinical Nutrition. 2005;24(2):314–320. doi: 10.1016/j.clnu.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Ferriday D, et al. Variation in the Oral Processing of Everyday Meals Is Associated with Fullness and Meal Size; A Potential Nudge to Reduce Energy Intake? Nutrients. 2016;8(5):315. doi: 10.3390/nu8050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klesges RC, et al. Parental influences on children's eating behavior and relative weight. Journal of Applied Behavior Analysis. 1983;16(4):371–378. doi: 10.1901/jaba.1983.16-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haidet KK, et al. Methods to Improve Reliability of Video Recorded Behavioral Data. Research in nursing & health. 2009;32(4):465–474. doi: 10.1002/nur.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.WHO. Child Growth Standards. 2003 Available from: http://www.who.int/childgrowth/standards/Technical_report.pdf.
- 47.Wang Y, Chen H-J. Handbook of anthropometry. Springer; 2012. Use of percentiles and z-scores in anthropometry; pp. 29–48. [Google Scholar]
- 48.CDC. Defining Childhood Obesity. Centres for Disease Control and Prevention] 2015 Available from: https://www.cdc.gov/obesity/childhood/defining.html.
- 49.Sadananthan SA, et al. Automated segmentation of visceral and subcutaneous (deep and superficial) adipose tissues in normal and overweight men. J Magn Reson Imaging. 2015;41(4):924–34. doi: 10.1002/jmri.24655. [DOI] [PubMed] [Google Scholar]
- 50.Zhu Y, Hollis JH. Relationship between chewing behavior and body weight status in fully dentate healthy adults. Int J Food Sci Nutr. 2015;66(2):135–9. doi: 10.3109/09637486.2014.979317. [DOI] [PubMed] [Google Scholar]
- 51.Bolhuis D, Keast R, Newman L. Consumption with fork or spoon? Effects on acute food intake, eating rate and palatability. Journal of Nutrition. 2016;4:1–48. [Google Scholar]
- 52.Hutchings JB, Lillford PJ. The perception of food texture - the philosophy of the breakdown path. Journal of Texture Studies. 1988;19(2):103–115. [Google Scholar]
- 53.Hill AJ, Magson LD, Blundell JE. Hunger and palatability: tracking ratings of subjective experience before, during and after the consumption of preferred and less preferred food. Appetite. 1984;5(4):361–371. doi: 10.1016/s0195-6663(84)80008-2. [DOI] [PubMed] [Google Scholar]
- 54.Park S, Shin WS. Differences in eating behaviors and masticatory performances by gender and obesity status. Physiol Behav. 2015;138:69–74. doi: 10.1016/j.physbeh.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 55.White AK, et al. A comparison of chewing rate between overweight and normal BMI individuals. Physiology & Behavior. 2015;145:8–13. doi: 10.1016/j.physbeh.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 56.Moreno LA, Pigeot I, Ahrens W. Prevalence and etiology. Nueva York: Springer; 2011. Epidemiology of obesity in children and adolescents. [Google Scholar]
- 57.Hiiemae K, et al. Natural bites, food consistency and feeding behaviour in man. Arch Oral Biol. 1996;41(2):175–89. doi: 10.1016/0003-9969(95)00112-3. [DOI] [PubMed] [Google Scholar]
- 58.Viskaal-van Dongen M, Kok FJ, de Graaf C. Eating rate of commonly consumed foods promotes food and energy intake. Appetite. 2011;56(1):25–31. doi: 10.1016/j.appet.2010.11.141. [DOI] [PubMed] [Google Scholar]
- 59.Forde CG, et al. Texture and savoury taste influences on food intake in a realistic hot lunch time meal. Appetite. 2013;60:180–186. doi: 10.1016/j.appet.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 60.Salazar Vázquez B, et al. Control of overweight and obesity in childhood through education in meal time habits. The ‘good manners for a healthy future’programme. Pediatric obesity. 2015 doi: 10.1111/ijpo.12091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamilton-Shield J, et al. Changing eating behaviours to treat childhood obesity in the community using Mandolean: the Community Mandolean randomised controlled trial (ComMando) - a pilot study. Health Technol Assess. 2014;18(47) doi: 10.3310/hta18470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ford AL, et al. Treatment of childhood obesity by retraining eating behaviour: randomised controlled trial. Bmj. 2010;340:b5388. doi: 10.1136/bmj.b5388. [DOI] [PubMed] [Google Scholar]
- 63.Galhardo J, et al. Normalizing eating behavior reduces body weight and improves gastrointestinal hormonal secretion in obese adolescents. The Journal of Clinical Endocrinology & Metabolism. 2011;97(2):E193–E201. doi: 10.1210/jc.2011-1999. [DOI] [PubMed] [Google Scholar]
- 64.Coulthard H, Harris G, Emmett P. Delayed introduction of lumpy foods to children during the complementary feeding period affects child's food acceptance and feeding at 7 years of age. Maternal & child nutrition. 2009;5(1):75–85. doi: 10.1111/j.1740-8709.2008.00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Northstone K, Emmett P, Nethersole F. The effect of age of introduction to lumpy solids on foods eaten and reported feeding difficulties at 6 and 15 months. Journal of Human Nutrition and Dietetics. 2001;14(1):43–54. doi: 10.1046/j.1365-277x.2001.00264.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.