Abstract
Objective
Frailty is a common condition in patients with severe aortic stenosis (AS) undergoing transcatheter aortic valve replacement (TAVR). The aim of this systematic review was to assess the impact of frailty status on acute kidney injury (AKI) and mortality after TAVR.
Methods
A systematic literature search was conducted using MEDLINE, EMBASE, and Cochrane databases from the inception through November 2016. The protocol for this study is registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42016052350). Studies that reported odds ratios, relative risks or hazard ratios comparing the risk of AKI after TAVR in frail vs. non-frail patients were included. Mortality risk was evaluated among the studies that reported AKI-related outcomes. Pooled risk ratios (RR) and 95% confidence interval (CI) were calculated using a random-effect, generic inverse variance method.
Results
Eight cohort studies with a total of 10,498 patients were identified and included in the meta-analysis. The pooled RR of AKI after TAVR among the frail patients was 1.19 (95% CI 0.97–1.46, I2 = 0), compared with non-frail patients. When the meta-analysis was restricted only to studies with standardized AKI diagnosis according to Valve Academic Research Consortium (VARC)-2 criteria, the pooled RRs of AKI in frail patients was 1.16 (95% CI 0.91–1.47, I2 = 0). Within the selected studies, frailty status was significantly associated with increased mortality (RR 2.01; 95% CI 1.44–2.80, I2 = 58).
Conclusion
The findings from our study suggest no significant association between frailty status and AKI after TAVR. However, frailty status is associated with mortality after TAVR and may aid appropriate patient selection for TAVR.
Introduction
Transcatheter aortic valve replacement (TAVR), also known as transcatheter aortic valve implantation (TAVI), has been increasingly utilized for patients with severe aortic stenosis (AS) who are at substantial or prohibitive risk for surgical aortic valve replacement (SAVR) [1–5]. To date, more than 200,000 TAVR procedures have been performed [6, 7]. Although TAVR is considered a less invasive treatment compared with SAVR, the one and two year mortality following TAVR is still considerable at 24% and 34%, respectively [8, 9]. Acute kidney injury (AKI) after TAVR is common with a varied reported incidence ranging from 15% to 57% [2, 6, 10, 11]. Studies have demonstrated significant associations between AKI and decreased survival in patients undergoing TAVR [7, 10, 12].
Frailty is a state of late-life deterioration and vulnerability, characterized by physical weakness, wasting (involving both loss of muscle mass and weight), loss of endurance, decreased balance and mobility, slowed performance, increased sedentary behaviour, and decreased cognitive function [13, 14]. Studies have demonstrated that frailty is associated with adverse health outcomes including postoperative complications, increased hospital length of stay, dependency, falls, discharges to skilled nursing or assisted living facilities, and increased mortality in both general and cardiac surgery populations [15–18].
Among hospitalized patients, a recent retrospective study demonstrated a potential association between frailty and AKI [19]. There is a paucity of data regarding the impact of frailty on AKI incidence, for patients who undergo TAVR. Previous studies of patients undergoing TAVR have not shown such an association [20–27]. Nevertheless, it is possible that previous studies have been underpowered due to sample size. Also, the impact of frailty on AKI after TAVR is unclear due also to the diversity of definitions used for frailty [20–27]. Thus, we conducted this systematic review to assess the impact of frailty on AKI after TAVR comprehensively.
Materials and methods
Search strategy
The protocol of this study is registered with PROSPERO (International Prospective Register of Systematic Reviews; no. CRD42016052350). We also followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (S1 File) [28]. Two investigators (CT and WC) systematically searched and reviewed the published literature and conference abstracts indexed in MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and the Cochrane Database of Systematic Reviews from database inception through November 2016 without language restrictions, using the search strategy described in Text A in S2 File. Other pertinent references were obtained via manual review of these retrieved references.
Inclusion criteria
The studies fulfilled the following inclusion criteria: 1) randomized controlled trials (RCTs) or observational studies (cohort, cross-sectional, or case-control studies) published as original articles or conference abstracts that evaluated the risk of AKI after TAVR in frail patients; 2) available data with odds ratio, relative risk, or hazard ratio with 95% confidence intervals (CIs); and 3) a reference group composed of non-frail patients. The primary outcome was AKI after TAVR. Mortality risk was also evaluated among the studies that reported AKI-outcome. Study eligibility was defined by the two investigators noted previously. Differing decisions were solved by mutual consensus. The quality of each study was quantified via the validated Newcastle-Ottawa quality assessment scale for cohort and case-control studies [29] and modified Newcastle-Ottawa scale [30] for cross-sectional studies.
Data extraction
Two investigators (CT and WC) performed data extraction and analysis of study quality. A standardized data collection template was used to extract the following information: last name of the first author, article title, study design, year of study, country of origin, year of publication, sample size, AKI definition, and definition of frailty.
Statistical analysis
The data analysis was completed using Review Manager software (Version 5.3, Copenhagen, Denmark) from the Cochrane Collaboration. Point estimates and standard errors were derived from each included study and were combined by the generic inverse variance method of DerSimonian and Laird [31]. Given the likelihood of increased inter-observation variance, a random-effect model was applied. Statistical heterogeneity was evaluated utilizing Cochran’s Q test. These results complemented the I2 statistic, which quantifies the proportion of the total variation across studies due to heterogeneity rather than chance. The I2 values of <25%, 26%-50%, 51%-75%, and >75% were deemed to represent insignificant, low, moderate, and high heterogeneity, respectively [32]. The presence of publication bias was screened via funnel plots of the logarithm of odds ratios vs. standard errors [33].
Results
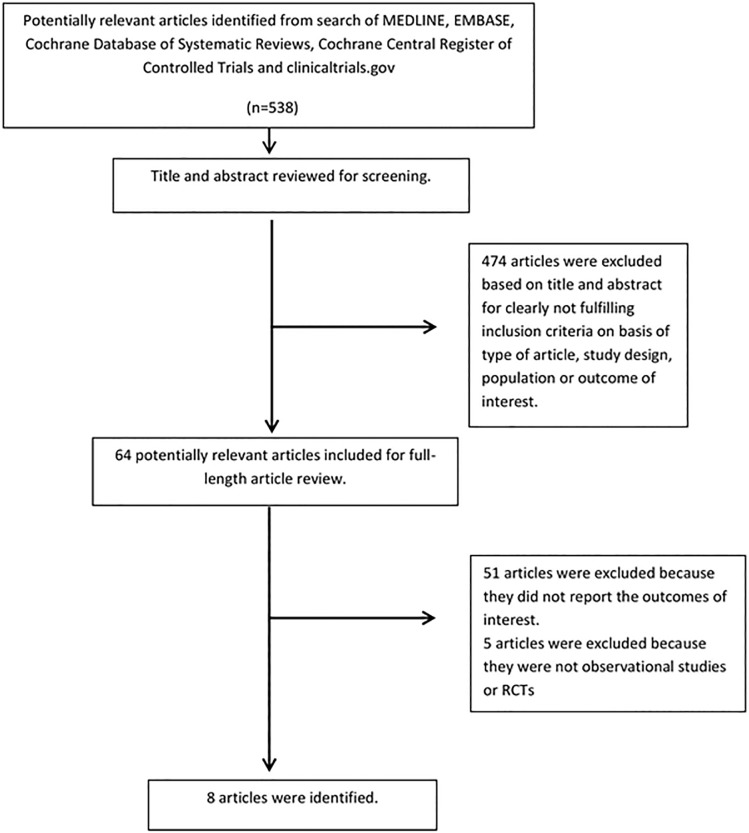
Our search strategy yielded 538 articles. Of these, 474 were excluded based on relevance and eligibility criteria following the review of the title and abstract. The remaining 64 articles underwent full-length review, and subsequently, 56 were excluded for failing to meet all eligibility requirements. Of these, 51 articles did not report the outcome of interest, and five articles were not observational studies or RCTs. Eight cohort studies [20–27] with a total of 10,498 patients were included in the meta-analysis to assess the risk of AKI following TAVR in frail vs. non-frail patients (Table 1). Fig 1outlines our search methodology and selection process.
Table 1. Main characteristics of the studies included in this meta-analysis[20–27].
| Study | Year | Total number | Frailty definition | AKI definition | Risk ratio for AKI | Risk ratio for mortality | Quality assessment |
|---|---|---|---|---|---|---|---|
| Green et al[20] | 2012 | 159 | Frailty score, based on gait speed, grip strength, serum albumin and activities of daily living status, > 5 | Acute kidney injury stage 2 or 3 according to VARC-2 definition | 1.10 (0.21–5.60) | 3.51 (1.43–8.62) | S 4, C 0, O 3 |
| Stortecky et al[21] | 2012 | 256 | BMI < 20 kg/m2 | Acute kidney injury stage 3 according to RIFLE definition | 1.35 (0.13–13.56) | 2.43 (0.92–6.39) | S 4, C 0, O 3 |
| Puls et al[22] | 2014 | 300 | Katz index < 6 | Acute kidney injury according to modified RIFLE definition | AKI; 1.23 (0.76–2.00), AKI stage 3; 2.23 (1.12–4.47) | 2.67 (1.7–4.3) | S 4, C 0, O 3 |
| Green et al[23] | 2015 | 244 | Frailty score, based on gait speed, grip strength, serum albumin and activities of daily living status, ≥ 6 | Renal failure requiring dialysis | 1.62 (0.58–4.49) | 2.50 (1.40–4.35) | S 4, C 0, O 3 |
| Yamamoto et al[24] | 2015 | 777 | BMI < 20 kg/m2 | Acute kidney injury according to VARC-2 definition | 0.84 (0.26–2.70) | 0.68 (0.29–1.61) | S 4, C 2, O 3 |
| Alfredson et al[25] | 2016 | 8,039 | 5-m gait speed < 10 s | Acute kidney injury according to VARC-2 definition | 1.18 (0.91–1.53) | 1.35 (1.01–1.80) | S 4, C 0, O 3 |
| Koifman et al[26] | 2016 | 491 | BMI < 20 kg/m2 | Acute kidney injury stage 2 or 3 according to VARC-2 definition | 1.04 (0.30–3.94) | 2.45 (1.26–4.75) | S 4, C 0, O 3 |
| Saji et al[27] | 2016 | 232 | The lowest tertile of normalized psoas muscle mass (cross-sectional areas of the psoas muscle at the L4 vertebra level measured by CT and normalized to body surface area) | Acute kidney injury stage 2 or 3 according to AKIN definition | 1.20 (0.52–2.80) | 2.30 (1.09–4.86) | S 4, C 0, O 3 |
Abbreviation: AKI, acute kidney injury; AKIN, Acute Kidney Injury Network; BMI, body mass index; S, selection; C, comparability; O, outcome; VARC, Valve Academic Research Consortium.
Fig 1. Outline of our search methodology.
AKI definition
All included studies identified AKI occurrence, based on the change in serum creatinine (SCr) or glomerular filtration rate (GFR) after TAVR. Seven [20–22, 24–27] of the eight studies used standard AKI definitions as shown in Table 1. Four [20, 24–26] of the eight studies used standardized AKI diagnosis according to Valve Academic Research Consortium (VARC)-2 (definition consisting of the Acute Kidney Injury Network (AKIN) or the Kidney Disease Improving Global Outcomes (KDIGO) criteria with the timing for AKI diagnosis up to 7 days following a TAVR procedure) [34].
Frailty and AKI risk after TAVR
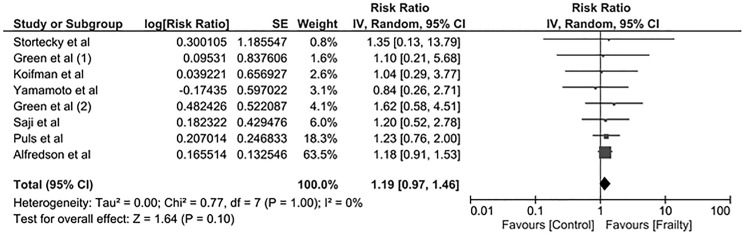
The pooled RR of AKI after TAVR in frail patients was 1.19 (95% CI 0.97–1.46), compared with non-frail patients, as shown in Fig 2. The statistical heterogeneity was insignificant with an I2 of 0%. When the meta-analysis was restricted only to studies with standardized AKI diagnosis according to VARC-2 criteria, the pooled RRs of AKI in frail patients was 1.16 (95% CI 0.91–1.47, I2 = 0).
Fig 2. Forest plot of included studies comparing the risk of AKI after TAVR in frail vs. non-frail patients.
Square data markers represent risk ratios (RRs), and horizontal lines represent the 95% confidence intervals (CIs) with marker size reflecting the statistical weight of the study using random-effects model. A diamond data marker represents the overall RR and 95% CI for the outcome of interest.
Of the eight studies, frailty status was identified by frailty score or gait speed in four studies [20, 22, 23, 25] and body mass index (BMI) or psoas muscle mass in the other four studies [21, 24, 26, 27] (Table 1). The pooled RR of AKI after TAVR in frail patients based on frailty score, or gait speed was 1.21 (95% CI 0.97–1.51, I2 = 0). The pooled RR of AKI after TAVR in frail patients based on BMI or psoas muscle mass was 1.07 (95% CI 0.60–1.93, I2 = 0).
Frailty and mortality risk after TAVR
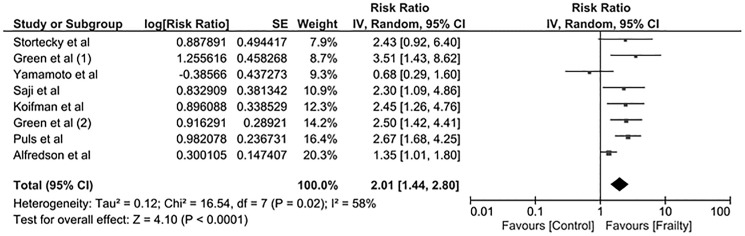
All included studies [20–27] evaluated the association between mortality (within 1 year after TAVR) and frailty status. The pooled RR of mortality in frail patients was 2.01 (95% CI 1.44–2.80, I2 = 58), as shown in Fig 3. The pooled RR of mortality after TAVR in frail patients based on frailty score or gait speed was 2.19 (95% CI 1.38–3.47, I2 = 70). The pooled RR of mortality after TAVR in frail patients based on BMI or psoas muscle mass was 1.78 (95% CI 0.99–3.21, I2 = 54).
Fig 3. Forest plot of included studies comparing the risk of mortality after TAVR in frail vs. non-frail patients.
Square data markers represent risk ratios (RRs); horizontal lines represent the 95% CIs with marker size reflecting the statistical weight of the study using random-effects model. A diamond data marker represents the overall RR and 95% CI for the outcome of interest.
Evaluation for publication bias
Funnel plots to evaluate publication bias for the risks of AKI and mortality after TAVR in frail patients are summarized in Fig A and B in S2 File. The graphs suggested no significant publication bias.
Study quality
All included cohort studies were of moderate to high quality [20–27], with a median Newcastle-Ottawa quality assessment scale of 7 (range 7–9) as shown in Table 1.
Discussion
In this systematic review, we demonstrated a statistically insignificant and potentially clinically relevant association between frailty and AKI after TAVR. This association remained insignificant after limiting studies to those that only utilized an AKI diagnosis according to VARC-2 criteria. However, within all included studies, frailty was significantly associated with increased mortality within 1 year in patients undergoing TAVR.
Studies have demonstrated abnormalities in frail patients including immune dysfunction, chronic low-grade systemic inflammation, and endocrine dysregulation such as insulin resistance and testosterone deficiency [13, 35]. Since it is well known that renal ischemia-reperfusion injury incites inflammation that subsequently exacerbates further injury, it has been postulated that frailty in cardiac surgery patients (especially with cardiopulmonary bypass) [36] may predispose patients to AKI [19]. However, based on the findings of this meta-analysis despite higher statistical power, there was no observed significant association between frailty and the risk of AKI after TAVR. These results suggests that the magnitude of frailty impact on AKI after TAVR is likely small compared to other known risk factors for AKI after TAVR (baseline renal function, transapical approach, blood transfusion and the need for circulatory support) [37–42].
Despite an insignificant association between frailty and AKI after TAVR, frail patients still carry a higher mortality risk after TAVR [20–27, 43–46]. Compared with non-frail patients, our meta-analysis demonstrated that frail patients had an approximately two-fold increased mortality risk within one year after TAVR. The increased mortality after TAVR in frail patients is likely multifactorial [15–18], but less likely due to AKI based on the findings of our study. Thus, frailty and AKI may independently affect patients' mortality without significant synergistic effects. Despite limited data on cause of death in the included studies, it has been previously shown that frailty is associated with increased cardiovascular diseases and events that result in increased mortality [47].
Although the studies included in our meta-analysis were all of moderate to high quality, there are some limitations that worth mentioning. First, there are multiple methods to measure or characterize AKI and frailty in the literature [20–27]. VARC-2 standardized the timing for the AKI diagnosis, extending from 72 hours to 7 days following a TAVR procedure using standardized criteria, i.e., the AKIN criteria and KDIGO criteria. Although VARC-2 definition is widely adopted as the standardized endpoint definition for TAVR, these three definitions are very similar with minor differences. Thus, the use of different AKI definition should not result in significant heterogeneity and the findings of our study suggest a statistically insignificant association between frailty and AKI after TAVR in different definitions. The definition of frailty is currently not standardized, and greater than 20 instruments for measurements of frailty have been developed [5]. BMI is used as a simple surrogate for wasting, whereas frailty score might provide more comprehensive functional assessment of the frailty condition [5]. Although there was no significant heterogeneity in our meta-analysis, a standardized definition of frailty and universal frailty assessment tools are needed in order to better understanding the impact of frailty on mortality. Second, there was limited data regarding the cause of death after TAVR. Thus, future studies are warranted to identify the underlying mechanisms on frailty-related mortality after TAVR. In addition, future studies are needed to evaluate if interventions to prevent and improve frailty can reduce mortality after TAVR. Lastly, this is a meta-analysis of observational studies subject to the inherent limitations for which a causal relationship cannot be inferred.
Our meta-analysis indicates no association between frailty and the risk of AKI after TAVR. However, frailty assessment should be part of comprehensive evaluation before TAVR and may aid patient selection process since it is significantly associated with higher mortality in patients undergoing TAVR.
Supporting information
(DOC)
Text A in S2 File: Search Strategy
Fig A in S2 File: Funnel plot of included studies in the meta-analysis for the risk of AKI after TAVR in frail patients. RR = risk ratio, SE = standard error.
Fig B in S2 File: Funnel plot of included studies with adjusted analysis in the meta-analysis for the risk of mortality after TAVR in frail patients. RR = risk ratio, SE = standard error.
(DOC)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.O'Brien SM, Shahian DM, Filardo G, Ferraris VA, Haan CK, Rich JB, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 2—isolated valve surgery. Ann Thorac Surg. 2009;88(1 Suppl): S23–42. Epub 2009/07/09. [DOI] [PubMed] [Google Scholar]
- 2.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. The New England journal of medicine. 2014;370(19):1790–8. Epub 2014/04/01. doi: 10.1056/NEJMoa1400590 [DOI] [PubMed] [Google Scholar]
- 3.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521–643. Epub 2014/03/05. doi: 10.1161/CIR.0000000000000031 [DOI] [PubMed] [Google Scholar]
- 4.Reardon MJ, Adams DH, Kleiman NS, Yakubov SJ, Coselli JS, Deeb GM, et al. 2-Year Outcomes in Patients Undergoing Surgical or Self-Expanding Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2015;66(2):113–21. Epub 2015/06/10. doi: 10.1016/j.jacc.2015.05.017 [DOI] [PubMed] [Google Scholar]
- 5.Thongprayoon C, Cheungpasitporn W, Kashani K. The impact of frailty on mortality after transcatheter aortic valve replacement. Ann Transl Med 2017;5(6):144 doi: 10.21037/atm.2017.01.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elhmidi Y, Bleiziffer S, Deutsch MA, Krane M, Mazzitelli D, Lange R, et al. Acute kidney injury after transcatheter aortic valve implantation: incidence, predictors and impact on mortality. Archives of cardiovascular diseases. 2014;107(2):133–9. Epub 2014/02/22. doi: 10.1016/j.acvd.2014.01.002 [DOI] [PubMed] [Google Scholar]
- 7.Cheungpasitporn W, Thongprayoon C, Kashani K. Transcatheter Aortic Valve Replacement: a Kidney's Perspective. Journal of renal injury prevention. 2016;5(1):1–7. Epub 2016/04/14. PubMed Central PMCID: PMCPMC4827378. doi: 10.15171/jrip.2016.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kodali SK, Williams MR, Smith CR, Svensson LG, Webb JG, Makkar RR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. The New England journal of medicine. 2012;366(18):1686–95. Epub 2012/03/27. doi: 10.1056/NEJMoa1200384 [DOI] [PubMed] [Google Scholar]
- 9.Hinterbuchner L, Strohmer B, Hammerer M, Prinz E, Hoppe UC, Schernthaner C. Frailty scoring in transcatheter aortic valve replacement patients. European journal of cardiovascular nursing: journal of the Working Group on Cardiovascular Nursing of the European Society of Cardiology. 2016;15(6):384–97. Epub 2015/07/29. [DOI] [PubMed] [Google Scholar]
- 10.Thongprayoon C, Cheungpasitporn W, Srivali N, Ungprasert P, Kittanamongkolchai W, Greason KL, et al. Acute kidney injury after transcatheter aortic valve replacement: a systematic review and meta-analysis. American journal of nephrology. 2015;41(4–5):372–82. Epub 2015/06/27. doi: 10.1159/000431337 [DOI] [PubMed] [Google Scholar]
- 11.Thongprayoon C, Cheungpasitporn W, Kittanamongkolchai W, Srivali N, Greason KL, KB K. Changes in kidney function among patients undergoing transcatheter aortic valve replacement. J Renal Inj Prev 2017;6(3):216–21. [Google Scholar]
- 12.Thongprayoon C, Cheungpasitporn W, Srivali N, Harrison AM, Gunderson TM, Kittanamongkolchai W, et al. AKI after Transcatheter or Surgical Aortic Valve Replacement. Journal of the American Society of Nephrology: JASN. 2016;27(6):1854–60. Epub 2015/10/22. PubMed Central PMCID: PMCPMC4884118. doi: 10.1681/ASN.2015050577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen MA. Frailty and cardiovascular disease: potential role of gait speed in surgical risk stratification in older adults. Journal of geriatric cardiology: JGC. 2015;12(1):44–56. Epub 2015/02/14. PubMed Central PMCID: PMCPMC4308458. doi: 10.11909/j.issn.1671-5411.2015.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. Journal of the American Medical Directors Association. 2013;14(6):392–7. Epub 2013/06/15. PubMed Central PMCID: PMCPMC4084863. doi: 10.1016/j.jamda.2013.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Revenig LM, Canter DJ, Kim S, Liu Y, Sweeney JF, Sarmiento JM, et al. Report of a Simplified Frailty Score Predictive of Short-Term Postoperative Morbidity and Mortality. Journal of the American College of Surgeons. 2015;220(5):904–11.e1. Epub 2015/04/25. doi: 10.1016/j.jamcollsurg.2015.01.053 [DOI] [PubMed] [Google Scholar]
- 16.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. Journal of the American College of Surgeons. 2010;210(6):901–8. Epub 2010/06/01. doi: 10.1016/j.jamcollsurg.2010.01.028 [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59(3):255–63. Epub 2004/03/20. [DOI] [PubMed] [Google Scholar]
- 18.Sepehri A, Beggs T, Hassan A, Rigatto C, Shaw-Daigle C, Tangri N, et al. The impact of frailty on outcomes after cardiac surgery: a systematic review. The Journal of thoracic and cardiovascular surgery. 2014;148(6):3110–7. Epub 2014/09/10. doi: 10.1016/j.jtcvs.2014.07.087 [DOI] [PubMed] [Google Scholar]
- 19.Baek SH, Lee SW, Kim SW, Ahn SY, Yu MY, Kim KI, et al. Frailty as a Predictor of Acute Kidney Injury in Hospitalized Elderly Patients: A Single Center, Retrospective Cohort Study. PloS one. 2016;11(6):e0156444 Epub 2016/06/04. PubMed Central PMCID: PMCPMC4892677. doi: 10.1371/journal.pone.0156444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green P, Woglom AE, Genereux P, Daneault B, Paradis JM, Schnell S, et al. The impact of frailty status on survival after transcatheter aortic valve replacement in older adults with severe aortic stenosis: a single-center experience. JACC Cardiovascular interventions. 2012;5(9):974–81. Epub 2012/09/22. PubMed Central PMCID: PMCPMC3717525. doi: 10.1016/j.jcin.2012.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stortecky S, Windecker S, Pilgrim T, Huber C, Khattab AA, Kadner A, et al. The association of body mass index with clinical outcome in patients with severe aortic stenosis undergoing transcatheter aortic valve implantation. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2012;8:N215. [DOI] [PubMed] [Google Scholar]
- 22.Puls M, Sobisiak B, Bleckmann A, Jacobshagen C, Danner BC, Hunlich M, et al. Impact of frailty on short- and long-term morbidity and mortality after transcatheter aortic valve implantation: risk assessment by Katz Index of activities of daily living. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2014;10(5):609–19. Epub 2014/08/20. [DOI] [PubMed] [Google Scholar]
- 23.Green P, Arnold SV, Cohen DJ, Kirtane AJ, Kodali SK, Brown DL, et al. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). The American journal of cardiology. 2015;116(2):264–9. Epub 2015/05/13. PubMed Central PMCID: PMCPMC4475494. doi: 10.1016/j.amjcard.2015.03.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto M, Hayashida K, Watanabe Y, Mouillet G, Hovasse T, Chevalier B, et al. Effect of body mass index <20 kg/m(2) on events in patients who underwent transcatheter aortic valve replacement. The American journal of cardiology. 2015;115(2):227–33. Epub 2014/12/24. doi: 10.1016/j.amjcard.2014.10.026 [DOI] [PubMed] [Google Scholar]
- 25.Alfredsson J, Stebbins A, Brennan JM, Matsouaka R, Afilalo J, Peterson ED, et al. Gait Speed Predicts 30-Day Mortality After Transcatheter Aortic Valve Replacement: Results From the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2016;133(14):1351–9. Epub 2016/02/28. doi: 10.1161/CIRCULATIONAHA.115.020279 [DOI] [PubMed] [Google Scholar]
- 26.Koifman E, Kiramijyan S, Negi SI, Didier R, Escarcega RO, Minha S, et al. Body mass index association with survival in severe aortic stenosis patients undergoing transcatheter aortic valve replacement. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions. 2016;88(1):118–24. Epub 2015/12/31. [DOI] [PubMed] [Google Scholar]
- 27.Saji M, Lim DS, Ragosta M, LaPar DJ, Downs E, Ghanta RK, et al. Usefulness of Psoas Muscle Area to Predict Mortality in Patients Undergoing Transcatheter Aortic Valve Replacement. The American journal of cardiology. 2016;118(2):251–7. Epub 2016/05/30. doi: 10.1016/j.amjcard.2016.04.043 [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535 Epub 2009/07/23. PubMed Central PMCID: PMC2714657. doi: 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 30.Herzog R, Alvarez-Pasquin MJ, Diaz C, Del Barrio JL, Estrada JM, Gil A. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC public health. 2013;13:154 PubMed Central PMCID: PMC3602084. doi: 10.1186/1471-2458-13-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. PubMed Central PMCID: PMC192859. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337(8746):867–72. Epub 1991/04/13. [DOI] [PubMed] [Google Scholar]
- 34.Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. European heart journal. 2012;33(19):2403–18. Epub 2012/10/03. doi: 10.1093/eurheartj/ehs255 [DOI] [PubMed] [Google Scholar]
- 35.Travison TG, Nguyen AH, Naganathan V, Stanaway FF, Blyth FM, Cumming RG, et al. Changes in reproductive hormone concentrations predict the prevalence and progression of the frailty syndrome in older men: the concord health and ageing in men project. The Journal of clinical endocrinology and metabolism. 2011;96(8):2464–74. Epub 2011/06/17. doi: 10.1210/jc.2011-0143 [DOI] [PubMed] [Google Scholar]
- 36.Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clinical journal of the American Society of Nephrology: CJASN. 2015;10(3):500–14. Epub 2014/11/08. PubMed Central PMCID: PMCPMC4348689. doi: 10.2215/CJN.07830814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thongprayoon C, Cheungpasitporn W, Srivali N, Kittanamongkolchai W, Greason KL, Kashani KB. Incidence and risk factors of acute kidney injury following transcatheter aortic valve replacement. Nephrology (Carlton, Vic). 2016;21(12):1041–6. Epub 2015/12/30. [DOI] [PubMed] [Google Scholar]
- 38.Thongprayoon C, Cheungpasitporn W, Gillaspie EA, Greason KL, Kashani KB. The risk of acute kidney injury following transapical versus transfemoral transcatheter aortic valve replacement: a systematic review and meta-analysis. Clinical kidney journal. 2016;9(4):560–6. Epub 2016/08/02. PubMed Central PMCID: PMCPMC4957730. doi: 10.1093/ckj/sfw055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thongprayoon C, Cheungpasitporn W, Srivali N, Harrison AM, Kittanamongkolchai W, Greason KL, et al. Transapical versus transfemoral approach and risk of acute kidney injury following transcatheter aortic valve replacement: a propensity-adjusted analysis. Renal failure. 2016:1–6. Epub 2016/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thongprayoon C, Cheungpasitporn W, Gillaspie EA, Greason KL, Kashani KB. Association of blood transfusion with acute kidney injury after transcatheter aortic valve replacement: A meta-analysis. World journal of nephrology. 2016;5(5):482–8. Epub 2016/09/21. PubMed Central PMCID: PMCPMC5011255. doi: 10.5527/wjn.v5.i5.482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thongprayoon C, Cheungpasitporn W, Podboy AJ, Gillaspie EA, Greason KL, Kashani KB. The effects of contrast media volume on acute kidney injury after transcatheter aortic valve replacement: a systematic review and meta-analysis. Journal of evidence-based medicine. 2016;9(4):188–93. Epub 2016/06/18. doi: 10.1111/jebm.12208 [DOI] [PubMed] [Google Scholar]
- 42.Cheungpasitporn W, Thongprayoon C, Kashani K. Updates on the risk factors of acute kidney injury after transcatheter aortic valve replacement. J Renal Inj Prev. 2017;6(1):16–17. doi: 10.15171/jrip.2017.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kleczynski P, Dziewierz A, Bagienski M, Rzeszutko L, Sorysz D, Trebacz J, et al. Impact of frailty on mortality after transcatheter aortic valve implantation. American heart journal. 2017;185:52–8. Epub 2017/03/08. doi: 10.1016/j.ahj.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 44.Kleczynski P, Dziewierz A, Bagienski M, Rzeszutko L, Sorysz D, Trebacz J, et al. Long-Term Mortality and Quality of Life After Transcatheter Aortic Valve Insertion in Very Elderly Patients. The Journal of invasive cardiology. 2016;28(12):492–6. Epub 2016/10/16. [PubMed] [Google Scholar]
- 45.Yamamoto M, Shimura T, Kano S, Kagase A, Kodama A, Sago M, et al. Prognostic Value of Hypoalbuminemia After Transcatheter Aortic Valve Implantation (from the Japanese Multicenter OCEAN-TAVI Registry). The American journal of cardiology. 2017;119(5):770–7. Epub 2016/12/27. doi: 10.1016/j.amjcard.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 46.Garg L, Agrawal S, Pew T, Hanzel GS, Abbas AE, Gallagher MJ, et al. Psoas Muscle Area as a Predictor of Outcomes in Transcatheter Aortic Valve Implantation. The American journal of cardiology. 2017;119(3):457–60. Epub 2016/12/10. doi: 10.1016/j.amjcard.2016.10.019 [DOI] [PubMed] [Google Scholar]
- 47.Barzilay JI, Blaum C, Moore T, Xue QL, Hirsch CH, Walston JD, et al. Insulin resistance and inflammation as precursors of frailty: the Cardiovascular Health Study. Archives of internal medicine. 2007;167(7):635–41. Epub 2007/04/11. doi: 10.1001/archinte.167.7.635 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Text A in S2 File: Search Strategy
Fig A in S2 File: Funnel plot of included studies in the meta-analysis for the risk of AKI after TAVR in frail patients. RR = risk ratio, SE = standard error.
Fig B in S2 File: Funnel plot of included studies with adjusted analysis in the meta-analysis for the risk of mortality after TAVR in frail patients. RR = risk ratio, SE = standard error.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.